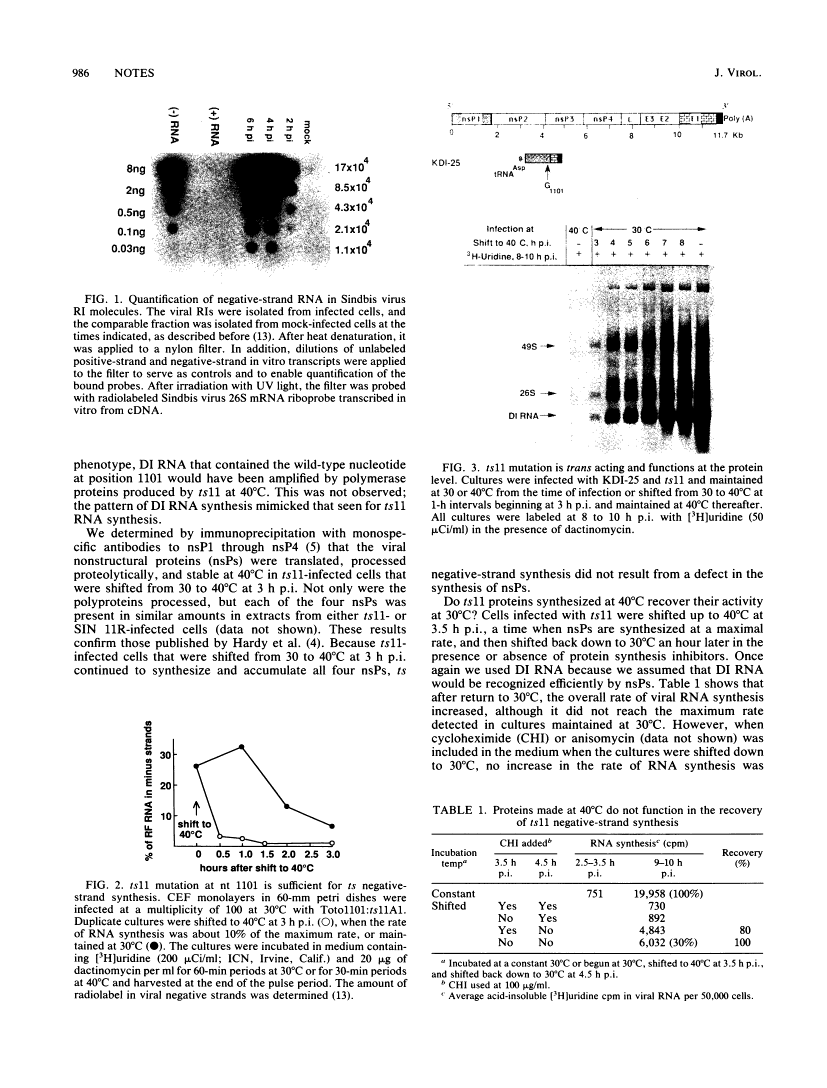
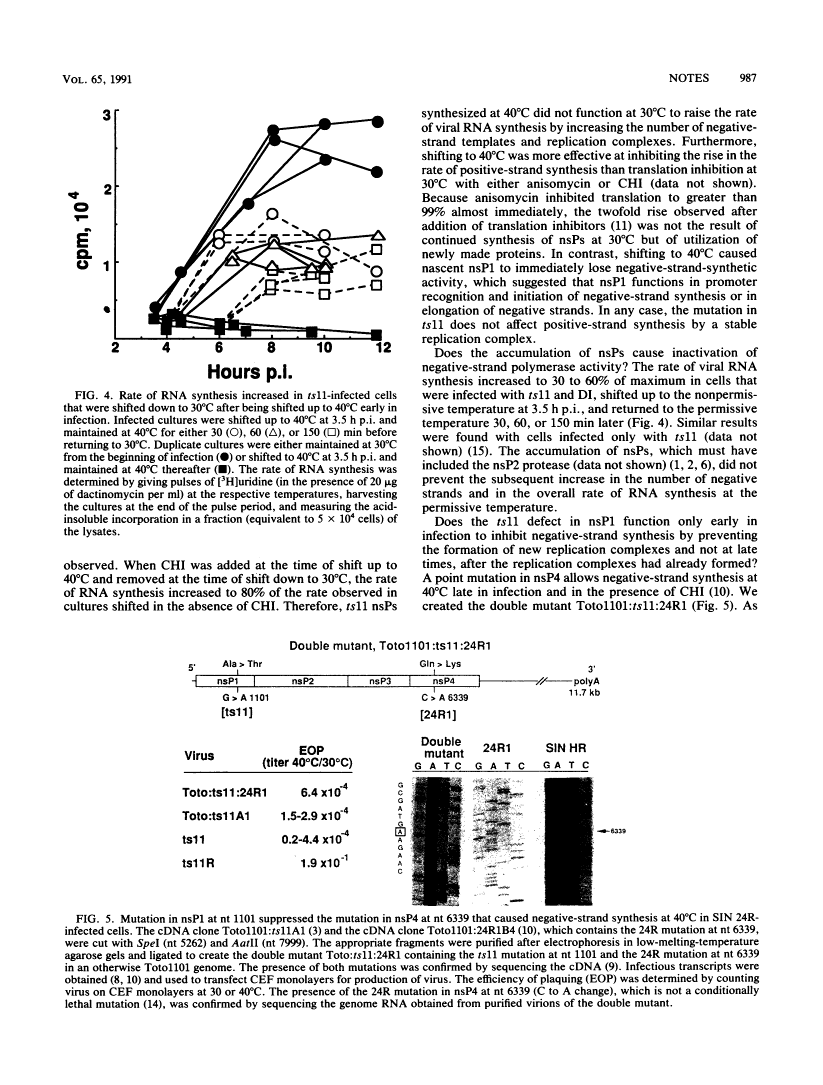
Abstract
Free full text

Sindbis virus nsP1 functions in negative-strand RNA synthesis.
Abstract
A mutation at nucleotide 1101 of Sindbis virus ts11 nsP1 caused temperature-sensitive negative-strand synthesis and suppressed the 24R phenotype, which is caused by a mutation in nsP4. Nonstructural proteins synthesized and accumulated by ts11 at 40 degrees C did not cause the reactivation of negative-strand synthesis upon return to 30 degrees C and did not prevent the formation of new replication complexes at 30 degrees C.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- de Groot RJ, Hardy WR, Shirako Y, Strauss JH. Cleavage-site preferences of Sindbis virus polyproteins containing the non-structural proteinase. Evidence for temporal regulation of polyprotein processing in vivo. EMBO J. 1990 Aug;9(8):2631–2638. [Europe PMC free article] [Abstract] [Google Scholar]
- Ding MX, Schlesinger MJ. Evidence that Sindbis virus NSP2 is an autoprotease which processes the virus nonstructural polyprotein. Virology. 1989 Jul;171(1):280–284. [Abstract] [Google Scholar]
- Hahn YS, Strauss EG, Strauss JH. Mapping of RNA- temperature-sensitive mutants of Sindbis virus: assignment of complementation groups A, B, and G to nonstructural proteins. J Virol. 1989 Jul;63(7):3142–3150. [Europe PMC free article] [Abstract] [Google Scholar]
- Hardy WR, Hahn YS, de Groot RJ, Strauss EG, Strauss JH. Synthesis and processing of the nonstructural polyproteins of several temperature-sensitive mutants of Sindbis virus. Virology. 1990 Jul;177(1):199–208. [Abstract] [Google Scholar]
- Hardy WR, Strauss JH. Processing the nonstructural polyproteins of Sindbis virus: study of the kinetics in vivo by using monospecific antibodies. J Virol. 1988 Mar;62(3):998–1007. [Europe PMC free article] [Abstract] [Google Scholar]
- Hardy WR, Strauss JH. Processing the nonstructural polyproteins of sindbis virus: nonstructural proteinase is in the C-terminal half of nsP2 and functions both in cis and in trans. J Virol. 1989 Nov;63(11):4653–4664. [Europe PMC free article] [Abstract] [Google Scholar]
- Levis R, Weiss BG, Tsiang M, Huang H, Schlesinger S. Deletion mapping of Sindbis virus DI RNAs derived from cDNAs defines the sequences essential for replication and packaging. Cell. 1986 Jan 17;44(1):137–145. [Abstract] [Google Scholar]
- Rice CM, Levis R, Strauss JH, Huang HV. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987 Dec;61(12):3809–3819. [Europe PMC free article] [Abstract] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. [Europe PMC free article] [Abstract] [Google Scholar]
- Sawicki D, Barkhimer DB, Sawicki SG, Rice CM, Schlesinger S. Temperature sensitive shut-off of alphavirus minus strand RNA synthesis maps to a nonstructural protein, nsP4. Virology. 1990 Jan;174(1):43–52. [Abstract] [Google Scholar]
- Sawicki DL, Sawicki SG. Short-lived minus-strand polymerase for Semliki Forest virus. J Virol. 1980 Apr;34(1):108–118. [Europe PMC free article] [Abstract] [Google Scholar]
- Sawicki DL, Sawicki SG, Keränen S, Käriäinen L. Specific Sindbis virus-coded function for minus-strand RNA synthesis. J Virol. 1981 Aug;39(2):348–358. [Europe PMC free article] [Abstract] [Google Scholar]
- Sawicki SG, Sawicki DL. The effect of loss of regulation of minus-strand RNA synthesis on Sindbis virus replication. Virology. 1986 Jun;151(2):339–349. [Europe PMC free article] [Abstract] [Google Scholar]
- Sawicki SG, Sawicki DL, Käriäinen L, Keränen S. A Sindbis virus mutant temperature-sensitive in the regulation of minus-strand RNA synthesis. Virology. 1981 Nov;115(1):161–172. [Abstract] [Google Scholar]
- Tuomi K, Kädäridäinen L, Söderlund H. Quantitation of Semlike Forest virus RNAs in infected cells using 32-P equilibrium labelling. Nucleic Acids Res. 1975 Apr;2(4):555–565. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jvi.65.2.985-988.1991
Read article for free, from open access legal sources, via Unpaywall:
https://jvi.asm.org/content/jvi/65/2/985.full.pdf
Free to read at jvi.asm.org
http://jvi.asm.org/cgi/content/abstract/65/2/985
Free after 4 months at jvi.asm.org
http://jvi.asm.org/cgi/reprint/65/2/985
Citations & impact
Impact metrics
Citations of article over time
Article citations
Current challenges in the discovery of treatments against Mayaro fever.
Expert Opin Ther Targets, 28(5):345-356, 08 May 2024
Cited by: 0 articles | PMID: 38714500
Review
Chikungunya virus nonstructural protein 1 is a versatile RNA capping and decapping enzyme.
J Biol Chem, 299(12):105415, 31 Oct 2023
Cited by: 0 articles | PMID: 37918803 | PMCID: PMC10687048
Cell-Type-Dependent Role for nsP3 Macrodomain ADP-Ribose Binding and Hydrolase Activity during Chikungunya Virus Infection.
Viruses, 14(12):2744, 09 Dec 2022
Cited by: 2 articles | PMID: 36560748 | PMCID: PMC9787352
Selective Estrogen Receptor Modulators Limit Alphavirus Infection by Targeting the Viral Capping Enzyme nsP1.
Antimicrob Agents Chemother, 66(3):e0194321, 18 Jan 2022
Cited by: 2 articles | PMID: 35041501 | PMCID: PMC8923187
The Putative Roles and Functions of Indel, Repetition and Duplication Events in Alphavirus Non-Structural Protein 3 Hypervariable Domain (nsP3 HVD) in Evolution, Viability and Re-Emergence.
Viruses, 13(6):1021, 28 May 2021
Cited by: 3 articles | PMID: 34071712 | PMCID: PMC8228767
Review Free full text in Europe PMC
Go to all (61) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Temperature sensitive shut-off of alphavirus minus strand RNA synthesis maps to a nonstructural protein, nsP4.
Virology, 174(1):43-52, 01 Jan 1990
Cited by: 46 articles | PMID: 2136781
Synthesis and processing of the nonstructural polyproteins of several temperature-sensitive mutants of Sindbis virus.
Virology, 177(1):199-208, 01 Jul 1990
Cited by: 37 articles | PMID: 2141204
Modification of Asn374 of nsP1 suppresses a Sindbis virus nsP4 minus-strand polymerase mutant.
J Virol, 76(17):8641-8649, 01 Sep 2002
Cited by: 23 articles | PMID: 12163583 | PMCID: PMC136982
Mapping of RNA- temperature-sensitive mutants of Sindbis virus: complementation group F mutants have lesions in nsP4.
J Virol, 63(3):1194-1202, 01 Mar 1989
Cited by: 105 articles | PMID: 2521674 | PMCID: PMC247815