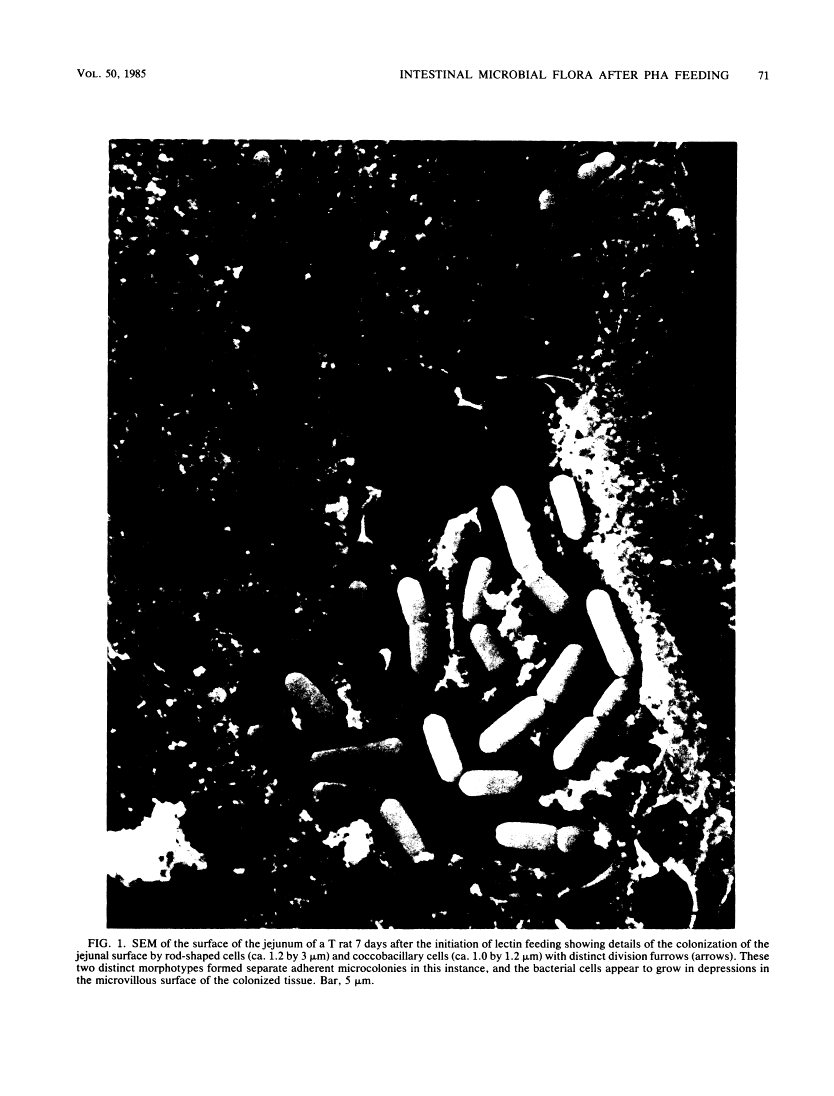
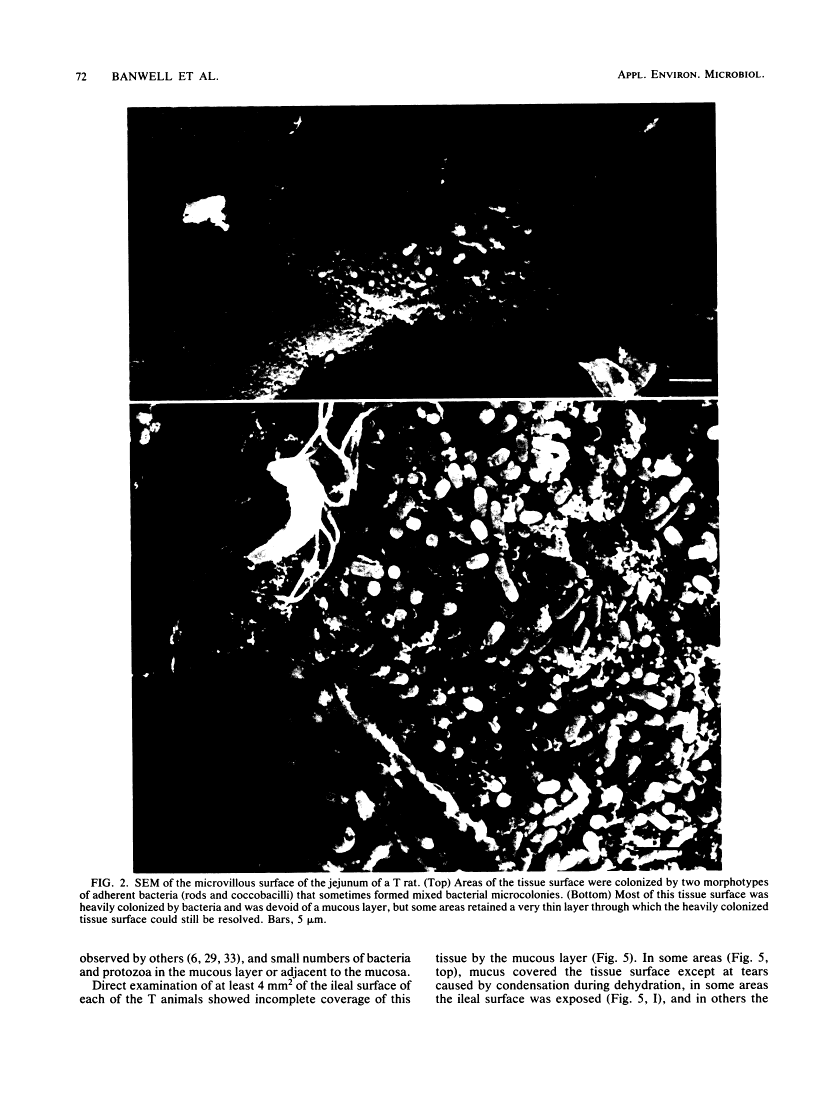
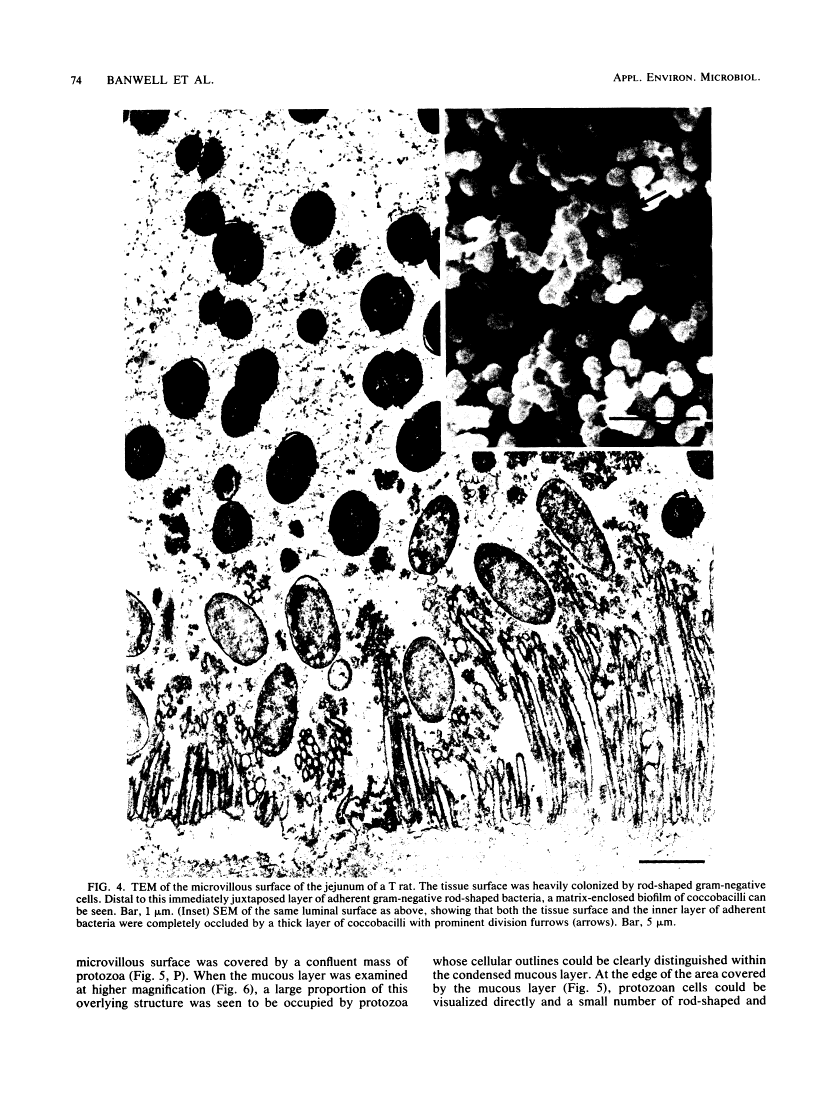
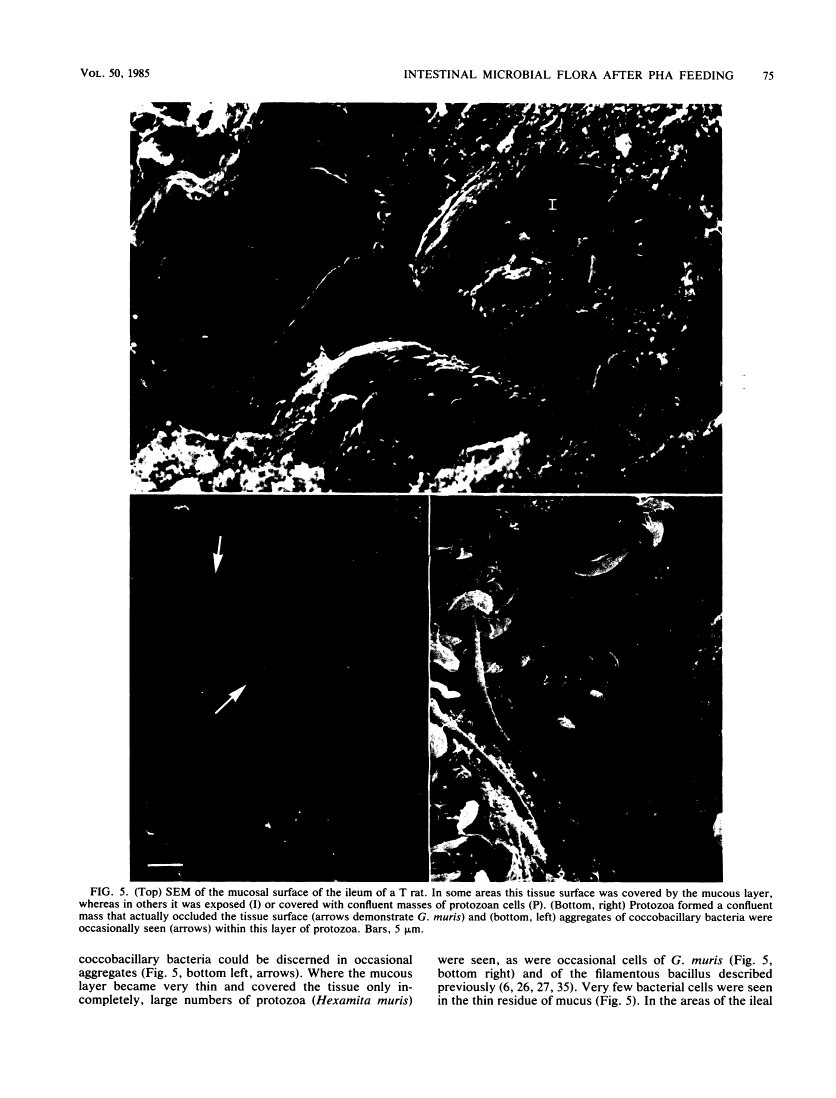
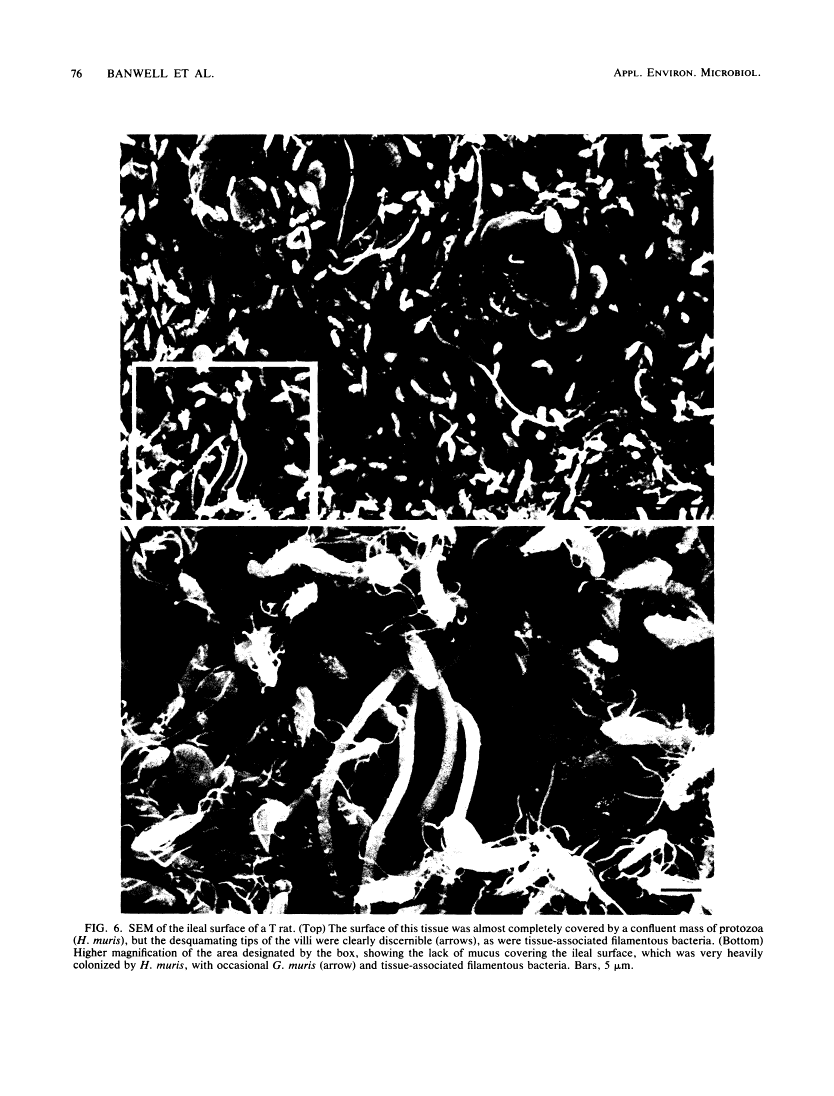
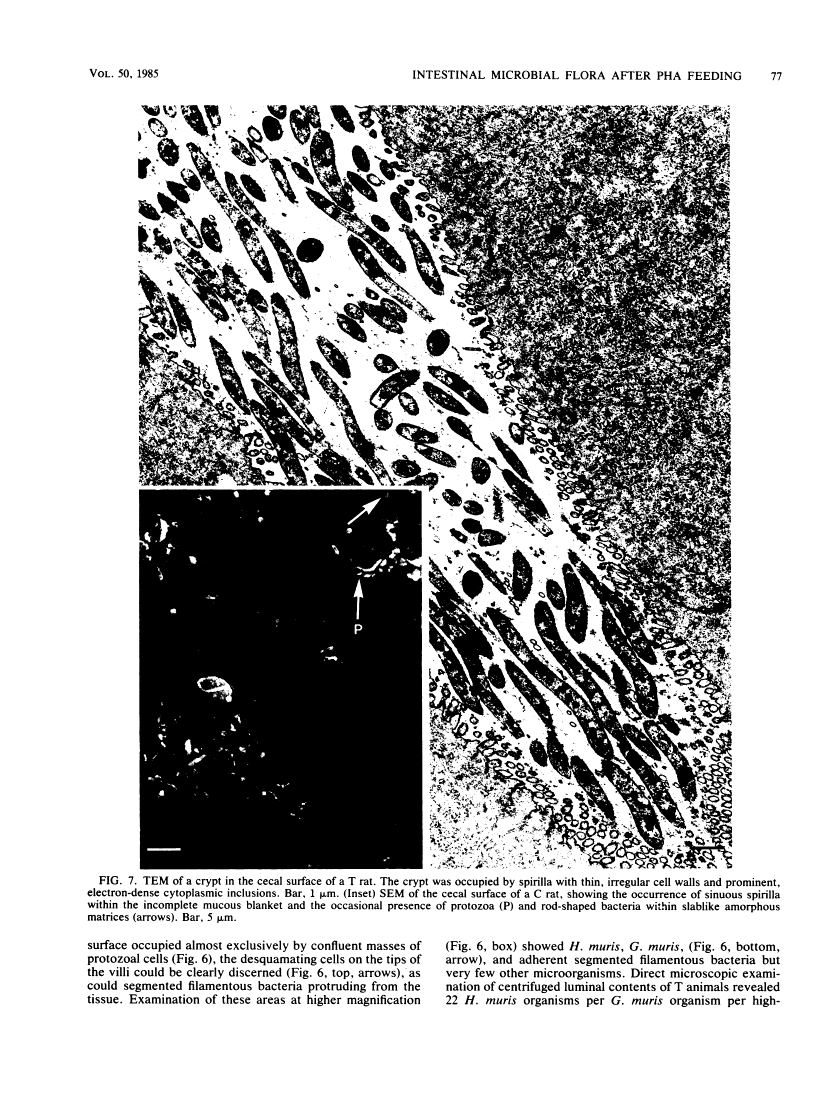
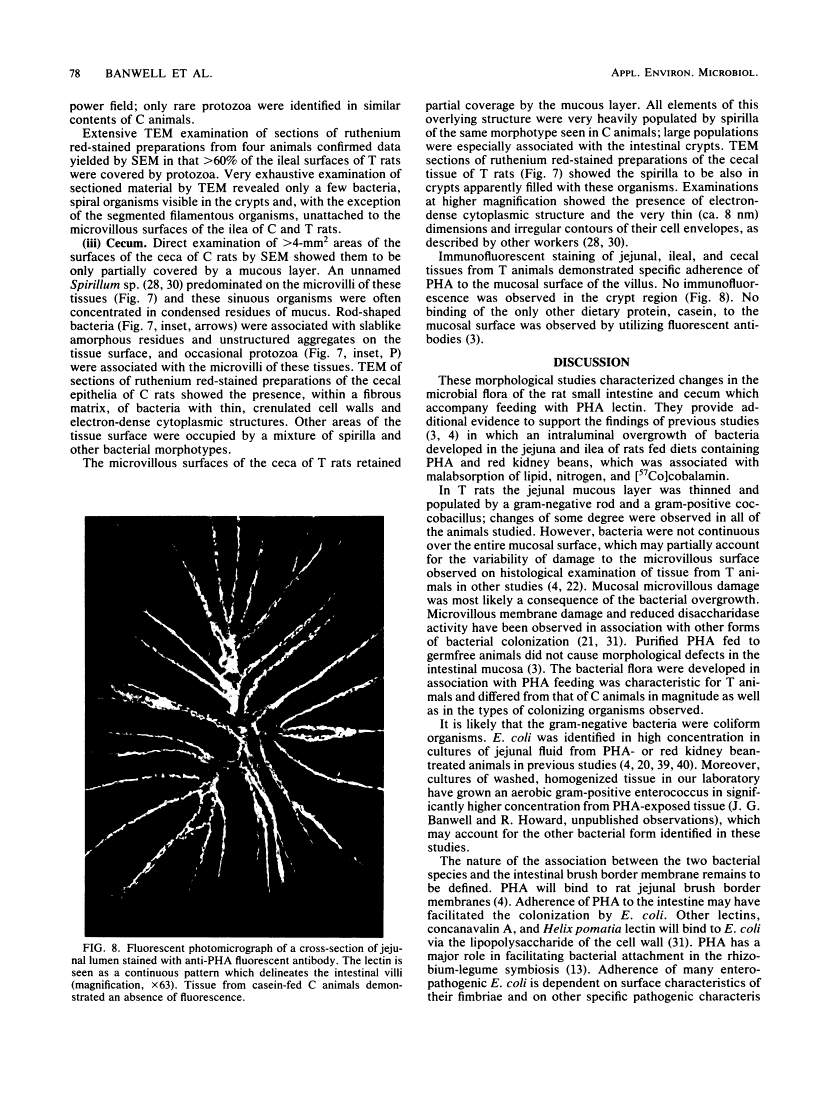
Abstract
Free full text

Intestinal microbial flora after feeding phytohemagglutinin lectins (Phaseolus vulgaris) to rats.
Abstract
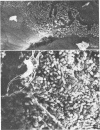
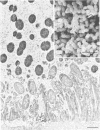
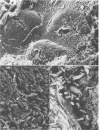
Incorporation of purified phytohemagglutinin (PHA) lectins derived from red kidney beans (Phaseolus vulgaris) in the diet of weanling rats will cause growth failure, malabsorption of nutrients, and bacterial overgrowth in the small intestine. These effects are not caused by feeding a similar quantity of PHA to germfree rats. To define the morphological and bacterial changes on the mucosal surfaces of the jejunum, ileum, and cecum in greater detail, we pair fed two groups of weanling rats isocaloric, isonitrogenous diets with or without 0.5% PHA protein. On the jejunal surfaces of control rats, the mucous layer was a confluent covering with sparsely scattered bacteria and protozoa. In PHA-treated rats, the mucous layer was thin and discontinuous, and the microvillous surface of the tissue was extensively populated by bacterial cells of two distinct morphotypes--a gram-negative rod and a gram-positive coccobacillus. In all PHA-treated animals, these bacteria formed adherent monospecific or mixed adherent microcolonies on the tissue surface. Tissue damage was observed in PHA-exposed jejunal tissue as evidenced by vesiculation of the microvillous plasma membrane and by damage to the brush border membrane. On the ileal surfaces of control rats, there was a thick mucous layer within which small numbers of bacteria and protozoa were seen. Segmented filamentous bacteria were anchored in the tissue surface. In PHA-treated rats, the ileal surface was only incompletely covered by a mucous layer, and the overlying mucosal surface was extensively covered by large numbers of protozoan cells (predominantly Hexamita muris). Most of the ileal surfaces not covered by the mucous layer were occupied and virtually occluded by an overgrowth of these protozoan cells with occasional cells of Giardia muris and the tissue-associated segmented bacillus. In the ceca of control rats, the mucosa was incompletely covered by a discontinuous mucous layer and colonized by an unnamed Spirillum sp., other bacteria, and occasional protozoa. The cecal surfaces of PHA-treated rats retained most of their incomplete overlying mucous layer, which was heavily colonized by the same type of Spirillum sp. seen in untreated animals; intestinal crypts were colonized. These descriptive morphological studies demonstrate that exposure to purified PHA in the diet caused characteristic changes in the microbial ecology of the small intestine. The changes in microbial flora contributed to the malabsorption of nutrients in the small intestines of PHA-fed animals.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (6.9M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. Why microbial predators and parasites do not eliminate their prey and hosts. Annu Rev Microbiol. 1981;35:113–133. [Abstract] [Google Scholar]
- Banwell JG, Abramowsky CR, Weber F, Howard R, Boldt DH. Phytohemagglutinin-induced diarrheal disease. Dig Dis Sci. 1984 Oct;29(10):921–929. [Abstract] [Google Scholar]
- Banwell JG, Boldt DH, Meyers J, Weber FL., Jr Phytohemagglutinin derived from red kidney bean (Phaseolus vulgaris): a cause for intestinal malabsorption associated with bacterial overgrowth in the rat. Gastroenterology. 1983 Mar;84(3):506–515. [Abstract] [Google Scholar]
- Brockett M, Tannock GW. Dietary components influence tissue-associated lactobacilli in the mouse stomach. Can J Microbiol. 1981 Apr;27(4):452–455. [Abstract] [Google Scholar]
- Chase DG, Erlandsen SL. Evidence for a complex life cycle and endospore formation in the attached, filamentous, segmented bacterium from murine ileum. J Bacteriol. 1976 Jul;127(1):572–583. [Europe PMC free article] [Abstract] [Google Scholar]
- Cohen PS, Rossoll R, Cabelli VJ, Yang SL, Laux DC. Relationship between the mouse colonizing ability of a human fecal Escherichia coli strain and its ability to bind a specific mouse colonic mucous gel protein. Infect Immun. 1983 Apr;40(1):62–69. [Europe PMC free article] [Abstract] [Google Scholar]
- Costerton JW, Ingram JM, Cheng KJ. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. [Europe PMC free article] [Abstract] [Google Scholar]
- Cummings RD, Kornfeld S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem. 1982 Oct 10;257(19):11230–11234. [Abstract] [Google Scholar]
- Evans RJ, Pusztai A, Watt WB, Bauer DH. Isolation and properties of protein fractions from navy beans (Phaseolus vulgaris) which inhibit growth of rats. Biochim Biophys Acta. 1973 Mar 23;303(1):175–184. [Abstract] [Google Scholar]
- Felsted RL, Leavitt RD, Bachur NR. Purification of the phytohemagglutinin family of proteins from red kidney beans (Phaseolus vulgaris) by affinity chromatography. Biochim Biophys Acta. 1975 Sep 9;405(1):72–81. [Abstract] [Google Scholar]
- Finkelstein RA, Boesman-Finkelstein M, Holt P. Vibrio cholerae hemagglutinin/lectin/protease hydrolyzes fibronectin and ovomucin: F.M. Burnet revisited. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1092–1095. [Europe PMC free article] [Abstract] [Google Scholar]
- FLOREY HW. The secretion and function of intestinal mucus. Gastroenterology. 1962 Sep;43:326–329. [Abstract] [Google Scholar]
- Gaastra W, de Graaf FK. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. [Europe PMC free article] [Abstract] [Google Scholar]
- Hewitt D, Coates ME, Kakade ML, Liener IE. A comparison of fractions prepared from navy (haricot) beans (Phaseolus vulgaris L.) in diets for germ-free and convential chicks. Br J Nutr. 1973 May;29(3):423–435. [Abstract] [Google Scholar]
- Jayne-Williams DJ, Burgess CD. Further observations on the toxicity of navy beans (Phaseolus vulgaris) for Japaneses quail (Coturnix coturnix japonica). J Appl Bacteriol. 1974 Mar;37(1):149–169. [Abstract] [Google Scholar]
- Jonas A, Flanagan PR, Forstner GG. Pathogenesis of mucosal injury in the blind loop syndrome. Brush border enzyme activity and glycoprotein degradation. J Clin Invest. 1977 Dec;60(6):1321–1330. [Europe PMC free article] [Abstract] [Google Scholar]
- King TP, Pusztai A, Clarke EM. Kidney bean (Phaseolus vulgaris) lectin-induced lesions in the small intestine: 1. Light microscope studies. J Comp Pathol. 1980 Oct;90(4):585–595. [Abstract] [Google Scholar]
- Leavitt RD, Felsted RL, Bachur NR. Biological and biochemical properties of Phaseolus vulgaris isolectins. J Biol Chem. 1977 May 10;252(9):2961–2966. [Abstract] [Google Scholar]
- Luft JH. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. [Abstract] [Google Scholar]
- Malick LE, Wilson RB. Modified thiocarbohydrazide procedure for scanning electron microscopy: routine use for normal, pathological, or experimental tissues. Stain Technol. 1975 Jul;50(4):265–269. [Abstract] [Google Scholar]
- Neutra MR. Prokaryotic-eukaryotic cell junctions: attachment of spirochetes and flagellated bacteria to primate large intestinal cells. J Ultrastruct Res. 1980 Feb;70(2):186–203. [Abstract] [Google Scholar]
- Owen RL, Nemanic PC, Stevens DP. Ultrastructural observations on giardiasis in a murine model. I. Intestinal distribution, attachment, and relationship to the immune system of Giardia muris. Gastroenterology. 1979 Apr;76(4):757–769. [Abstract] [Google Scholar]
- Phillips M, Lee A, Leach WD. The mucosa-associated microflora of the rat intestine: a study of normal distribution and magnesium sulphate induced diarrhoea. Aust J Exp Biol Med Sci. 1978 Dec;56(6):649–662. [Abstract] [Google Scholar]
- Pistole TG. Interaction of bacteria and fungi with lectins and lectin-like substances. Annu Rev Microbiol. 1981;35:85–112. [Abstract] [Google Scholar]
- Prizont R. Glycoprotein degradation in the blind loop syndrome: identification of glycosidases in jejunal contents. J Clin Invest. 1981 Feb;67(2):336–344. [Europe PMC free article] [Abstract] [Google Scholar]
- REISFELD RA, LEWIS UJ, WILLIAMS DE. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. [Abstract] [Google Scholar]
- Rozee KR, Cooper D, Lam K, Costerton JW. Microbial flora of the mouse ileum mucous layer and epithelial surface. Appl Environ Microbiol. 1982 Jun;43(6):1451–1463. [Europe PMC free article] [Abstract] [Google Scholar]
- Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. [Abstract] [Google Scholar]
- Schneider DR, Parker CD. Purification and characterization of the mucinase of Vibrio cholerae. J Infect Dis. 1982 Apr;145(4):474–482. [Abstract] [Google Scholar]
- Smithson KW, Millar DB, Jacobs LR, Gray GM. Intestinal diffusion barrier: unstirred water layer or membrane surface mucous coat? Science. 1981 Dec 11;214(4526):1241–1244. [Abstract] [Google Scholar]
- Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. [Abstract] [Google Scholar]
- Untawale GG, Pietraszek A, McGinnis J. Effect of diet on adhesion and invasion of microflora in the intestinal mucosa of chicks. Proc Soc Exp Biol Med. 1978 Nov;159(2):276–280. [Abstract] [Google Scholar]
- Wilson AB, King TP, Clarke EM, Pusztai A. Kidney bean (Phaseolus vulgaris) lectin-induced lesions in rat small intestine: 2. Microbiological studies. J Comp Pathol. 1980 Oct;90(4):597–602. [Abstract] [Google Scholar]
- Winne D. Unstirred layer thickness in perfused rat jejunum in vivo. Experientia. 1976 Oct 15;32(10):1278–1279. [Abstract] [Google Scholar]
Associated Data
Articles from Applied and Environmental Microbiology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/aem.50.1.68-80.1985
Read article for free, from open access legal sources, via Unpaywall:
https://aem.asm.org/content/aem/50/1/68.full.pdf
Free to read at aem.asm.org
http://aem.asm.org/cgi/content/abstract/50/1/68
Free after 4 months at aem.asm.org
http://aem.asm.org/cgi/reprint/50/1/68
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/aem.50.1.68-80.1985
Article citations
Rhubarb extract rebuilding the mucus homeostasis and regulating mucin-associated flora to relieve constipation.
Exp Biol Med (Maywood), 248(23):2449-2463, 11 Dec 2023
Cited by: 0 articles | PMID: 38073524 | PMCID: PMC10903230
Artificial intelligence model for analyzing colonic endoscopy images to detect changes associated with irritable bowel syndrome.
PLOS Digit Health, 2(2):e0000058, 17 Feb 2023
Cited by: 4 articles | PMID: 36812592 | PMCID: PMC9937744
Issues for patchy tissues: defining roles for gut-associated lymphoid tissue in neurodevelopment and disease.
J Neural Transm (Vienna), 130(3):269-280, 30 Oct 2022
Cited by: 8 articles | PMID: 36309872 | PMCID: PMC10033573
Review Free full text in Europe PMC
Electrostatic interactions mediate the nucleation and growth of a bacterial functional amyloid.
Front Mol Biosci, 10:1070521, 12 Jan 2023
Cited by: 0 articles | PMID: 36756360 | PMCID: PMC9900396
Mucosal Biofilms Are an Endoscopic Feature of Irritable Bowel Syndrome and Ulcerative Colitis.
Gastroenterology, 161(4):1245-1256.e20, 17 Jun 2021
Cited by: 47 articles | PMID: 34146566 | PMCID: PMC8527885
Go to all (23) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Phytohemagglutinin derived from red kidney bean (Phaseolus vulgaris): a cause for intestinal malabsorption associated with bacterial overgrowth in the rat.
Gastroenterology, 84(3):506-515, 01 Mar 1983
Cited by: 31 articles | PMID: 6822324
Small intestinal growth caused by feeding red kidney bean phytohemagglutinin lectin to rats.
Gastroenterology, 104(6):1669-1677, 01 Jun 1993
Cited by: 22 articles | PMID: 8500725
Bacterial overgrowth by indigenous microflora in the phytohemagglutinin-fed rat.
Can J Microbiol, 34(8):1009-1013, 01 Aug 1988
Cited by: 22 articles | PMID: 3061624
Microbial flora of the mouse ileum mucous layer and epithelial surface.
Appl Environ Microbiol, 43(6):1451-1463, 01 Jun 1982
Cited by: 63 articles | PMID: 7103492 | PMCID: PMC244253