Abstract
Free full text

Immunobiology
Platelet-mediated modulation of adaptive immunity: unique delivery of CD154 signal by platelet-derived membrane vesicles
Abstract
Although mounting evidence indicates that platelets participate in the modulation of both innate and adaptive immunity, the mechanisms by which platelets exert these effects have not been clearly defined. The study reported herein uses a previously documented adoptive transfer model to investigate the ability of platelet-derived membrane vesicles to communicate activation signals to the B-cell compartment. The findings demonstrate for the first time that platelet-derived membrane vesicles are sufficient to deliver CD154 to stimulate antigen-specific IgG production and modulate germinal center formation through cooperation with responses elicited by CD4+ T cells. The data are consistent with the hypothesis that platelets modulate inflammation and adaptive immunity at sites distant from the location of activation and that platelet-derived membrane vesicles are sufficient to mediate the effect.
Introduction
Platelets are a nucleated cellular fragments produced by megakaryocytes and are best known for their role in hemostasis.1 However, accumulating evidence from recent studies indicates an additional role in modulating adaptive immune responses.2–4 In this regard, platelets have been shown to modulate dendritic cell activation,5–8 enhance T-cell responses,5,9 induce B-cell production of IgG antibodies,5,10 and enhance germinal center (GC) formation in cooperation with T cells.11 Despite this support for the idea that platelet activation is necessary for modulation of immunity, the underlying mechanisms by which platelets communicate signals have not been clearly defined. Platelets are activated by diverse stimuli, including infection, inflammation, and injury, and these cause the rapid release of numerous bioactive mediators capable of modulating innate immune cells, activating endothelial cells, and influencing systemic immune responses. The exquisite sensitivity of platelets to the environment, their location and number within the circulation, and the variety of chemical modulators released on their activation make them uniquely suited to play a sentinel role and to provide early signals to immune cells
For approximately a half century, researchers have known that platelets modulate inflammatory cell responses.12,13 Recently, the list of proposed effector functions for platelets was expanded to include a regulatory role in adaptive immune responses.4,5,7,11,14 Most of the studies on which these proposals are based have focused on CD154 (CD40L).15 CD154 is critical to the initiation and propagation of the adaptive immune response and is expressed by several cell types, including CD4+ T cells, CD8+ T cells, γδ-T cells, and platelets.16 Although a wide variety of cells express CD154 messenger RNA, expression of the protein seems to be tightly regulated, adding further evidence of the importance of this molecule in normal biology. Best known as “signal 2” delivered through ligating to its receptor, CD40, during CD4+ T cell–mediated activation of B cells, CD154 is crucial for the development of T cell–dependent humoral immune responses. Humans lacking functional CD154 fail to isotype switch from the IgM antibody isotype, producing a hyper-IgM syndrome. Consistent with this finding, CD154 gene knockout (CD154−/−) mice are incapable of producing IgA, IgE, or IgG in response to T cell–dependent antigens and are also unable to produce the GC response necessary for the differentiation of memory B cells and plasma cells.17
The established paradigm is that the CD154 signal is delivered solely by CD4+ T cells to B cells. Recent reports, however, suggest that this paradigm may need to be revised to include a role for platelet-derived CD154. In vitro, platelets are capable of activating B cells to proliferate and produce antibodies,10 and in vivo they augment IgG production in CD154−/− mice.5 The physiologic relevance is supported by several studies. Experiments in which wild-type mice were depleted of platelets before priming showed a reduction in antigen-specific IgG production, suggesting that platelet-derived CD154 is necessary for an optimized antibody response.5 Further experiments showed, that under conditions of low precursor T cell numbers, platelets deliver a CD154 signal in cooperation with T cells, enhancing GC responses and increasing specific IgG production.11 These data support the hypothesis that platelets are an important early source of CD154 signals that promote optimal immune response and IgG production.
The mechanism whereby platelet-derived CD154 is delivered to splenic B cells has not been investigated. On their activation at a site of injury or inflammation, platelets translocate adhesion molecules to the surface to aid in self-aggregation, and bind to other cells and matrices to prevent the loss of blood and to form a thrombus around the site of injury.1 The aggregation and binding properties of activated platelets make it difficult for fully activated platelets to travel through the circulatory system, through the lungs and to the spleen to act on B cells, although circulating platelets expressing activation markers have been reported.18 An alternative to intact platelets is that factors released after activation mediate platelet-induced responses. Platelet activation results in release of a myriad of soluble factors, including the release 2 types of membrane vesicles: microparticles and exosomes.19 On activation, platelets vesiculate, forming cell membrane-derived microparticles expressing and/or containing membrane and cytoplasmic molecules released on activation. These platelet-derived microparticles (PMPs) are approximately 0.1 to 1.0 μm in diameter in humans and express P-selectin (CD62P) and glycoprotein IIb-IIIa (GP IIb-IIIa).20 PMPs are elevated in peripheral blood as a result of chronic platelet activation in various disease states.21 Moreover, PMPs adhere to a variety of cells, can activate endothelial cells, leukocytes and other platelets, and deliver signals through chemokines, such as RANTES.22–24 Another vesicle released by platelets, exosomes, range in size from 0.04 to 0.1 μm and arise from the internal membrane vesicles of multivesicular bodies and granules in platelets.25 Unlike PMP, exosomes do not share a similar surface phenotype of activated platelets. However, both PMP and exosomes are known to carry and deliver cellular signals, suggesting a potential role in platelet-derived signaling to the adaptive immune compartment that often is far removed from the site of platelet activation.22,25–30 Studies reported herein use a previously documented adoptive transfer model to investigate the ability of platelet-derived membrane vesicles to communicate activation signals to the B-cell compartment to augment antigen-specific IgG production and GC formation.5,11
Methods
Mice and materials
C57Bl/6 (B6) mice were purchased from the National Cancer Institute (Frederick, MD). Breeding pairs of CD154 gene knockout mice (CD154−/−; H-2b background) and B6.RAG1 knockout mice (RAG 1−/−; H-2b background) were purchased from Jackson Laboratories (Bar Harbor, ME). Thrombin, apyrase, and prostaglandin E1 (PGE1) were purchased from Sigma-Aldrich (St Louis, MO). Agonistic anti-CD40 antibody 1C10 and the CD154 blocking antibody MR-1 were purified from serum-free medium (HB101) using conditions that minimize introduction of endotoxin. Antimouse IgG, IgG1, IgG2b, IgG2c, IgG3, and IgM antibodies purchased from Jackson Immunoresearch Laboratories (West Grove, PA). Antimouse tumor necrosis factor-α (TNF-α; clone TN3-19.12) was purchased from eBiosciences (San Diego, CA). All adenovirus vectors were produced by the Gene Transfer Vector Core at the University of Iowa.
Platelet and platelet-derived membrane vesicle preparation
Murine platelets were isolated essentially as described.31 In brief, mice were anesthetized and bled by severing the abdominal aorta. Blood was collected into syringes containing 1.0 mL acid-citrate-dextrose (12.5 g/L Na citrate, 10.0 g/L D-glucose, and 6.85 g/L citric acid), added to 6 mL of piperazine-N,N-bis2-ethanesulfonic acid (150 mM NaCl and 20 mM of piperazine-N,N-bis2-ethanesulfonic acid [pH 6.5]), and spun at 100g for 15 minutes. The platelet-rich supernatant was collected, and 1 U/mL of apyrase and 1 μM of PGE1 (final concentrations) were added and spun at 1000g for 10 minutes. The platelet pellet was resuspended in Tyrode's buffer (134 mM NaCl, 2.9 mM KCL 0.34 mM Na2PO4, 12 mM NaHCO3, 20 mM HEPES, 1 mM MgCl2, 5 mM glucose, and 0.5 mg/mL bovine serum albumin, pH to 6.5), and counted using a Coulter Particle Counter (Coulter, Miami, FL). To activate platelets, 0.5U thrombin/mL was added to platelet suspension. All platelet manipulations were performed at room temperature. Platelets were allowed to activate for 20 minutes. at 37°C. The activated platelet suspension was spun at 13 000g for 5 minutes. to pellet whole platelets and platelet aggregates. Platelet-derived membrane vesicles (PDMVs) were pelleted by modifying previously described protocols.32–36 Briefly, the activated platelet supernatant (AP Sup) was collected and fractionated into PDMV pellet and PDMV-poor supernatant by centrifugation at 20
000g for 5 minutes. to pellet whole platelets and platelet aggregates. Platelet-derived membrane vesicles (PDMVs) were pelleted by modifying previously described protocols.32–36 Briefly, the activated platelet supernatant (AP Sup) was collected and fractionated into PDMV pellet and PDMV-poor supernatant by centrifugation at 20 000g for 2 hours at 4°C. Total protein analysis was performed using BCA Protein Assay Kit (Pierce, Rockford. IL) with a stated working range from 0.02 to 2 mg/mL.
000g for 2 hours at 4°C. Total protein analysis was performed using BCA Protein Assay Kit (Pierce, Rockford. IL) with a stated working range from 0.02 to 2 mg/mL.
Adenovirus specific IgG enzyme-linked immunosorbent assay
Serum was collected from mice 7 to 14 days after immunization with adenovirus; 96-well plates were coated overnight at 4°C with 109 Ad5-βgal particles per well in 50 μL 0.1 M NaHCO3 (pH 9.2). Wells were then washed and blocked 2 to 4 hours at room temperature with 3% bovine serum albumin in 0.01% Tween 20 and 0.02% NaN3. Blocking solution was decanted, and 100 μL diluted plasma was incubated per well for 2 to 4 hours at room temperature. After 6 washes, 100 μL peroxidase-labeled secondary antibody was incubated for 1 to 2 hours at room temperature per well. Wells were washed 7 times, after which 100 μL fresh substrate (ortho-phenylenediamine in 0.04 M Na2HPO4 and 0.02 M citric acid, pH 5.0) was added. Samples were incubated at room temperature in the dark for 30 minutes. The reaction was then stopped by adding 25 μL of 4.5 M H2SO4 per well. Sample absorbances were measured at 490 nm. Total IgG was quantified using a standard mouse adenovirus monoclonal IgG1 from Fitzgerald Industries International (Concord, MA).
Transmission electron microscopy
Platelet and PDMV pellets were washed and fixed with 2% paraformaldehyde/1% glutaraldehyde solution in phosphate-buffered saline (PBS) for 1 hour at 4°C. Rinse 3 times with PBS. Add 1% OsO4 with 1.5% potassium ferrocyanide in PBS for 2 hours. Rinse 3 times with PBS and once with distilled water. Dehydrate with ethanol through successive steps then embed within epoxy medium. Place in 70°C oven for 8 hours and section using microtome. Examine using a Hitachi H-7000 Transmission Electron Microscope (Central Microscopy Research Facilities, University of Iowa). Images were acquired on Kodak 4489 negative sheet film for image recording.
In vitro stimulations of B cells
Primary splenic B cells were isolated from C57BL/6 mice between the ages of 8 and 10 weeks. Mice were anesthetized and killed. Spleens were removed and ground between frosted microscope slides in balanced salt solution (BSS). Cells were washed and resuspended in 2 mL of BSS. Percoll stock was made adding 1 mL 10× BSS and 50 μL of 7.5% sodium bicarbonate solution to 9 mL Percoll. Dilutions of 50%, 60%, 70%, and 75% of the Percoll stock were made with BSS. The cell suspension was underlain with the 50%, 60%, 70%, 75%, and the Percoll stock, in that order, in a 15-mL tube. The tube was centrifuged at 2900g in a Jouan CR412 centrifuge for 15 minutes at 4°C. Cells collected from 60% to 70% interface and 70% to 75% interface. Cells were washed and resuspended in magnetic cell sorting (MACS) buffer according to manufacturer's protocol for isolation of untouched B cells using CD43 microbeads (Miltenyi Biotec, Auburn, CA). Cells were stained for CD19 and CD45R and analyzed by flow cytometry to assess purity of B cells.
Quantification of CD154 activity
MS-1 cells, an immortalized pancreatic endothelial cell line, were obtained from ATCC (Manassas, VA) and routinely cultured in Dulbecco modified Eagle medium with 5% fetal bovine serum. After 6-hour exposure to platelets or sCD154, total cellular RNA was isolated using the RNAeasy kit (Qiagen, Valencia, CA). First strand synthesis was performed using Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA) in a reaction using 2 μg total RNA primed with random hexamers following the manufacturer's instructions; 2 μL of each reverse transcription reaction was subjected to real-time quantitative PCR using proprietary TaqMan primer and probe sets for mouse MCP-1 and 18S rRNA (Applied Biosystems, Foster City, CA). For each sample, 3 PCRs were performed. The resulting relative increase in reporter fluorescent dye emission was monitored by the TaqMan system (GeneAmp 5700 sequence detection system and software; PerkinElmer Life and Analytical Sciences, Waltham, MA). The level of MCP-1 mRNA, relative to 18S rRNA, was calculated using the formula: relative mRNA expression = 2 − (Ct of MCP-1 − Ct of 18S rRNA), where Ct is the threshold cycle value.
GC formation assessment
GC formation was assessed as previously described.11 Platelets were depleted using 10 μg of antibody p0p3/4 (Emfret Analytics, Eibelstadt, Germany) 24 hours before intravenous adoptive transfer of 4 × 106 negatively selected, naive B6 CD4+ T cells along with intravenous adoptive transfer of either B6 platelets, B6 PDMV, or CD154−/− PDMV. Four hours later, the mice received via intravenous injection 108 pfu of adenovirus. Twelve days after injection, spleens were harvested and processed for histologic examination of frozen sections. Fluoresence was immediately visualized at room temperature in staining buffer (1 mg/mL 2.4G2 [Fc block], 10% fetal calf serum, 0.1% bovine serum albumin, 0.05% Tween 20, all in 80 mM Tris-buffered saline) with a BX-51 digital light microscope (Olympus, Melville, NY) using a 10×/0.3 numeric aperture phase lens. Images were acquired using a Spot RT Slider camera and Spot Software version 4.6.4.8 (Diagnostic Instruments, Sterling Heights, MI). Images were processed using Adobe Photoshop version 6 (Adobe Systems, San Jose, CA). GCs were visualized and reported as GCs/periarteriolar T-cell-rich lymphoid sheath (PALS). A PALS unit is the combined follicular zone/T-cell zone surrounding a single central arteriole in thin sections.
Results
Activated platelets release functional CD154 into the supernatant
To investigate the role of released CD154 in the induction of IgG production, experiments were undertaken to compare the adenovirus-specific IgG response stimulated in CD154−/− mice by wild-type, intact activated platelets (AP) from B6 mice versus that stimulated by the activated platelet supernatant alone (AP Sup). AP and AP Sup were produced from platelets purified from whole blood. There is between 0.03% and 0.05% contamination by CD3+ cells (data not shown). This number is equivalent to 150 to 200 possible T cells in the 5 × 108 transferred platelets. Serum was collected and tested by ELISA to assess relative adenovirus-specific IgG titers elicited by these and control treatments (Figure 1). When mice were treated with the supernatant from wild-type activated platelets, adenovirus-specific IgG production increased. Supernatant-derived augmentation was enhanced by a second injection, with antibody production approaching the levels observed after injection of activated platelets. In contrast, supernatant from platelets harvested from CD154−/− mice failed to confer this stimulatory effect. These data suggest that activated platelets release factors into the supernatant that are sufficient by themselves to mediate CD154 signaling to B cells for production of IgG.
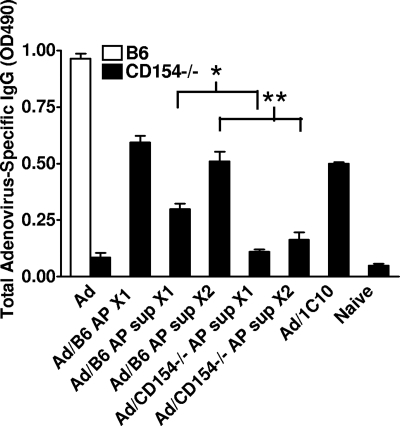
Whole platelets are not necessary for the delivery of CD154 signal. CD154−/− mice were injected with 5 × 107 activated platelets (AP) or activated platelet supernatant (AP Sup) from 5 × 108 wild-type (B6) or CD154−/− platelets. Positive controls were given 500 μg anti-CD40 antibody (1C10) IP. Mice were immunized on day (−1) with 108 pfu of adenovirus. On day 0, all mice received one injection of either AP or AP Sup. A second injection was given to half the mice on day 6 (X1 indicates mice receiving one injection; X2, mice receiving 2 injections). Serum was collected on day 9 for adenovirus-specific IgG analysis by ELISA. This experiment was performed 3 times with 5 mice per group. The graph is from a representative experiment (Wilcoxon rank sum test: *2-tailed P = .002, **2-tailed P = .006).
Characterization of PDMV
To more rigorously ascertain whether platelet components released on activation are sufficient to signal the adaptive immune compartment, and further to determine which components might be involved, highly purified platelet fractions were isolated. One signaling candidate is the cleaved, soluble CD154 (sCD154), which is released during the degranulation of activated platelets at sites of injury and infection.37 A second is CD154-containing PDMV released from platelets as microparticles (PMPs) and exosomes.37 PMPs, but not platelet-derived exosomes, have been shown to express CD154,22,38 although CD154 expression on exosomes from other cell types has been demonstrated.39 Thus, to address whether soluble or PDMV-associated CD154 is responsible for the augmentation of IgG production, PDMV and soluble protein fractions were isolated as described in “Methods.” The literature cites several different protocols for the isolation of platelet PDMV.32,33,36,40,41 Preliminary studies using various centrifugation speeds ranging from 10 000g to 100
000g to 100 000g demonstrated that centrifuging AP Sup at more than or equal to 20
000g demonstrated that centrifuging AP Sup at more than or equal to 20 000g for 2 hours was sufficient for the isolation of PDMV of the anticipated size that contained the CD154 fraction (Figure 2; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Based on the data reported in Figure S1, all PDMVs in this report were isolated at 20
000g for 2 hours was sufficient for the isolation of PDMV of the anticipated size that contained the CD154 fraction (Figure 2; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Based on the data reported in Figure S1, all PDMVs in this report were isolated at 20 000g. The purity and efficiency of the PDMV preparation were assessed using electron microscopy (TEM) and protein analyses. TEM visualization of membrane-bound microparticles approximately 100 nm in diameter within the purified PDMV preparation demonstrated the isolation of PDMVs (Figure 2A). Because of the lack of an effective means to quantify PDMV before adoptive transfer or to track them afterward, all experiments were standardized based on the number of platelets activated to generate the PDMV sample. Total protein content showed equivalent protein levels in comparable fractions generated from B6 and CD154−/− platelets (Figure 2B). Of note, the lowest protein levels (near the lower limit of detectability) were observed in the PDMV fractions.
000g. The purity and efficiency of the PDMV preparation were assessed using electron microscopy (TEM) and protein analyses. TEM visualization of membrane-bound microparticles approximately 100 nm in diameter within the purified PDMV preparation demonstrated the isolation of PDMVs (Figure 2A). Because of the lack of an effective means to quantify PDMV before adoptive transfer or to track them afterward, all experiments were standardized based on the number of platelets activated to generate the PDMV sample. Total protein content showed equivalent protein levels in comparable fractions generated from B6 and CD154−/− platelets (Figure 2B). Of note, the lowest protein levels (near the lower limit of detectability) were observed in the PDMV fractions.
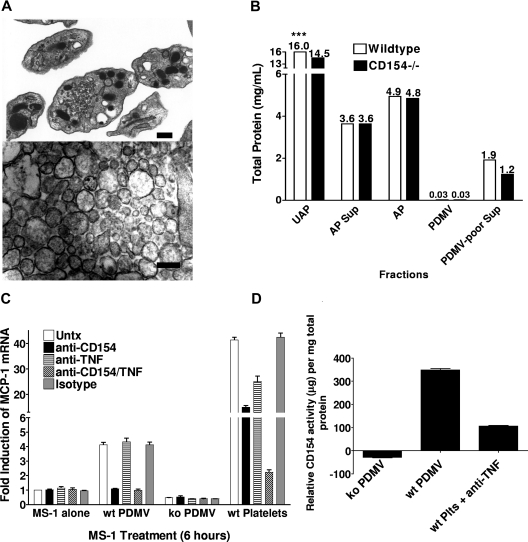
Characterization of PDMVs. PDMVs were isolated and visualized by TEM and analyzed for total protein. (A) TEM images of whole platelets (top) and PDMV (bottom). Bar represents 100 nm. (B) Total protein analysis was performed on 5 × 108 unactivated platelets (UAP) and activated platelets (AP); and activated platelet supernatant (AP Sup), PDMV pellet (PDMV), and PDMV-poor supernatant (PDMV-poor Sup) made from 5 × 108 platelets. ***Total protein values (mg/mL) above each bar. (C) 105 MS-1 cells were plated in each well of a 24-well plate and allowed to grow to near confluence over 2 days. PDMVs from 4.5 × 108 CD154 wt or ko platelets, or 108 platelets were added to each well; 10 μg/mL of anti-CD154 and/or anti-TNF-α antibodies added to designated wells. The experiment was performed in triplicate. (D) Quantitative real-time PCR was performed using purchased primers for MCP-1 and 18S mRNA. Standard curve ranged from 10 μg/mL to 4.9 ng/mL.
Functional quantification of CD154 on PDMV using MS-1 endothelial cell line
Because of the lack of reagents necessary to quantify the amount of mouse CD154 contained within intact or subfractions of activated platelets, we established a biologic assay using CD154-induced MCP-1 production by MS-1 endothelial cells. Previous studies showed that both CD154 and TNF induced MCP-1 production; thus, antibody neutralization was used to segregate activity to the respective stimuli. Recombinant soluble CD154 (Axxora Life Sciences, San Diego, CA) was used as a positive control. Initially, MS-1 cells and PDMVs were cocultured for 6 hours and assessed for increased expression of the endothelial activation marker MCP-1 mRNA levels using quantitative real-time PCR. PDMVs from B6 mice increased in MCP-1 message approximately 4-fold, which was completely blocked by MR-1, but not anti-TNF-α blocking antibody, indicating that CD154 is the only factor activating MS-1 cells (Figure 2C). Whole activated platelets also induced MCP-1, but this was partially blocked by both CD154 and TNF-α blocking antibodies.
To quantify the relative CD154 activity in the PDMV fraction, we ran the assay with a standard curve and, surprisingly, calculated that the CD154 activity per milligram total protein in the PDMV pellet was equivalent to the activity induced by 350 μg of the standard protein (Figure 2D). Whole activated platelets, when blocked with anti-TNF, showed approximately 100 μg of CD154 activity. These data not only confirm the presence of CD154 activity within the PDMV but also indicate that the CD154 activity becomes concentrated on these structures, lending support to the importance of PDMV in the delivery of CD154 signals.
PDMV delivery of CD154 to B cells
The isolated fractions were then tested for their ability to activate adenovirus-specific IgG production in our model. Each fraction, including the PDMV pelleted from activated platelet supernatant (PDMV Pellet) and the PDMV-poor supernatant after fractionation, was transferred into CD154−/− mice (Figure 3A) in conjunction with adenovirus immunization. Only the unfractionated supernatant and the PDMV pellet from B6 mice induced adenovirus-specific IgG production. The fact that neither unfractionated AP supernatant nor the PDMV fraction from CD154−/− platelets elicited a response demonstrated that CD154 within the PDMV pellet was sufficient to facilitate the augmentation of adenovirus-specific IgG. This was further verified by the use of the CD154 blocking antibody MR-1 (Figure 3B). Addition of MR-1 to B6 PDMV before injection into immunized CD154−/− mice abrogated the antibody augmentation induced by B6 PDMV alone, whereas control Ig had no effect. Taken together, these data confirm the finding that whole activated platelets are not necessary for the delivery of the CD154 signal. Moreover, because total protein analysis of the injected fractions shows that PDMVs represent a small percentage of the total protein from the initial platelet pellet, these data suggest that CD154 activity strongly fractionates with the PDMV pellet. Finally, the augmentation elicited by the PDMV fraction was comparable with that produced by unfractionated, activated platelet supernatants, suggesting that PDMVs may play a role in the delivery of the platelet-derived CD154 signal.
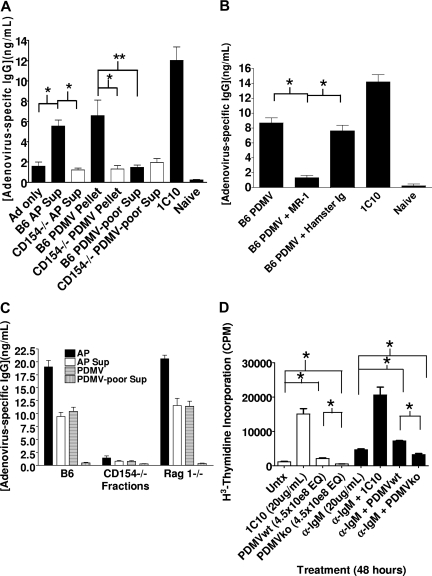
PDMV delivery of CD154 signal. (A) CD154−/− mice were immunized on day (−1) and then injected on days 0 and 6 with activated platelet supernatant (AP Sup), PDMV pellet, or PDMV-poor supernatant from 5 × 108 wild-type (B6) or CD154−/− platelets, or 500 μg 1C10 intraperitoneally. Serum was collected on day 9 for quantification of total adenovirus-specific IgG using a commercially available mouse anti-adenovirus IgG standard. (B) PDMVs derived from 5 × 108 B6 platelets were injected intravenously into CD154−/− mice 24 hours after immunization with 108 particles Ad-OVA; 10 μg/mL CD154 blocking antibody MR-1 was added to AP Sup before isolation of PDMVs and to PDMVs after resuspension. Mice were injected with 100 μg MR-1 just before receiving PDMV + MR-1. Serum was collected on day 7 for quantification of total adenovirus specific IgG production by ELISA. (C) PDMVs derived from 5 × 108 platelets injected 24 hours after immunization. Serum collected on day 7. (D) Primary B cells were isolated from spleens of 8-week-old C57BL/6 mice by Percoll gradient enrichment and negative selection over magnetic beads (Miltenyi Biotec). 6 × 105 B cells were plated in each well of a 96-well plate and PDMVs from 4.5 × 108 wt or ko platelets added to each designated well, with or without anti-IgM, 5 wells per experimental condition. PDMVs and B cells were coincubated for 48 hours, with the final 6 hours in the presence of 1 μCi of 3H-T. Cells were harvested and thymidine incorporation measured (Wilcoxon rank sum test: *2-tailed P = .012, **2-tailed P = .019).
Although the platelets have been purified using the described centrifugation techniques, there is still a minimal amount of contamination by T cells and B cells (0.03%-0.05%; data not shown). Because this level of contamination resulted in only 150 to 200 T cells in the transferred platelets, it is unlikely that T-cell CD154 affected the outcome of the response. However, to determine whether this contamination contributed to the increase in IgG production, we performed the experiments with platelets from B6.RAG 1−/− mice (Figure 3C). The use of RAG−/− mice eliminates any possible contribution from T or B cells, which may be contaminating our platelet preparations, as these mice do not produce any lymphocytes. The data show that the increase in IgG is due solely to the platelets in our preparations and the products produced by the platelets during activation and not by any contaminating T or B cells.
Although PDMV induces IgG production in vivo, it is not known whether PDMVs are able to interact and stimulate B cells directly. To determine whether PDMVs and B cells are capable of interacting directly, the impact of CD154 signaling on B-cell proliferation was assessed in an in vitro coculture using primary splenic B cells isolated from B6 mice. Proliferation was measured by tritiated thymidine incorporation into DNA (Figure 3D). Under conditions with or without anti-IgM acting as “signal 1” in B-cell activation, wt PDMVs were observed to induce B-cell proliferation. Although significant proliferation was observed with or without signal 1 activation, the proliferative response driven by PDMV was weak. These data suggest that in vivo signaling by PDMV is more complex than simply engaging B-cell CD40. Interestingly, the data repeatedly showed that CD154−/− PDMVs seemed to inhibit proliferation of B cells.
Characterization of the PDMV-mediated response
To verify that the time course of PDMV-induced antibody production observed in our system is similar to what had been reported previously for whole, activated platelets,5 we performed an adoptive transfer experiment evaluating adenovirus-specific IgG production over time (Figure 4A). PDMVs produced a response that peaks at day 7 and decreases rapidly to background, as previous studies investigating platelet-induced IgG production have shown, sug-gesting that the mechanism of delivery is the same. Dose-dependence of the PDMV-induced IgG production was determined using PDMVs that were prepared from platelets suspended at 4.5 × 108 platelets/mL and serially diluted 2-fold before transfer into CD154−/− mice (Figure 4B). PDMV from the equivalent of 4.5 × 108 platelets represents the undiluted, neat sample. Because of the significant loss of PDMVs during sample preparation (Figure 2B) and the inability to quantify the amount of PDMV in the final samples, it cannot be assumed that PDMV from 2.25 × 108 platelets is the minimum effective dose. Rather, it is likely that only a fraction of the starting sample remains by completion of the preparation. Thus, these data demonstrate that the effect of PDMVs is titratable but does not provide a basis for determining the number of PDMV necessary to deliver an activating signal.
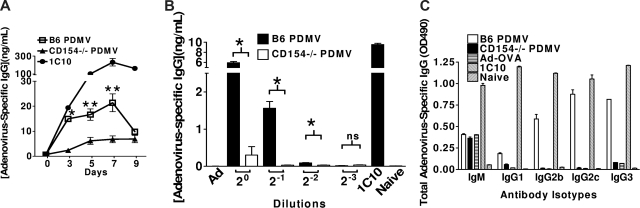
Characterization of PDMV-induced augmentation of IgG production. (A) Time course of PDMV response. CD154−/− mice injected with PDMV pellet from 5 × 108 B6 or CD154−/− platelets. Serum collected on days 3, 5, 7, and 9. Total IgG quantified by ELISA using commercial mouse antiadenovirus IgG standard (Wilcoxon rank sum test: *2-tailed P = .020, **2-tailed P = .012 comparing the B6 PDMV time point to the corresponding CD154−/− PDMV time point). (B) Dose-response study. Neat samples contain PDMV from 4.5 × 108 activated platelets. Dilutions made from resuspended PDMV pellets. Total IgG quantified by ELISA using commercial mouse antiadenovirus IgG standard (Wilcoxon rank sum test: *2-tailed P = .012, **2-tailed P = .012; ns indicates not significant). (C) Analysis of antibody isotypes produced. Samples pooled from neat groups in dose-response experiment. ELISAs were performed for each antibody isotype. Each experiment was performed with 5 mice per group and repeated once.
To further characterize PDMV-induced IgG stimulation, serum antibody production was analyzed at the isotype level, which based on the isotype secretion pattern might also provide clues to the subset of splenic B cells activated. IgG1, IgG2b, IgG2c, IgG3, and IgM were analyzed by ELISA, and the results were consistent with previous reports characterizing IgG production in response to adenovirus. PDMVs strongly induced IgG2b, IgG2c, and IgG3 (Figure 4C),42,43 as well as leading to a slight increase in IgG1. IgG3 is associated with marginal zone (MZ) B cells, which play a significant role in T-cell independent responses and are among the first B cells to respond to blood-borne pathogens. IgG2b, IgG2c, and IgG1 are produced primarily by follicular B cells.44
PDMV enhances the GC reaction
Further studies were performed to determine whether PDMV functions in conjunction with T cells to augment GC formation. Previous studies have shown that, in a nontransgenic system in which antigen-specific T-cell precursors are limiting, platelet-associated CD154 enhances GC formation and antibody production in cooperation with CD4+ T cells.45 To further validate a role for PDMVs in immune modulation, experiments were performed to determine whether PDMVs are sufficient to cooperate with CD4+ T cells in stimulating GC formation (Figure 5C). CD154−/− mice injected with B6 PDMVs showed a similar percentage of GC-positive PALS units compared with mice injected with B6 platelets, indicating that PDMVs are indeed able to enhance T cell–mediated GC formation in a CD154-dependent fashion. Taken together, these data begin to unravel a possible mechanism by which PDMVs deliver platelet-derived CD154 to B cells to modulate IgG production and GC formation.
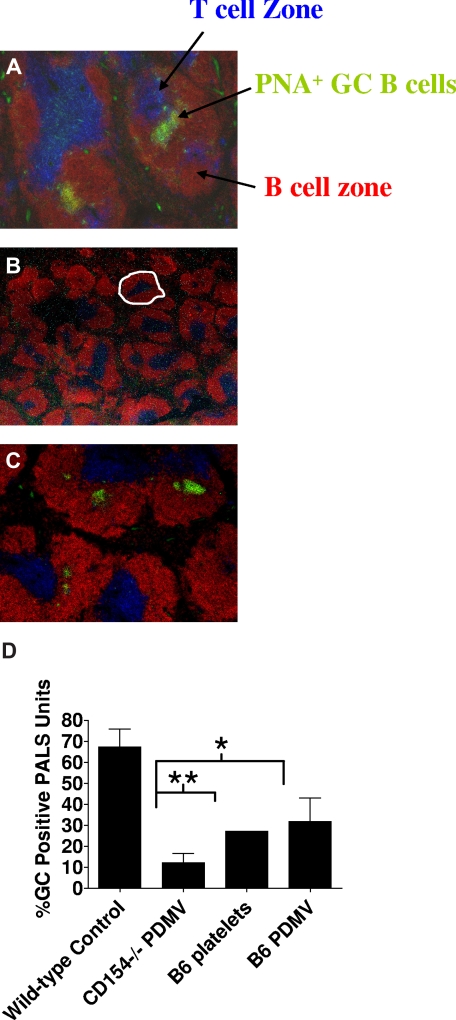
B6 PDMVs enable a limited number of normal B6 CD4+ T cells to induce GC reactions in CD154−/− mice depleted of platelets. Platelets were depleted using 10 μg p0p3/4 24 hours before intravenous adoptive transfer of 4 × 106 negatively selected, naive B6 CD4+ T cells intravenously and 108 B6 platelets, B6 PDMV from 5 × 108 platelets, or CD154−/− PDMV from 5 × 108 platelets. Twelve days after injection, spleens were harvested and processed for histologic examination of frozen sections. Blue represents T-cell zone (anti-CD4 and CD8 staining); red, B-cell follicles (anti-B220); green, peanut agglutinin (PNA; GC B cells stain PNAhi). GCs are visualized as B220 PNA double-positive (green-yellow) areas of cells. Representative histology from the wild-type control consisting of B6 mice immunized with adenovirus alone (A) and CD154−/− mice immunized with adenovirus and treated with naive B6 CD4+ T cells with CD154−/− PDMV (B) or B6 PDMV (C). GCs were visualized and reported as GCs/PALS (D). A PALS unit consists of the follicular and T-cell zones surrounding a central arteriole. An example of a PALS unit is delineated in panel B with a dashed white line. The experiment was performed with 5 mice per group and repeated once (*2-tailed P = .014; **2-tailed P = .058).
Discussion
Previous studies showed that platelet activation results in the modulation of T- and B-cell immunity; however, the mechanism(s) by which the signal is delivered remains unclear.5,45 When platelets become activated, they express a variety of surface adhesion molecules, leading to aggregation at the site of activation. Aggregation limits the mobility of platelets and, thus, also their capacity to communicate with sites distant from the activation site. We hypothesized that platelet communication with the adaptive immune compartment is mediated at least in part by PDMVs. The data presented herein demonstrate that PDMVs are sufficient to induce IgG production, enhance GC formation in vivo, and induce proliferation of B cells and activation of MS-1 cells in vitro, in a CD154-dependent manner. Intravenous injection of PDMV under the experimental conditions described here mimics PDMV release during platelet activation, suggesting that PDMVs are sufficient to carry a CD154 signal through the circulatory system to distant sites where signaling can occur. Although our platelet preparations contain minimal contaminating T cells and B cells, we showed that RAG−/− preparations, which are free of T and B cells, function as well as PDMV isolated from wt mice. Likewise, in data previously published, we showed that, in platelet and T cell cooperative studies, a minimum of 4 × 106 T cells alone was necessary to observe an increase in GC formation and IgG production.11 Taken together, these data demonstrate the sufficiency of platelet-derived CD154 in PDMV preparations to mediate IgG production.
In an attempt to show the presence and quantity of CD154 in the platelets and PDMV preparations, we had purchased and used ELISA kits from 2 different manufacturers (Bender MedSystems, Burlingame, CA, and R&D Systems, Minneapolis, MN). However, neither kit was able to quantify mouse CD154 from lysed platelets or PDMV. We were unable to confirm the presence or quantity of CD154 by electron microscopy or Western blot, as well, because of the general lack of quality antibodies to detected fixed or denatured mouse CD154. The use of CD154−/− platelets or PDMVs and also neutralization of CD154 function in wild-type platelet preparations directly links CD154 in PDMV to IgG production. Using a functional assay to quantify CD154 activity compared with a standard, we showed that PDMVs contain CD154 and that it becomes concentrated within this fraction after platelet activation.
It is clear that PDMVs are available for carrying platelet-derived signals to other cells under physiologic conditions. Platelets release 2 types of membrane vesicles: PMPs and exosomes. PMPs bud from the plasma membrane of activated platelets and hence are suspected of carrying surface markers from activated platelets as membrane-bound molecules. Early studies showed PMPs to be an important source of integrins and selectins for leukocyte attachment to endothelial cells, a process that is important for recruitment to and transmigration at sites of injury.35,46–47 In addition, platelets and PMPs are known to modulate the activity of the cells with which they interact, including monocytes, neutrophils, and endothelial cells.27,29,35,46,49,50 For example, PMPs are important for platelet-derived CD154 to stimulate the maturation of monocyte-derived dendritic cells.14 Because circulating PMPs and CD154 increase during inflammation, understanding the role of PMPs in the delivery of CD154 signals and the impact of this delivery on damage has broad implications.2,51,52 Unlike PMPs, exosomes would not acquire surface markers from the plasma membrane of activated platelets. Rather, exosomes bud into the granules themselves during development. Platelet-derived exosomes have not been shown to have any function in vivo or in vitro. Because circulating PDMV and CD154 increase during inflammation, understanding the role of PDMV in the delivery of CD154 signals and the impact of this delivery on damage has broad implications.2,51,52 Given the current demonstration of the sufficiency of PDMVs to modulate B-cell responses, PDMV-associated CD154 signaling likely plays a role in inflammation and other conditions associated with platelet activation.53
Signal delivery via membrane vesicles is not a property unique to platelets.23,54 Endothelial microparticles have been shown to activate monocytes and to promote adhesion.55 Membrane vesicles released by dendritic cells and B cells have also been shown to enhance antigen presentation.26,28,56 Mast cells have been shown to produce exosomes, which express a variety of immunoregulatory molecules, including CD154. These mast cell–derived exosomes are capable of activating both B and T cells.57 One interesting feature common to microparticles from many cell types, including platelets, is their ability to transfer membrane-associated molecules to other cell types, including B cells.58–62 Our study expands the role of membrane vesicles in general, and of PDMV specifically, by showing that platelet-derived PDMVs are able to deliver a CD154 signal that is able to modulate the activity of immune cells and the subsequent immune responses.
The mechanisms by which PDMV communicate with the adaptive immune compartment are not known. Our experiments, which take advantage of the availability of CD154−/− mice, have shown that platelet or PDMV-induced antibody production is dependent on platelet-derived CD154. However, the experiments do not address whether the CD154 signal is delivered by PMP or exosomes. Likewise, it is not known whether the CD154 signal is delivered directly to B cells or indirectly through other cell types. A direct interaction would involve platelet-derived PDMVs interacting directly with B cells, as has been shown for membrane vesicles derived from other cell types.63 Alternatively, PDMVs could signal indirectly through phagocytic cells present in the spleen. In this regard, it is interesting to note that CD154 ligation to CD40 activates monocytes and dendritic cells64,65 and that activated macrophages and dendritic cells modulate antibody production by B cells.66–69 Similarly, PDMVs could indirectly deliver a signal to B cells via PDMV-associated CD154 activated phagocytes, either through another molecule, such as B-cell activating factor of the TNF family, or using these phagocytes as a scaffold for platelet-derived CD154 signaling to B cells. Taken together, these observations support the potential for platelets and PDMVs to signal B cells in an indirect manner. Further studies are in progress to more clearly define the signaling mechanism.
In a wild-type mouse, adenovirus is cleared by a Th1-mediated response with an antibody profile made up of IgG2a, in BALB/c mice, or IgG2c, in C57BL/6 mice, and IgG2b.70,71 In the PDMV-induced response to adenovirus, IgG2b and IgG2c are the predominant isotypes, with some IgG3 being produced as well. IgG3 is also induced in a Th1 response, suggesting that PDMV can induce the same type of response in CD154−/− mice as found in wild-type mice immunized with adenovirus. The presence of a high level of IgG3 is interesting because of the possible role of MZ B cells in PDMV-induced antibody production. IgG3 and, to a lesser extent, IgG2a and IgG2c are strongly associated with MZ B-cell activation. However, other B cells, including follicular B cells, are also capable of producing these isotypes in significant quantities.72 MZ B cells, along with B1 cells, are thought to be the major source of “natural antibodies” linking innate and adaptive immune responses.73 IgG3 antibodies from MZ B cells appear early in infection, independent of T-cell help.74 Because this response does not require T-cell help, it is limited in duration and lacks memory. This is similar to the characteristics of the PDMV-induced antibody response, suggesting a possible role for platelet-derived CD154 in inducing an early antibody response by MZ B cells in mice, although this has not been fully investigated.
Platelet depletion data show a role for platelets in the early splenic antibody response in wild-type and CD154−/− mice.5 However, the physiologic role platelets and/or PDMVs play in the modulation of IgG production during an infection remains to be clearly defined. Our studies clearly demonstrate the sufficiency of PDMVs to signal the B-cell compartment; however, in the context of a normal animal, the contribution of PDMVs in establishing antibody responses to pathogens remains to be clearly established. The current study indicates that PDMVs may play an important role by acting as a vector for the CD154 signal by concentrating the CD154 activity and carrying it away from the site of platelet activation and aggregation, thereby making it possible for it to encounter the cells necessary to produce the antigen-specific response.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant AI060924. B.D.E. was supported by NIH grant DK071712 and DOD fellowship DAMD17-03-1-0079. S.A.C. was supported by NIH grant DK067338-02.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.L.R. contributed vital reagents, designed research, and analyzed data; R.J.J. performed research; T.J.W. contributed vital reagents, designed research, and analyzed data; S.A.C. designed and performed research, and analyzed data; B.D.E. performed research and analyzed data; D.L.S. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Timothy L. Ratliff, Director, Purdue Cancer Center, Hansen Life Sciences Research Bldg, Rm 145, 201 S University St, West Lafayette, IN 47907-2064; e-mail: ude.eudrup@ffiltarlt.
References
Articles from Blood are provided here courtesy of The American Society of Hematology
Full text links
Read article at publisher's site: https://doi.org/10.1182/blood-2007-06-097410
Read article for free, from open access legal sources, via Unpaywall:
http://www.bloodjournal.org/content/111/10/5028.full.pdf
Free to read at www.bloodjournal.org
http://www.bloodjournal.org/cgi/content/abstract/111/10/5028
Free after 12 months at www.bloodjournal.org
http://www.bloodjournal.org/cgi/content/full/111/10/5028
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Current status and future perspectives of platelet-derived extracellular vesicles in cancer diagnosis and treatment.
Biomark Res, 12(1):88, 26 Aug 2024
Cited by: 0 articles | PMID: 39183323 | PMCID: PMC11346179
Review Free full text in Europe PMC
Breakthrough infections after COVID-19 vaccinations do not elicit platelet hyperactivation and are associated with high platelet-lymphocyte and low platelet-neutrophil aggregates.
Res Pract Thromb Haemost, 7(8):102262, 14 Nov 2023
Cited by: 0 articles | PMID: 38193050 | PMCID: PMC10772876
Extracellular vesicles in acute respiratory distress syndrome: Understanding protective and harmful signaling for the development of new therapeutics.
Histol Histopathol, 39(2):131-144, 01 Sep 2023
Cited by: 0 articles | PMID: 37712224
Review
Mesenchymal Stem Cell-Derived Extracellular Vesicles: An Emerging Diagnostic and Therapeutic Biomolecules for Neurodegenerative Disabilities.
Biomolecules, 13(8):1250, 16 Aug 2023
Cited by: 6 articles | PMID: 37627315 | PMCID: PMC10452295
Review Free full text in Europe PMC
Multi-omics analysis of naïve B cells of patients harboring the C104R mutation in TACI.
Front Immunol, 13:938240, 16 Aug 2022
Cited by: 1 article | PMID: 36072607 | PMCID: PMC9443529
Go to all (143) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cooperation between platelet-derived CD154 and CD4+ T cells for enhanced germinal center formation.
J Leukoc Biol, 78(1):80-84, 17 May 2005
Cited by: 64 articles | PMID: 15899982
The role of platelet CD154 in the modulation in adaptive immunity.
Immunol Res, 39(1-3):185-193, 01 Jan 2007
Cited by: 29 articles | PMID: 17917065
Review
Platelet influence on T- and B-cell responses.
Arch Immunol Ther Exp (Warsz), 57(4):235-241, 04 Jul 2009
Cited by: 36 articles | PMID: 19578816
Review
The emerging role of platelets in adaptive immunity.
Cell Immunol, 238(1):1-9, 01 Nov 2005
Cited by: 94 articles | PMID: 16442516
Review
Funding
Funders who supported this work.
NIAID NIH HHS (1)
Grant ID: R01 AI060924
NIDDK NIH HHS (4)
Grant ID: K01 DK071712
Grant ID: DK071712
Grant ID: K01 DK067338
Grant ID: DK067338-02
NIGMS NIH HHS (1)
Grant ID: T32 GM007337
 1-3,6,7
1-3,6,7




