| PMC full text: |
|
Figure 2
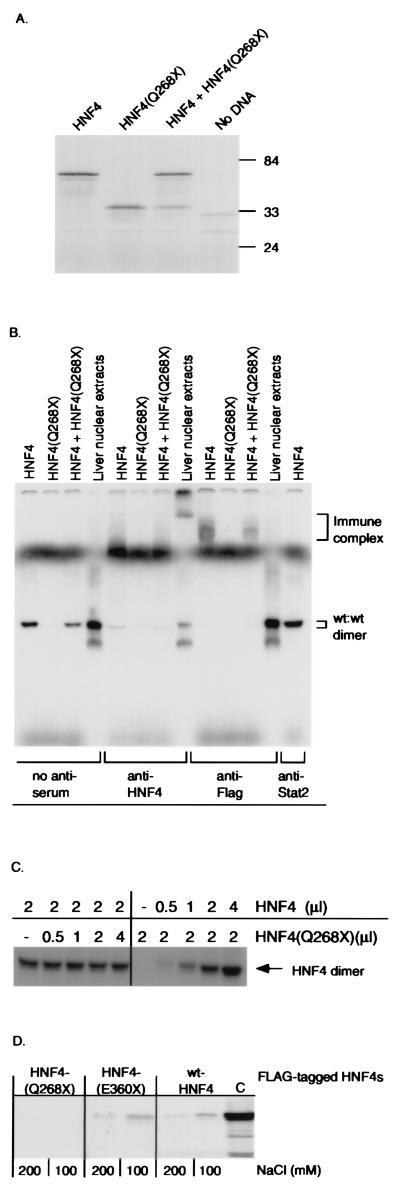
Expression, DNA binding, and dimerization properties of HNF4α and deletion mutant (Q268X). (A) SDS/PAGE analysis of in vitro-translated wild-type HNF4α and mutant HNF4(Q268X), followed by autoradiography. Numbers indicate molecular mass protein markers in kDa. (B) EMSA analysis of DNA binding activity of in vitro-translated HNF4α and deletion mutant (Q268X) (Top) by using the 32P-labeled double-stranded oligonucleotide LF-A1 as a probe. Supershift analysis was carried out with a polyclonal anti-HNF4 antiserum, an anti-Flag mAb, or an anti-STAT-2 control antiserum. (C) EMSA analysis of DNA binding activity of in vitro-translated wild-type HNF4α with increasing amounts of HNF4(Q268X) protein. (D) Both in vitro-translated wild-type HNF4 and HNF4(E360X), but not HNF4(Q268X), dimerize with HNF4. FLAG-tagged HNF4(Q268X), HNF4(E360X), or wild-type HNF4α bound to a anti-FLAG mAb attached to agarose was incubated with [35S]-methionine-labeled HNF4α protein. Immunoprecipitates were run on a 10% SDS/PAGE and bound HNF4α was detected by autoradiography. Each reaction was performed in buffers containing 100 and 200 mM NaCl, respectively (32). (C) 35S-methionine-labeled HNF4α.