Abstract
Free full text

NADPH OXIDASES IN LUNG BIOLOGY AND PATHOLOGY. HOST DEFENSE ENZYMES, AND MORE
Abstract
The deliberate production of reactive oxygen species (ROS) by phagocyte NADPH oxidase is widely appreciated as a critical component of antimicrobial host defense. Recently, additional homologs of NADPH oxidase (NOX) have been discovered throughout the animal and plant kingdoms, which appear to possess diverse functions in addition to host defense, including cell proliferation, differentiation, and regulation of gene expression. Several of these NOX homologs are also expressed within the respiratory tract, where they participate in innate host defense as well as in epithelial and inflammatory cell signaling and gene expression, and fibroblast and smooth muscle cell proliferation, in response to bacterial or viral infection and environmental stress. Inappropriate expression or activation of NOX/DUOX during various lung pathologies suggests their specific involvement in respiratory disease. This review summarizes the current state of knowledge regarding the general functional properties of mammalian NOX enzymes, and their specific importance in respiratory tract physiology and pathology.
INTRODUCTION
The regulated production of reactive oxygen species (ROS) by NADPH oxidase has long been considered a unique property of phagocytic cells, which use this enzyme system to assist in their killing of invading microorganisms. This critical property of NADPH oxidase was supported by discovery of genetic defects in this enzyme system, which are responsible for the development of chronic granulomatous disease (CGD), a condition characterized by a failure to mount effective defense against bacteria and fungi, and resulting in severe and recurrent infections. Conversely, excessive production of reactive oxygen species (ROS) by NADPH oxidase is commonly thought to be responsible for tissue injury associated with a range of chronic inflammatory diseases, as supported by detection of characteristic stable oxidation products within inflamed tissues, indicative of collateral oxidative cell or tissue injury due to exaggerated or dysregulated activation of this host defense system. This relatively simplistic view of ROS biology has been substantially challenged and refined with the recent discovery of several homologs of the phagocyte NADPH oxidase. We now know that a number of NADPH homologs are present in many diverse aerobic organisms, with appear to have evolved by using these enzyme systems for regulated ROS production in various aspects of cell biology. Indeed, recent studies over the past several years have linked NADPH oxidases (NOX’s) to cellular processes as diverse as cell proliferation, migration, differentiation, immunomodulation, and oxygen sensing (1–4).
The respiratory tract is a unique organ system that has evolved to provide an optimal area of contact with the external environment to allow for efficient O2 delivery for appropriate tissue oxygenation and function. As a result, the respiratory tract is highly exposed and vulnerable to environmental challenges, including airborne microbes, viruses, and other irritants and allergens, and therefore relies on a potent innate defense system that enables it to succesfully combat these environmental dangers without compromising lung function. It is therefore not surprising that several NOX isozymes are present within the airway, within a number of different cell types, as a critical component of local host defense, analogous to the phagocyte NADPH oxidase system. NOX-dependent host defense mechanisms are not simply restricted to oxidative killing mechanisms, but also entail their contribution to cell signaling mechanisms that regulate innate host defenses or airway responses to injury. Furthermore, several NOX enzymes that are present within structural lung cell types are known regulate cell proliferation, migration, and/or differentiation, suggesting that altered expression or activation of these NOX isoforms may also contribute to several lung pathologies by participating in tissue repair and/or remodeling. This review will discuss some general aspects of NOX regulation and biology, and summarizes current knowledge regarding the presence and functional roles of various NOX isoforms within the respiratory tract, and their proposed involvement in airway biology and disease.
THE NOX FAMILY
The first described involvement of a mammalian NADPH oxidase in biological ROS formation stems from studies in phagocytic cells, and extensive research over the past several decades have revealed many details about molecular and biochemical aspects of this enzyme system (5, 6). The phagocyte NADPH oxidase centers around a membrane-associated flavocytochrome b558 (gp91phox), which contains binding sites for NADPH and FAD as well as two haems that are critical for transmembrane electron transport from NADPH to molecular O2, to generate superoxide anion (O2•−) and subsequently hydrogen peroxide (H2O2). The gp91phox protein does not act independently, but is associated with membrane-bound protein, p22phox, and is activated after recruitment and phosphorylation of 3 regulatory cytosolic proteins (p47phox, p67phox, and p40phox) and the GTPase, Rac1, which assemble with the membrane-bound subunits to form a functional NADPH oxidase (2, 6). This allows for NADPH oxidase, which is silent in resting phagocytes, to be rapidly activated in response to microbial infection or cell stimulation.
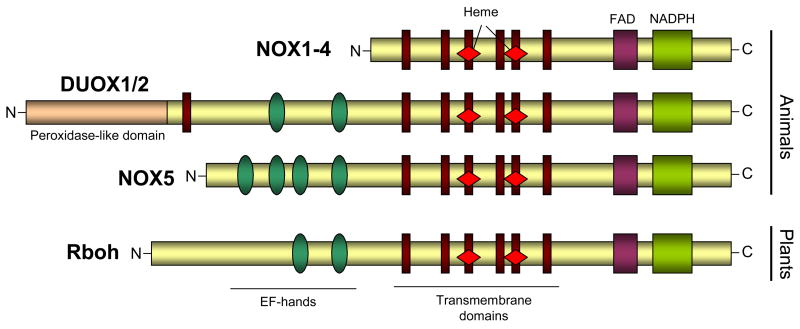
With the completion of the human genome project, various homologs of the phagocyte NADPH oxidase were found to exist, the first of which was identified by two groups in 1999 and 2000, and initially referred to as mitogen oxidase 1 (MOX1) or NADPH oxidase homolog 1 (NOH-1) (7, 8). This homolog is ~60% homologous to gp91phox, and has been renamed NOX1 (2, 6). Soon afterwards, additional homologs were cloned, termed NOX3, NOX4, and NOX5, as well as two larger homologs that are now known as dual oxidase (DUOX) 1 and DUOX2. As illustrated in Fig. 1, the various NOX isoforms share a number of critical structural and functional domains with the “traditional” gp91phox (now also known as NOX2), as well as with NADPH oxidases in non-mammalian organisms such as plants.
In analogy to NOX2, activation of NOX1, NOX3 and NOX4 also requires association with p22phox, and in case of NOX1 (and perhaps NOX3) also requires assembly with Rac and with cytosolic co-factors known as NOX organizer 1 (NOXO1) and NOX activator 1 (NOXA1), which share functional similarities with p47phox and p67phox, respectively (1, 2, 6). However, in contrast to NOX2 activation, the interaction of NOX1 with NOXO1 and NOXA1 results in O2•− production without the need for subunit phosphorylation (5, 6). NOX4 does not appear to rely on cytosolic co-factors and Rac activation and it thought to be a constitutively active enzyme, although this is still a matter of controversy (2, 6). NOX5 and DUOX1/2 differ from the other NOX homologs and contain additional intracellular Ca2+-binding EF-hand domain regions. Consequently, these homologs can be activated by Ca2+-mobilizing stimuli and do not rely on association with p22phox or with cytosolic co-factors (9–12). In this regard, NOX5 and DUOX1/2 relate closely to NADPH oxidases in plants and in protists (Fig. 1), consistent with suggestions that the acquisition of Ca2+ binding domains within these NOX’s may have occurred relatively early in evolution (13–15). The two DUOX proteins contain an additional transmembrane region and N-terminal extracellular peroxidase homology domain with homology to other mammalian heme peroxidases, but the functionality of this extracellular domain of DUOX1/2 is still unknown (1, 2). General features of NOX/DUOX and their regulators and activation mechanisms are summarized in Table I. Additional details regarding the molecular biology and regulation of the various NOX/DUOX enzymes are summarized in several excellent recent reviews (1, 2, 6, 16).
TABLE I
Summary of the various NOX/DUOX enzymes, co-factors, activation mechanisms, and expression in lung cells.
| NOX/DUOX | Regulatory proteins/subunits | Activation mechanism | Expression in lung |
|---|---|---|---|
| NOX1 (NOH-1, MOX1) | p22phox, NOXO1, NOXA1, Rac1 | ? | Airway and alveolar epithelium (?) |
| NOX2 (gp91phox) | p22phox, p47phox (NOXO2), p67phox (NOXA2), p40phox, Rac1/2 | Ca2+, phosphorylation | Myeloid cells (e.g. macrophages, dendritic cells), endothelial cells smooth muscle cells, fibroblasts. |
| NOX3 | p22phox, NOXO1 | ? | ? |
| NOX4 (RENOX) | p22phox | Constitutively active | Endothelial cells, smooth muscle cells, fibroblasts |
| DUOX1 (Thox1, LNOX1) | DUOXA1 | Ca2+, phosphorylation | Airway and alveolar epithelial cells, lymphocytes (?) |
| DUOX2 (Thox2, LNOX2) | DUOXA2 | Ca2+, phosphorylation | Airway and alveolar epithelial cells. |
Alternative names for NOX/DUOX are presented in parentheses. References and clarifications of abbreviations are presented throughout the main text.
GENERAL ASPECTS OF NOX-DEPENDENT SIGNALING
Production of cellular and extracellular mediators
The principal enzymatic function of NOX/DUOX is to catalyze transmembrane transfer of electrons from the cytosolic electron donor NADPH to the principal electron acceptor, O2, within the extracellular space or within specialized cell compartments (e.g. phagosomes) (Fig. 2). This action initially generates superoxide anion (O2•−), and subsequently hydrogen peroxide (H2O2), though spontaneous or superoxide dismutase (SOD)-catalyzed reactions. DUOX1/2 and NOX4 appear to directly generate H2O2 as the primary product, without detectable intermediate production of O2•− (1, 10, 17). The biological actions of NOX/DUOX are therefore thought to be mediated by either H2O2 or O2•− (or indirect secondary ROS production), through their ability to directly (in)activate specific oxidant-sensitive cellular or extracellular targets, thereby affecting various cellular signaling pathways or biological processes. In addition to producing ROS, NOX-mediate electron transfer also induces membrane depolarization and intracellular acidification due to one-electron oxidation of NADPH to generate H+ (Fig. 2). As a result, activation of NOX leads to the activation of voltage-gated H+ channels and/or other ion channels (18–20), as mechanisms of charge compensation. Original studies of NOX1 gene regulation suggested the presence of a short NOX1 splice variant with intrinsic H+ channel activity (8), although this was later realized to be an artifact due to the formation of a stable loop within NOX1 mRNA (6). Intracellular acidification by NOX/DUOX activation may also induce the activation of Na+/H+ exchangers (NHE), which are ubiquitously expressed and are critically involved in the regulation of cell volume and shape, and cell adhesion, migration, and proliferation (21, 22). Therefore, cellular actions of NOX/DUOX may also include ROS-independent mechanisms. Similarly, the antimicrobial action of NOX2 in phagocytes not only involves ROS production, but is also related to NOX-catalyzed extrusion of K+ into the phagosomal vacuole through BKCa channels, which promotes the activation of neutral proteases (23).
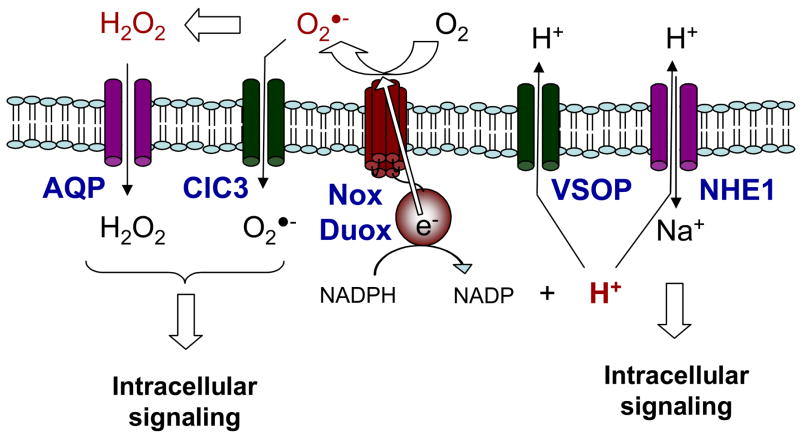
Second messenger production in response to NOX/DUOX activation and association with membrane channels. Activation of NOX/DUOX proteins to produce ROS results in transmembrane electron transfer, causing membrane depolarization and intracellular acidification, leading to activation of e.g. voltage-gated proton channels (VSOP) and Na+/H+ exchange (HNE), which may regulate downstream cellular responses. In addition, intracellular signaling by O2•−/H2O2 may involve their transmembrane transport through aquaporins (AQP) or the chloride channel ClC3.
Targets for NOX-derived ROS: Heme peroxidases
The most widely appreciated mechanism NOX-mediated host defense in phagocytes involves H2O2-mediated activation of locally secreted heme peroxidases, such as myeloperoxidase (MPO) and eosinophil peroxidase (EPO), to generate secondary oxidants that are more potently antimicrobial than H2O2 and/or O2•− themselves (24). Intraphagosomal production of these peroxidase-derived oxidants, which are more reactive and less diffusible than H2O2, may also act on locally secreted antimicrobial proteins, and alter their antimicrobial properties (25). However, compared to the dramatic consequences of NADPH oxidase deficiency (due to mutations in NOX2 or associated proteins), genetic deficiencies in either MPO or EPO are relatively non-consequential and do not result in significant antimicrobial or antifungal defects (26). This suggests that NOX2-mediated antimicrobial actions must also include mechanisms that are independent of heme peroxidases (23).
Similar cooperativity of NADPH oxidase with a locally secreted heme peroxidase also exists within the thyroid gland, which secretes the heme peroxidase thyroperoxidase (TPO) to promote the synthesis of thyroid hormone. The H2O2 that is responsible for TPO activation originates from the NOX homologues DUOX1 and DUOX2 that are highly expressed within thyrocytes (27). The importance of DUOX2 in thyroid hormone synthesis is supported by clinical observations of hypothyroidism in association with missense mutations in the DUOX2 gene (28, 29). DUOX1 and 2 are also expressed in airway and intestinal epithelia, where they may contribute to host defense by a similar cooperative mechanism with lactoperoxidase (LPO), which is secreted by submucosal glands (1, 30, 31). A structurally related dual oxidase, Udx1, is expressed in sea urchin eggs and is activated upon fertilization to produce H2O2 which stimulates ovoperoxidase-mediated protein cross-linking within the fertilization envelope (32).
The close association of DUOX with locally secreted heme peroxidases in these various biological systems is peculiar, since DUOX proteins themselves contain a conserved peroxidase homology domain. However, since the peroxidase-like domain of DUOX lacks conserved histidine residues that are considered essential for heme binding, and its ability to function as a peroxidase function has been questioned (1, 6). Natural missense mutations within the extracellular peroxidase-like domain of DUOX2 result in impaired oxidative protein folding in the endoplasmic reticulum (ER) and trafficking to the plasma membrane, suggesting an alternative functional role for this domain (33, 34). Nevertheless, some studies have claimed intrinsic peroxidase activity associated with DUOX proteins in C. elegans or Drosophila (35, 36), and of airway epithelial DUOX2 (37), although the presence of a separate, as yet unidentified, peroxidase cannot be ruled out. Other possible functions of the peroxidase homology domain of DUOX include a superoxide dismutase-like activity, to promote exclusive H2O2 production (32, 34), or a docking site for secreted peroxidases that utilize DUOX-generated H2O2.
Targets for NOX-derived ROS: Protein cysteine residues
In contrast to phagocyte NOX2 and DUOX1/2, other NOX isozymes have not been functionally associated with locally produced heme peroxidases, and their biological actions are therefore thought to be mediated by interactions of O2•−/H2O2 with other target proteins. In light of the broad range of biological processes that appear to be controlled by the various NOX isozymes (3, 6), it would seem logical that NOX-dependent signaling is mediated by specific oxidative regulation of a relatively small subset of proteins that are involved in widely used cellular signaling mechanisms. This notion is consistent with the relatively limited chemical reactivity of O2•−/H2O2 which would only affect a limited set of cellular targets. As summarized in Fig. 3, the cellular actions of NOX/DUOX appear to be associated primarily with two general signaling mechanisms, Ca2+-mediated signaling and tyrosine phosphorylation, which mediate their diverse overall effects on cell mitosis, differentiation, migration, and immune regulation, in various cellular systems and organisms (1, 2, 38).

General oxidative mechanisms involved in the diverse cellular responses to NOX/DUOX activation. Direct oxidant-sensitive targets for NOX/DUOX (including Ca2+ channels and various protein tyrosine phosphatases, PTPs) are indicated in red, and relationships with downstream affected pathways are illustrated.
The relationship between NOX-mediated oxidant signaling and tyrosine phosphorylation was first indicated by pioneering studies by Finkel and Rhee and their co-workers (39, 40), who demonstrated that the activation of receptor tyrosine kinases by growth factors, such as epidermal growth factor (EGF) or platelet-derived growth factor (PDGF), is associated with transient and regulated cellular production of H2O2, which amplifies tyrosine kinase signaling by temporally inactivating a protein tyrosine phosphatase (PTP). Because of the presence of an invariant catalytic cysteine residue within PTPs, which possesses an unusually low pKa due to the presence of vicinal basic residues, these enzymes are uniquely susceptible to oxidative inactivation, and a number of PTPs or other cysteine-containing phosphatases have been shown to be similarly subjected to oxidative inactivation in relation to cytokine and/or growth factor signaling due to the activation of NOX/DUOX enzymes, including PTP1B, low molecular weight (LMW-) PTP, SHP-1 and SHP-2, and PTEN (3, 41–47) (Fig. 3). Since the PTP superfamily contains over 100 different members (48, 49), NOX/DUOX can be expected to be involved in a highly diverse number of cell signaling pathways by this basic inactivation mechanism. Moreover, NOX/DUOX signaling may also be mediated by similar modifications of susceptible cysteines in other enzyme systems. Intriguingly, the evolution of tyrosine phosphorylation-based signaling may have occurred in coordination with the appearance of NOX genes, and both are used almost exclusively in multicellular eukaryotes and appear to control broadly overlapping cellular functions (3, 38, 49).
Ca2+ signaling
Whereas Ca2+-mobilizing stimuli are capable of activating various NOX enzymes, especially those that contain Ca2+-binding EF-hand domains (NOX5 and DUOX1/2), NOX activation can in turn also regulate Ca2+ signaling, presumably to create a positive feedback loop that allows for rapid signal amplification in discreet cellular regions. NOX-dependent Ca2+ signaling may be mediated by increased voltage-dependent Ca2+ channel opening, as has been demonstrated in angiotensin II signaling in neural cells (50), in B cell receptor-mediated signaling (51), and during NOX-mediated plant root hair outgrowth (52). In addition, NOX-mediated ROS may also induce oxidative cysteine modifications within Ca2+ channel, as was demonstrated for with respect to activation of the ryanodine receptor (53, 54).
Superoxide as a signaling molecule
Based on observations that NOX-dependent signaling is in many cases mitigated by H2O2-degrading enzymes such as catalase or peroxiredoxins (39, 55, 56), it is commonly assumed that H2O2 is the active signaling molecule in these signaling pathways. Indeed, H2O2 readily oxidizes cysteine thiolate moieties, e.g. within PTPs to a sulfenic acid (RSOH) intermediate. Because RSOH usually chemically unstable, it reacts rapidly with either GSH to form a mixed disulfide (RSSG), or with neighboring Cys residues to form intra-molecular or inter-molecular disulfides (RSSR’) (41). Oxidative inactivation of PTP1B was found to be associated with the reversible formation of a cyclic sulfenyl amide intermediate, which causes a conformational change within the enzyme (57, 58). The precise modifications that are critical for ROS-mediated regulation of cysteine-containing proteins are not clear, and secondary modifications to (mixed) disulfides may serve primarily to avoid irreversible oxidations, and allow restoration of enzyme activity by cellular reducing systems (41, 59).
In addition to H2O2, the primary product of NOX activation, O2•−, may also directly participate in NOX-mediated signaling (60). For example, NOX-dependent O2•− can regulate cell signaling by reaction with nitric oxide (NO•), thereby reducing its bioactivity and generating peroxynitrite (ONOO−), a powerful oxidant that can regulate protein function by unique oxidative modifications (61–63). Alternatively, O2•− can directly target Fe-S cluster proteins, such as aconitase, and thereby regulate mitochondrial function and iron homeostasis (64, 65). In addition, O2•− has been shown to directly activate protein kinase C, by interacting with its cysteine-rich zinc finger motif and facilitating zinc release (66), and can inactivate the protein phosphatase calcineurin, presumably by direct oxidation of the binuclear Fe2+-Zn2+ center within the active site (67). Moreover, O2•− can also directly inactivate PTPs, with higher kinetic rate compared to H2O2, to similarly form RSOH and mixed disulfides (68). The relative involvement of either O2•− or H2O2 in NOX/DUOX-mediated signaling may largely depend on the association of specific antioxidant enzymes with these signaling complexes, that control localized O2•− or H2O2 levels. For example, recent studies indicate that cytokine-induced signaling involves endosomal internalization of a receptor signaling complex that includes NOX2 as well as Cu/Zn SOD, which may serve to locally regulate H2O2 production and downstream signaling (69).
Location matters
The involvement of NOX enzymes in such diverse and crucial biological actions suggests that their expression and activation must be under tight control (2, 3), which is illustrated by the diversity in the activation mechanisms of the various NOX/DUOX proteins and the contribution of various co-factors that control NOX cellular localization and activity. Additionally, NOX-mediated effects on e.g. cell proliferation or migration also depend on strict spatial localization of NOX activation in association with the cytoskeleton (70, 71) or in membrane rafts (43, 72, 73), and on direct interactions with target proteins (3, 43, 55). This allows for effective oxidative signaling in the presence of abundant cytosolic antioxidant mechanisms (Cu/Zn SOD, GSH peroxidase), and assures confined oxidant production and target specificity. For example, PDGF signaling was recently found to be subjected to regulation by peroxiredoxin II, through its direct association with PDGFR, but not by other H2O2-metabolizing enzymes (catalase, GSH peroxidase) that do not interact with this signaling complex (55).
Based on the protein topology of NOX proteins, as well as experimental evidence, these enzymes generate O2•−/H2O2 either extracellularly or within phagosomal/endosomal compartments (Fig. 2). In light of the tight spatial organization of NOX enzymes with their signaling partners (3), it is not intuitive how extracellular generation of O2•−/H2O2 would effectively target intracellular targets within such signaling complexes (e.g. PTPs), because this would require ROS to rapidly cross a membrane lipid bilayer before diffusing away or interacting with extracellular targets. Even though NOX activation may be spatially confined within membrane rafts or in internalized endosomal structures (69, 74), which limits the action sphere of NOX-generated O2•−/H2O2 and shields them from extracellular antioxidant enzymes, these molecules are still segregated from their putative intracellular targets by a lipid membrane. H2O2 is readily capable of freely diffusing across lipid bilayers, but recent studies indicate that such transmembrane diffusion is restricted and is facilitated by the presence of aquaporins (75, 76). Similarly, transmembrane diffusion of O2•− is limited because it exists primarily as an anion at physiological pH (pKa of protonated form HOO• is 4.8). Intriguing recent observations suggest that NOX-generated O2•− can enter cells through the chloride channel-3 (ClC3) and thereby activate intracellular signaling events (77). The ClC3 channel also appears to be critical for neutrophil phagocytic activity and migration, which is linked to affects on intracellular ROS formation (78). Thus autocrine NOX-mediated oxidative signaling may depend on the presence and activity of aquaporins or (an)ion channels, which regulate intracellular distribution of O2•−/H2O2 (Fig. 2). O2•−/H2O2-mediated signaling may then be mediated by intracellular diffusion towards their targets, although it is more likely that these channels may closely interact with NOX signaling complex and their oxidizable targets.
NOX ISOZYMES WITHIN THE RESPIRATORY TRACT
Analysis of total lung or airway mRNA revealed the presence of substantial amounts of NOX2 as well as DUOX1 and DUOX2, and low amounts of NOX1 and NOX4 (35, 79–81). Although expression of NOX originates primarily from alveolar macrophages and other inflammatory cell types, the other NOX/DUOX enzymes are largely expressed in non-phagocytic cells within the lung, including airway and alveolar epithelial cells, pulmonary endothelial cells, fibroblasts, and smooth muscle cells. The presence of these NOX/DUOX isozymes in these structural lung cell types suggests their functional involvement in various aspects of lung biology in addition to their presumed roles as host defense enzymes. General features of NOX/DUOX and their expression in lung cells is summarized in Table I. The remainder of this review will summarize current knowledge regarding the regulation and functional aspects of the major lung NOX homologs, and discuss emerging concepts regarding their roles in either physiological and pathological conditions.
NADPH oxidases within the airway epithelium: DUOX1 and DUOX2
Pioneering studies published in 1992 demonstrated that airway epithelial cells are capable of producing H2O2 in response to cell stimulation, although the source of epithelial ROS production was unclear at the time (82). The fact that ROS production by polarized epithelia occurred only apically and could be stimulated by activators of protein kinase C (82), known to activate the phagocyte NADPH oxidase (5), suggested regulated and compartmentalized epithelial ROS production by an NADPH oxidase. The source of epithelial ROS was identified only a few years ago, after initial discovery of prominent expression of the NOX homologs DUOX1 and DUOX2 within the lung (35), and studies on lung tissues using in situ hybridization and immunohistochemistry, which revealed the presence of DUOX1 at the apical surface of tracheobronchial epithelial cells, and DUOX2 within salivary and submucosal glands (19, 79, 83, 84). Expression of other NOX isoforms (e.g. NOX1 and NOX2) and their regulatory proteins within airway or alveolar epithelial cells has been reported (19, 85), but studies using RNAi strategies demonstrated the importance of DUOX1/2 as primary sources of epithelial production of H2O2 (37, 79, 86, 87). Similarly, alveolar type II cells also generate NADPH oxidase-mediated O2•−/H2O2 (88, 89), which is due to the presence of DUOX1 (90). Although DUOX expression in the lung is primarily localized to airway or alveolar epithelial cells, recent studies have also suggest the presence of DUOX proteins in lymphocytes (51, 91).
Molecular biology of DUOX1/2
DUOX1 and DUOX2 were first cloned from thyroid tissues and were originally identified as p138Thox or ThOX1/2 (11, 92). Both DUOX proteins are highly similar N-glycosylated proteins (with 83% homology) with a molecular weight of ~180 kD. While mature N-glycosylated DUOX proteins are localized to the plasma membrane, substantial amounts of DUOX protein are also found intracellularly (27, 32), presumably in association with the ER. Heterologous DUOX1/2 expression in non-epithelial cell types typically does not result in a functional H2O2-generating enzyme at the plasma membrane, presumably because these proteins are not fully glycosylated and are retained in the ER (10, 93). This was recently resolved by the discovery of maturation factors of DUOX (DUOXA1/2), which are ER-resident transmembrane proteins that facilitate ER-to-Golgi transition and translocation of DUOX proteins to the plasma membrane (93). Analysis of particulate fractions of DUOX2-transfected HEK293 or Chinese hamster ovary cells, however, suggests that ER-retained DUOX may not be without function, and is capable of producing H2O2/O2•− upon Ca2+ stimulation in the absence of cytosolic activators or organizer subunits (10).
To date, relatively little is known about transcriptional regulation of DUOX (83, 90, 94). DUOX1 and its maturation factor DUOXA1 are both located on chromosome 15q15.3 in a complex locus in a head-to-head arrangement (92–94), and may share a bidirectional promoter. The same is true for their paralogs DUOX2/DUOXA2. Bidirectionally promoted gene pairs represent ~10% of all transcriptional units and have a higher probability of coordinated expression than random pairs of genes (95). Accordingly, expression of DUOX1/DUOXA1 and DUOX2/DUOXA2 in airway epithelial cells or thyrocytes have been found to be closely coordinated (90, 93). The promoter regions of human DUOX1 and DUOX2 are, however, markedly different. Both lack a TATA box, and the DUOX1 promoter is GC-rich and contains three putative SP1-binding elements, whereas the DUOX2 promoter does not contain any SP1-binding elements (94). Expression of DUOX1 and DUOX2 in thyrocytes was found to be inducible in response to cAMP stimulation by forskolin (12, 92), but this was not observed in human epithelial cells ((90) and unpublished results), and the human DUOX promoters does not appear to contain a cAMP-responsive element (94). The differences in DUOX1/2 promoter regions are consistent with the highly variable transcriptional regulation of airway epithelial DUOX1 and DUOX2 in response to pro-inflammatory cytokines or to bacterial or viral stimuli (36, 83, 96). For example, DUOX1 mRNA expression in primary airway epithelial cultures is induced 3–5 fold by the Th2 cytokines interleukin (IL)-4 and IL-13 (83), which induce ciliated-to-mucus cell transdifferentiation and mucus hyperplasia during allergic airway inflammation (97, 98). DUOX1 expression is also dramatically enhanced after hormonal differentiation of fetal lung epithelial cells to alveolar type II cells (90). In contrast, airway epithelial expression of DUOX2, but not DUOX1, is dramatically upregulated by the Th1 cytokine IFN-γ, and after rhinovirus or bacterial infection (36, 83, 96). DUOX1/2 expression in human thyrocytes was recently found to be downregulated in the presence of IL-1α/IFN-γ (99). The variable expression of DUOX1/2 by Th1 and Th2 cytokines suggests divergent roles of these enzymes in either innate or adaptive immune host defenses.
Functions of epithelial DUOX: Host defense and intracellular signaling
Because DUOX is located primarily at the apical surface of polarized airway or intestinal epithelia, and mediates luminal production of H2O2, it was originally hypothesized that epithelial DUOX functions as a component of innate host defense against commonly inhaled microorganisms, by producing H2O2 as a substrate for locally secreted LPO to generate antimicrobial oxidants (1, 30, 31, 86). LPO catalyzes H2O2-mediated oxidation of the pseudohalide thiocyanate (SCN−), a primary peroxidase substrate within airway secretions at concentrations up to 0.5 mM (30), to generate hypothiocyanate (HOSCN), a stronger oxidizing and antimicrobial agent compared to H2O2 (30, 100). Direct experimental evidence for the role of DUOX in fueling this LPO-SCN−-mediated antimicrobial host defense system was recently obtained in in vitro studies of rat and bovine tracheal epithelial cells (86). Although LPO is a secreted enzyme, interactions with hyaluronan at the apical epithelial surface protect it from mucocilliary clearance (101), and presumably facilitate efficient DUOX-LPO interaction at the airway surface. Acid secretion and acidification of the airway surface fluid by epithelial DUOX activation (Fig. 2) may further promote oxidant-mediated bacterial killing (19, 102).
The principal function of the respiratory epithelium is to provide a physical barrier to protect underlying structures from inhaled microorganisms or pollutants, but the airway epithelium also responds to various environmental stimuli by producing a range of biological mediators that signal to other lung cell types or cells of the immune system. Moreover, the airway epithelium also generates airway mucins that facilitate mucociliary clearance of inhaled particulates or microorganisms (103, 104). Recent studies suggest that DUOX may participate in these responses as well, as illustrated by DUOX1-mediated production of epithelial mucins, such as MUC1 and MUC5AC, in response to inflammatory mediators such as neutrophil elastase or tumor necrosis factor (TNF)-α (105, 106). Similarly, DUOX1 also mediates epithelial production of the neutrophil chemokine IL-8 in response to stimulation by bacterial lipopolysaccharide (LPS) (107). Hence, DUOX1 appears to be involved in other aspects of innate host defense in addition to direct microbial killing, and participates in epithelial signaling pathways that result in increased production of various epithelial mediators. The general role of DUOX1/2 in both innate and adaptive host defenses is supported more indirectly by their variable activation and induction by Th1 and Th2 cytokines and by bacterial stimuli (83, 96, 108), and by dramatic induction of alveolar epithelial DUOX1 during late gestation and alveolar maturation (90), which is consistent with its suggested importance in maintaining post-natal alveolar sterility.
Levels of DUOX1 expression in airway or alveolar epithelial cells increase markedly during air-liquid interface culture or after hormonal differentiation (83, 90) (van der Vliet, unpublished results), which suggests that DUOX1 may play additional roles related to lung development or epithelial cell differentiation. Accordingly, several recent studies have indicated critical functions of DUOX proteins in various developmental stages in other organisms, such as C. elegans or Drosophila (32, 35, 36, 109). Moreover, mammalian DUOX share structural and functional characteristics with NOX homologs in lower organisms and plants (e.g. Fig. 1), which are critically involved in cell growth and differentiation and morphogenesis (13, 38, 52, 110, 111). Because the airway epithelium is continuously subjected to injury due to environmental stress, it possesses an exceptional capacity to repair itself (112, 113), through a coordinated response involving rapid spreading and migration of neighboring cells, cell proliferation and redifferentiation, to restore the original mucociliary epithelium (103, 114, 115). Using in vitro wound models in cultured airway epithelial systems, DUOX1 was found to play a critical role in cell migration as part of the wound repair response (87, 108), illustrating an additional function of DUOX1 in maintenance of epithelial integrity. This is not a unique property of DUOX1, as other NOX isoforms have also been linked to proliferation and/or migration in various other cell types (70, 71, 116, 117), pointing to a possible common mechanism by which NOX/DUOX activation promotes these processes.
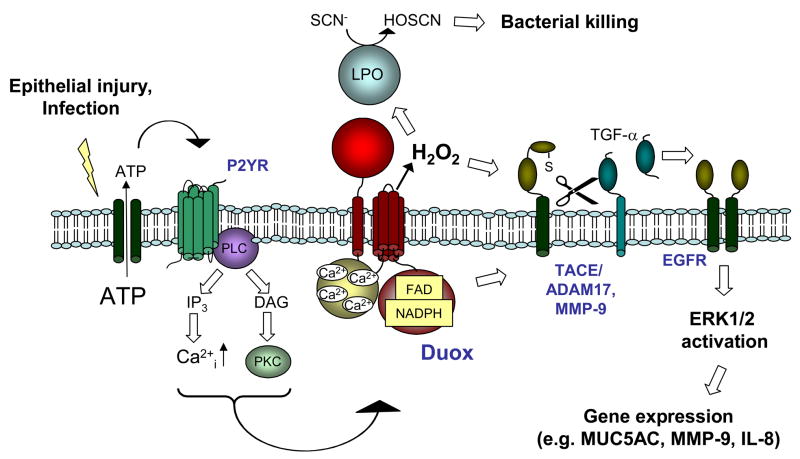
The mechanism by which DUOX promotes epithelial mucin or cytokine production, as well as epithelial cell migration and repair, appears to involve a common signaling pathway leading to the production of epidermal growth factor (EGF) family growth factors and the activation of EGFR and extracellular signal-regulated kinases (ERK1/2) (103, 106, 118–121), as illustrated in Fig. 4. Critical to the activation of these signaling pathways is the proteolytic cleavage of membrane-associated EGFR ligands by cell surface proteases, such as TNFα-converting enzyme (TACE; ADAM17), a member of the a disintegrin and metalloproteinase (ADAM) family of metzincin metalloproteinases (105, 108, 121), and matrix metalloproteinases (MMPs) such as gelatinase B (MMP-9), which play critical roles in epithelial host defense and in epithelial repair in response to injury (104, 122, 123). An important common structural and functional feature shared by the ADAM/MMP proteases is the presence of an invariant cysteine-containing pro-domain, which maintains latency by ligating the active site Zn2+, and is susceptible to oxidative disruption (124–127). Hence, the attractive hypothesis has been put forth that DUOX-derived H2O2 causes direct activation of cell surface MMP/ADAMs and thereby initiates EGFR-mediated cell signaling (87, 105, 108) (Fig. 4). However, experimental evidence for this is mostly indirect, and other (intracellular) mechanisms cannot be ruled out. For example, H2O2 itself is a poor direct activator of MMP-9, and oxidant-mediated MMP-9 activation appears to depend primarily on increases in MMP-9 expression (87, 128). Also, the Cys-containing prodomain of TACE (ADAM17) functions primarily as an intramolecular chaperone that prevents TACE degradation and aids in its secretion (129), activation of proTACE occurs primarily intracellularly in a late Golgi compartment by proteolytic removal of the prodomain by furin-like proconvertases (PC) (130), which depends primarily on Ca2+ signaling and intracellular acidification (131). Since DUOX may largely localize intracellularly in association with the ER, and is capable of promoting transmembrane H+ transport (19), DUOX-mediated activation of PC-TACE proteolytic cascades could conceivably involve intracellular and H2O2-independent mechanisms. Nevertheless, DUOX1 may also participate in cellular redox signaling, as indicated by a recent yeast-two-hybrid screen, which identified an EF-hand binding protein (EFP1), containing two thioredoxin domains, as a DUOX1-associated protein (132). However, no direct evidence exists at present for direct association of DUOX1 with e.g. tyrosine phosphorylation-mediated signaling.
Mechanisms of DUOX activation
The presence of EF-hand Ca2+ binding domains within DUOX suggests that it can be directly activated by Ca2+-mobilizing stimuli. Indeed, Ca2+ mobilization by various diverse stimuli were been found to promote apical H2O2 production in airway epithelial cells (10, 27, 84, 133). Moreover, in analogy to activation of NOX2 in phagocytes, H2O2 production by airway epithelial cells or thyrocytes was also stimulated in response to activation of protein kinase C (PKC), and Ca2+ and PKC stimulation appear to act synergistically in promoting H2O2 production (82, 105, 134, 135). Recent studies of DUOX1 activation in airway epithelial cells by LPS suggested the involvement of Ca2+-dependent isoforms of protein kinase C (PKCα,β) (108), although other stimuli appear to activate DUOX1 via activation of PKCδ (105, 106).
A major common mechanism involved in epithelial Ca2+ signaling and propagation of intercellular Ca2+ signaling in epithelial cell monolayers involves the regulated cellular release of ATP or related purines, which promote paracrine signaling by activating purinergic P2 receptors at the cell surface (136–139). Various forms of chemical or mechanical injury, and cell stimulation by bacterial products or dsRNA, result in release of ATP at levels that are sufficient to activate P2 receptors and mediate Ca2+ signaling (140–144). Extracellular levels of ATP and related purines are also actively regulated by cell surface ectoATPases and ecto-adenylate kinase (145, 146), further illustrating the dynamic nature and complexity of this signaling system. Purinergic receptors that are activated by ATP include P2X receptor channels and P2Y G-protein coupled receptors that mediate the activation of phospholipase C, intracellular Ca2+ mobilization and activation of PKC (139). Several P2X and P2Y isoforms are expressed in airway epithelial cells and are involved in regulating a number of epithelial properties including epithelial anion transport (139, 147), ciliary beat frequency (139), mucin secretion (148–151), production of inflammatory mediators such as CCL20, IL-8, and MMP-9 (87, 152–154), as well as epithelial cell migration and wound repair (155–157). Since P2 receptor stimulation induces Ca2+ mobilization, it is not surprising that extracellular ATP is capable of stimulating DUOX-mediated H2O2 production by airway epithelial cells. This appears to be mediated primarily by P2Y receptor stimulation (84, 87), which mediates intracellular Ca2+ mobilization and activates PKC (Fig. 4) (158, 159). Indeed, a number of downstream effects of P2 receptor activation, such as expression of MUC5AC, MMP-9 or IL-8 and stimulated cell migration, have been linked to activation of DUOX1 (87, 105, 107, 108), suggesting a common association of DUOX with epithelial purinergic signaling. Intriguingly, NOX activation has similarly been associated with ATP-mediated purinergic signaling in several other mammalian cell types (160–164), and in NADPH oxidase-mediated wound responses in plants (165, 166), which suggests that the general signaling pathway illustrated in Fig. 4 may be widely conserved as a common biological response mechanism to environmental stress.
NOX-mediated signaling in inflammatory-immune cells
The main NOX2-expressing cell types within the respiratory tract include alveolar macrophages and/or other resident or infiltrated inflammatory-immune cells (e.g. neutrophils, eosinophils, lymphocytes). The regulation of NOX2 expression and activation has been extensively reviewed (5, 6), and will therefore not be further discussed here. Although NOX2 in phagocytic cells may primarily serve a host defense function, by providing antimicrobial ROS or by activating cation channels, activation of NOX2 in neutrophils was also found to regulate intracellular signaling pathways, e.g. associated with Fc receptor function, that modulate neutrophil apoptosis and inflammatory gene regulation through the activation of transcription factors such as NF-κB and AP-1 (167). NOX2-regulated signaling in neutrophils primarily involves enhanced activation of tyrosine kinases such as Syk, Hck, Lyn, Fgr, Yes, and Btk, mediated by oxidative inhibition of tyrosine phosphatases such as CD45 and the SH2 domain-containing PTP, SHP-1 (167). Similarly, activation of macrophage NOX2, which produces markedly less ROS compared to neutrophils or eosinophils, has also been linked to regulation of NF-κB and AP-1 signaling, and production of pro-inflammatory cytokines (168), which appears to be associated with S-glutathionylation and inactivation of PTPs, such as PTP-1B and PTEN (164, 169).
NOX2 also appears contribute to antigen-dependent signaling in T and B lymphocytes. This was first suggested by initial observations that H2O2 can mimic antigen receptor stimulation, by negatively regulating the SH2 domain-containing PTPs, SHP-1 and SHP-2 (170). Subsequent studies confirmed that T cells express essential NADPH oxidase (NOX2) components, which contribute to oxidant production and signaling upon T cell receptor (TCR) activation, thereby regulating inflammatory cytokine production and T cell adhesion (47, 171). Similarly, antigen receptor stimulation in B cells has been found to involve the activation of NADPH oxidase, which serves to amplify BCR signaling by inactivation of the tyrosine phosphatase SHP-1, thereby promoting the activation of the protein tyrosine kinase Syk (170). Interestingly, a recent report suggests that NADPH oxidase-dependent BCR signaling is mediated by DUOX1 (51). Finally, dendritic cells (DC), which play a unique role in the initiation of primary specific immune responses, also express NOX2, which is thought to contribute to bacterial killing (172) but is also involved in regulation of antigen processing. This latter property does not seem to be a result of NOX-mediated oxidant production, but is related to NOX-dependent control of phagosomal pH, thereby preventing phagosomal antigen degradation (173, 174).
Functional aspects of NOX in other lung cell types
Oxygen sensing
One of the first reported examples of a role of NADPH oxidase activity in non-immune cells is its proposed involvement in oxygen sensing, thereby mediating various responses to changes in O2 tension (4). In the respiratory tract, pulmonary neuroepithelial bodies (NEBs) within the airway mucosa represent specialized pulmonary structures that are composed of clusters of innervated amine- and peptide-containing cells, and are involved in mediating airway responses to hypoxia. Based on studies with NADPH oxidase inhibitors and gp91phox (NOX2)-deficient mice, it has been demonstrated that these responses are due to the presence of NOX2, which acts as an oxygen sensor by regulating O2-sensitive K+ currents, via altered production of ROS (4, 175). NADPH oxidase (most likely NOX2) has been suggested to play a more universal role as oxygen sensor in various tissues, thereby mediating hypoxic pulmonary vasoconstriction, vascular smooth muscle function, and carotid and airway chemoreceptor activation (4). Accordingly, pulmonary arterial hypertension in response to chronic hypoxia was recently found to be associated with increased ROS production in intrapulmonary arteries, responses that were abolished in NOX2-deficient mice (176).
NOX in pulmonary endothelium
It has long been known that vascular endothelial cells are capable of producing O2•−/H2O2 by activation of a local NADPH oxidase, and endothelial express various NOX isoforms (NOX1, NOX2, and NOX4) and their critical cofactors (116, 177). These NOX’s appear to control several vascular processes, such as vascular tone, vascular cell growth and angiogenesis, and inflammation. Accordingly, pulmonary endothelial cells have been demonstrated to generate NADPH-derived oxidants in response to pulmonary ischemia (178, 179) as well as hyperoxia (180), which is associated with membrane depolarization due to the activation of K(ATP) channels, and resulting in stimulated endothelial cell proliferation and NO production (178, 181, 182). Although these responses were in some cases inhibited by catalase, suggesting the involvement of H2O2 (182), recent studies also suggest a role for O2•− in endothelial NOX signaling (77). Endothelial NOX activation in response to hyperoxia was found to occur in association with the actin cytoskeleton via the activation of the non-receptor tyrosine kinase Src, and has been suggested to contribute to decreased alveolar-capillary barrier function as a mechanism of hyperoxia-induced lung injury (183, 184).
Pulmonary smooth muscle cells and fibroblasts: NOX4
Studies in pulmonary fibroblasts and smooth muscle cells revealed the expression of p47phox, p67phox, p22phox and NOX4, and demonstrated increased production of ROS in response to inflammatory mediators, such as tumor necrosis factor β (TNF-β) or transforming growth factor β (TGF-β), which is primary associated with selective upregulation of NOX4 (185–188). In addition, NOX4 expression is also induced by other stimuli, including hypoxia/ischemia, shear stress, and ER stress, but to date no information appears to be available regarding the NOX4 promoter or its transcriptional regulators (6, 16, 81). NOX4 appears to be evolutionary more distant from NOX1-3, and only shares ~39% identity to NOX2. As mentioned earlier, NOX4 requires p22phox for functional activity, but in contrast to other NOX isozymes, NOX4 does not depend on other cytosolic subunits and is believed to be a constitutively active enzyme that appears to regulated primarily at the transcriptional level (6, 17). Recent studies using an inducible heterologous NOX4 expression system confirm the ability of NOX4 to produce ROS without need for a stimulus (189). Moreover, NOX4 produces primarily H2O2, although some evidence was also found for intracellular O2•− production (189).
Studies in isolated smooth muscle cells indicated that NOX4 expression in freshly isolated cells rapidly declines with multiple passages, in close association with loss of smooth muscle differentiation markers such as smooth muscle α-actin (SMA), myosin heavy chain (186, 190). Subsequent studies with NOX4-targeted siRNA demonstrated that NOX4 is required for the expression of smooth muscle differentiation markers, and maintenance of SMA-based stress fibers (190). Similarly, NOX4 was also found to be causally related to the transdifferentiation of cardiac fibroblasts into myofibroblasts in response to TGF-β1 (191), a critical mediator of pulmonary fibrosis. Indeed, while NOX4 is markedly upregulated by TGF-β1, its activity also appears to required for chronic SMAD2/3 activation by TGF-β1, and thus actively contributes to fibroblast activation and transdifferentiation as a major phenotype of fibrotic disease (191). In addition to studies supporting a role for NOX2 in pulmonary hypertension in response to hypoxia (176), it was recently demonstrated that pulmonary artery hypertension is also characterized by induction of NOX4, perhaps in response to initial activation of NOX2 within the pulmonary endothelium, which is responsible for increased ROS generation and smooth muscle cell proliferation (81). Thus, while NOX4 may be a normal component of differentiated pulmonary smooth muscle cells, its upregulation by inflammatory stimuli that promote fibrosis, such as TGF-β, suggests an important contribution of NOX4 in fibrotic lung disease. Recent studies suggest that NOX4 may also function as an oxygen sensor, by regulating the O2-sensitive K+ channel, TASK-1 (192).
Despite common induction of NOX4 by TGF-β1 in smooth muscle cells and fibroblasts, other aspects of NOX4 biology in these various cell types reveal intriguing differences. For example, NOX4-mediated ROS production in human pulmonary artery smooth muscle cells in response to TGF-β occurs primarily intracellularly, and NOX4 appears to localize to the ER or the nucleus (186). Accordingly, NOX4 activation was associated with intracellular ERK1/2 signaling and phosphorylation of nuclear or ER proteins, such as retinoblastoma protein and eukaryotic translation initiation factor 4E binding protein-1, thereby mediating smooth muscle cell proliferation and hypertrophy (186, 193). In contrast, myofibroblasts obtained from patients with iodopathic pulmonary fibrosis were found to produce primarily extracellular H2O2 in response to TGF-β1, and are thereby capable of inducing cell death in neighboring pulmonary epithelial cells, suggesting the association of NOX4 with the plasma membrane as a paracrine signaling molecule (187). Observations of NOX4 in association with SMA-based stress fibers in differentiated vascular smooth muscle cells, which appears to relocalize to focal adhesions during in vitro de-differentiation (190), would implicate that cellular localization of NOX4 varies with cell differentiation status, and that its functional properties may differ between normal healthy tissues and injured tissues that undergo repair and/or remodeling.
NOX ENZYMES IN LUNG DISEASE
The previous sections indicate that various NOX isoforms are expressed within several structural cell types in the respiratory tract, and appear to serve various salutary functions, e.g. in lung development, in cell responses to changes in oxygen tension, or in airway defenses against environmental stress. However, changes in NOX expression or activation during conditions of acute or chronic lung disease are also suggestive of a potential contributing role for NOX in disease pathology. Indeed, it is well appreciated that chronic diseases of the respiratory tract, such as chronic obstructive pulmonary disease (COPD), asthma, cystic fibrosis, or various forms of lung cancer, are associated with increased oxidative stress and enhanced ROS production (194–197), which may largely originate from enhanced and/or inappropriate NOX activation. To explain this paradox of beneficial and potentially detrimental effects of NOX enzymes, Lambeth recently proposed that NOX enzymes represent an example of antagonistic pleiotropy, meaning that potentially harmful genes are retained during evolution because they confer a reproductive advantage during early stages of life (198). In spite of considerable evidence that associates increased ROS production with various respiratory diseases, it is considerably less whether and how these ROS actually contribute to disease pathology, and multiple NOX isozymes may be responsible, with potentially unique consequences. The following sections briefly summarize available evidence linking specific NOX isozymes to lung disease pathology, based on changes in NOX activity or expression, genetic polymorphisms, and animal studies with genetically manipulated mice.
NOX2
It is commonly held that increased ROS production during acute or chronic inflammation largely originates from NOX2 activation in resident and/or recruited phagocytic cells (e.g. neutrophils, eosinophils) within the lung. However, direct evidence that NOX2 activation contributes to respiratory disease pathology is sparse. Genetic mutations in NOX2 (gp91phox), p47phox, p67phox and p22phox are known to be responsible for development of CGD, but minimal evidence exists for associations of these polymorphisms with other diseases, and a recent analysis failed to show an association of polymorphisms within gp91phox, p47phox, p67phox or p22phox with infectious or non-infectious lung diseases, such as tuberculosis or sarcoidosis (199). Attempts to implicate NOX2 or p47phox in models of lung inflammation and injury, using gp91phox−/− or p47phox−/− mice, demonstrated that genetic NADPH oxidase deficiency was in many cases associated with enhanced inflammation and injury (200–203), implicating that NOX2 also has beneficial functions in regulating inflammatory-immune responses. For example, infection of gp91phox−/− mice with the yeast C. neoformans or with influenza virus was in either case found to result in increased Th1-skewed inflammation and granuloma formation, compared to their wild-type counterparts, which led to increased influenza clearance and protection against C. neoformans infection (204, 205). Studies using chimera of gp91phox−/− mice indicated a contribution of non-hemapoetic cell NADPH oxidase to development of airways eosinophilia in a mouse model of allergic inflammation, suggesting a role for NOX2 within the vascular endothelium (206). Studies with gp91phox−/− mice also indicated a role for NOX2, presumbly within the endothelium, in development of pulmonary arterial hypertension in response to chronic hypoxia (176). Because of the various regulatory properties of NOX2 in inflammatory signaling, and their presence in non-hemapoeitic cell lineages, interpretation of studies with NOX2-deficient mice in modeling complex diseases such as asthma or COPD is clearly not straightforward.
DUOX
To date, very little is known regarding the potential contribution of DUOX1/2 to disease, and other than associations of DUOX2 mutations with mild hypothyroidism, no clear associations with lung disease have been reported (199). The fact that DUOX1/2 expression is highly variable under varying conditions of epithelial differentiation and/or inflammatory cytokine milieus, suggests that these enzymes might play important role in various acute and chronic lung diseases. Unfortunately, few studies to date have attempted to address this possibility and no knockout mice for DUOX1/2 are yet available. Analysis of bronchial biopsies from smokers or patients with COPD revealed decreased expression of DUOX1 and ~2-fold upregulation of DUOX2 (207, 208), which might be related to characteristic epithelial alterations within these subjects, such as goblet cell hyperplasia and squamous metaplasia (115, 209). Analysis of bronchial tissues from patients with severe cystic fibrosis indicated decreased DUOX2 expression (210), thus suppressing a component of host defense leading to enhanced respiratory infections in these subjects. The ability of the Th2 cytokines IL-4 and IL-13, which are commonly elevated in allergic asthma, to induce epithelial expression of DUOX1 (83, 87), might suggest a potential contribution of DUOX1 in the pathology of allergic airways disease. Indeed, reported associations of DUOX1 with airway acidification (19), enhanced EGFR activation (87, 105), or mucus production (105), all characteristic features of severe allergic asthma (211, 212), are consistent with this possibility. However, no evidence for this is available at present. Similarly, no information is yet available regarding a possible association of DUOX with lung cancer, although DUOX2 was recently found to be markedly upregulated in colon adenomas in association with MMP-7 (213).
Other NOX’s
Because of the recent discovery of various NOX homologs and incomplete understanding of their biological functions, it is not surprising that very little information exists regarding their potential involvement in lung disease. Observations of NOX4 induction by TGF-β in pulmonary fibroblasts or smooth muscle cells strongly suggest its importance in pulmonary fibrosis or airway remodeling during chronic lung diseases such as asthma (187, 193). Indeed, recent analysis of lung tissues from patients with pulmonary arterial hypertension revealed a ~2.5-fold upregulation of NOX4, which is believed to contribute to oxidant-mediated smooth muscle cell proliferation (81). Myofibroblasts from patients with iodopathic pulmonary fibrosis produce primarily extracellular H2O2 in response to TGF-β1, presumably due to the presence of NOX4 (187). Exposure of mice to cigarette smoke and/or LPS was found to result in increased lung expression of NOXO1, an activator of NOX1 (214), but the potential significance for cigarette smoke-induced emphysema or lung cancer is unclear. Intriguingly, development of pulmonary emphysema in mice due to genetic deficiency of Toll-like receptor (TLR)-4, was recently associated with upregulation of NOX3 in the pulmonary endothelium, which appears to contribute to elastolytic activity (215).
CONCLUDING REMARKS
As can be judged from this review, much has been learned over the past few years regarding the biological properties of NADPH oxidases (NOX) and their postulated functions, which extend significantly from their classically viewed roles as innate host defense enzymes. Moreover, the mechanisms by which NOX enzymes contribute to host defense are not restricted to oxidant-mediated bacterial killing, but clearly involve a number of additional signaling mechanisms that control inflammatory signaling and immune cell function. In spite of these beneficial properties of the NOX’s, alterations in their expression or activation as a result of environmental stresses such as hyp(er)oxia, cigarette smoke exposure, or bacterial or viral infection, and during conditions of chronic airway inflammation such as COPD or cystic fibrosis, would strongly suggest a potential contribution of NOX-derived ROS in various lung pathologies, although clinical evidence for this is still largely lacking. Global analysis of ROS production may not necessarily be very informative, because the contribution of individual NOX’s may be quite variable. Similarly, general strategies of NOX inhibition or antioxidant supplementation may not necessarily be useful, since they would also interfere with beneficial properties of NOX’s, which are still incompletely understood. In this regard, it is understandable why global strategies of antioxidant supplementation to suppress overall oxidant production have been relatively unsuccessful in prevention or treatment of airways diseases, as such approaches to not appreciate the various nuances of NOX biology.
Available studies with NOX-deficient mice to explore the contribution of NOX-derived ROS in models of lung disease clearly illustrate the complexity of NOX biology, and typically did not yield the anticipated outcome. So far, knockout mice for other critical NOX isoforms in the lung, such as DUOX1/2 or NOX4 are not yet available, perhaps due to the fact that these NOX’s may have important roles in cell proliferation and/or differentiation, which suggests that their genetic deletion may result in significant developmental defects or may be incompatible with survival. To better understand the contributions of individual NOX enzymes in lung biology or disease, strategies of cell- or tissue-specific NOX/DUOX gene deletion or overexpression, under the control of conditionally activated promoters, will be required to dissect the specific contributions of individual NOX isozymes within specific lung cell types. As the general field of NOX biology continues to mature, these model systems are likely to become available in the near future. Meanwhile, more studies will be needed to increase our understanding of NOX-mediated signaling, which appears to depend highly on the cellular location of NOX and its association with target proteins, and may include both ROS-dependent and –independent mechanisms. Also, our collective understanding of gene regulation of NOX/DUOX is still rather incomplete at present. Further development of critical research tools, such as specific antibodies against various NOX isoforms or genetic strategies to manipulate NOX/DUOX, which are only partially available at the present time, will be instrumental in this regard. These various deficiencies are hardly surprising in light of the recent discovery of most NOX isozymes. However, the NOX research field is rapidly evolving, with key observations being published almost weekly, and we can therefore anticipate significant new developments over the next several years, which will help sort out some apparent controversies and increase our understanding of NOX biology in general. Such advances will also be critical in evaluating their potential contribution to respiratory tract disease, and could pave the way for more directed and innovative therapeutic strategies.
Acknowledgments
The author wishes to thank Yvonne Janssen-Heininger, Nick Heintz, Gregory Conner, Richart Harper and Horst Fischer for frequent and stimulating discussions regarding lung NOX/DUOX biology and redox signaling, and the NIH (grants HL068865 and HL074295) for research support.
LIST OF ABBREVIATIONS
- ADAM
- a disintegrin and metalloproteinase
- BCR
- B cell receptor
- CGD
- chronic granulomatous disease
- ClC3
- chloride channel 3
- COPD
- chronic obstructive pulmonary disease
- DC
- dendritic cell
- DUOX
- dual oxidase
- DUOXA
- DUOX maturation factor
- EGF(R)
- epidermal growth factor (receptor)
- EPO
- eosinophil peroxidase
- ER
- endoplasmic reticulum
- ERK
- extracellular signal-regulated kinase
- IL
- interleukin
- IFN
- interferon
- LPO
- lactoperoxidase
- LPS
- lipopolysaccharide
- MUC
- mucin
- MMP
- matrix metalloproteinase
- MPO
- myeloperoxidase
- NEB
- neuroepithelial body
- NHE
- sodium-hydrogen exchanger
- NOX
- NADPH oxidase
- NOXA
- NOX activator
- NOXO
- NOX organizer
- PC
- protein convertase
- PDGF(R)
- platelet-derived growth factor (receptor)
- PKC
- protein kinase C
- PMA
- phorbol myristate acetate
- PTEN
- phosphatase and tensin homologue deleted on chromosome ten
- PTP
- protein tyrosine phosphatase
- ROS
- reactive oxygen species
- SHP
- SH2 domain-containing PTP
- SMA
- smooth muscle α-actin
- SOD
- superoxide dismutase
- TACE
- TNF-α converting enzyme
- TCR
- T cell receptor
- TGF-β
- transforming growth factor β
- TLR
- Toll-like receptor
- TNF-α
- tumor necrosis factor α
- TPO
- thyroperoxidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.freeradbiomed.2007.11.016
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2323509?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.freeradbiomed.2007.11.016
Article citations
Geometric constraint-triggered collagen expression mediates bacterial-host adhesion.
Nat Commun, 14(1):8165, 09 Dec 2023
Cited by: 0 articles | PMID: 38071397 | PMCID: PMC10710423
Soluble epoxide hydrolase inhibitors for smoking-associated inflammatory lung diseases and chronic obstructive pulmonary disease: a meta-analytical systematic review of preclinical and clinical studies.
Am J Transl Res, 15(11):6649-6659, 15 Nov 2023
Cited by: 1 article | PMID: 38074809 | PMCID: PMC10703658
Review Free full text in Europe PMC
Overview of the Mechanisms of Oxidative Stress: Impact in Inflammation of the Airway Diseases.
Antioxidants (Basel), 11(11):2237, 13 Nov 2022
Cited by: 34 articles | PMID: 36421423 | PMCID: PMC9687037
Review Free full text in Europe PMC
An Outlook on the Etiopathogenesis of Pulmonary Hypertension in HIV.
Cureus, 14(7):e27390, 28 Jul 2022
Cited by: 3 articles | PMID: 36046315 | PMCID: PMC9418639
Review Free full text in Europe PMC
A newly identified flavoprotein disulfide reductase Har protects Streptococcus pneumoniae against hypothiocyanous acid.
J Biol Chem, 298(9):102359, 09 Aug 2022
Cited by: 11 articles | PMID: 35952759 | PMCID: PMC9483559
Go to all (136) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology.
Physiol Rev, 87(1):245-313, 01 Jan 2007
Cited by: 3968 articles | PMID: 17237347
Review
Role of Nox family NADPH oxidases in host defense.
Antioxid Redox Signal, 8(9-10):1549-1561, 01 Sep 2006
Cited by: 149 articles | PMID: 16987010
Review
[The Nox/Duox family of ROS-generating NADPH oxidases].
Med Sci (Paris), 22(11):953-959, 01 Nov 2006
Cited by: 25 articles | PMID: 17101097
Review
Mucosal reactive oxygen species are required for antiviral response: role of Duox in influenza a virus infection.
Antioxid Redox Signal, 20(17):2695-2709, 15 Oct 2013
Cited by: 61 articles | PMID: 24128054
Funding
Funders who supported this work.
NHLBI NIH HHS (7)
Grant ID: R01 HL074295
Grant ID: HL068865
Grant ID: R01 HL085646
Grant ID: HL074295
Grant ID: R01 HL068865-05
Grant ID: R01 HL074295-04
Grant ID: R01 HL068865