Abstract
Free full text

Steady-State Kinetics and Mechanism of LpxD, the N-Acyltransferase of Lipid A Biosynthesis
Abstract
LpxD catalyzes the third step of lipid A biosynthesis, the R-3-hydroxymyristoyl-acyl carrier protein (R-3-OHC14-ACP)-dependent N-acylation of UDP-3-O-(R-3-hydroxymyristoyl)-α-D-glucosamine [UDP-3-O-(R-3-OHC14)-GlcN]. We have now over-expressed and purified E. coli LpxD to homogeneity. Steady state kinetics suggest a compulsory ordered mechanism in which R-3-OHC14-ACP binds prior to UDP-3-O-(R-3-OHC14)-GlcN. The product, UDP-2,3-diacylglucosamine, dissociates prior to ACP; the latter is a competitive inhibitor against R-3-OHC14-ACP and a noncompetitive inhibitor against UDP-3-O-(R-3-OHC14)-GlcN. UDP-2-N-(R-3-hydroxymyristoyl)-α-D-glucosamine, obtained by mild base hydrolysis of UDP-2,3-diacylglucosamine, is a noncompetitive inhibitor against both substrates. Synthetic R-3-hydroxylauroyl-methylphosphopantetheine is an uncompetitive inhibitor against R-3-OHC14-ACP and a competitive inhibitor against UDP-3-O-(R-3-OHC14)-GlcN, but R-3-hydroxylauroyl-methylphosphopantetheine is also a very poor substrate. A compulsory ordered mechanism is consistent with the fact that R-3-OHC14-ACP has a high binding affinity for free LpxD, whereas UDP-3-O-(R-3-OHC14)-GlcN does not. Divalent cations inhibit R-3-OHC14-ACP-dependent acylation but not R-3-hydroxylauroyl-methylphosphopantetheine-dependent acylation, indicating that the acidic recognition helix of R-3-OHC14-ACP contributes to binding. The F41A mutation increases the KM for UDP-3-O-(R-3-OHC14)-GlcN 30-fold, consistent with aromatic stacking of the corresponding F43 side chain against the uracil moiety of bound UDP-GlcNAc in the x-ray structure of Chlamydia trachomatis LpxD. Mutagenesis implicates E. coli H239 but excludes H276 as the catalytic base, and neither residue is likely to stabilize the oxyanion intermediate.
Lipid A is the hydrophobic moiety of lipopolysaccharide (LPS)1, which constitutes the outer leaflet of the outer membrane of most Gram-negative bacteria (1–3). The lipid A moiety of LPS is usually required for bacterial growth (3, 4) and is a potent activator of the mammalian innate immune system via the TLR4/MD-2 complex (5, 6). Over-production of cytokines due to excessive stimulation of TLR4/MD-2 may occur during severe Gram-negative infections and may contribute to the life-threatening complications of septic shock (7, 8).
The Kdo2-lipid A substructure of Escherichia coli LPS is synthesized by a conserved system of nine constitutive enzymes (Fig. 1A) (3). LpxD catalyzes the third reaction in this scheme, the R-3-hydroxymyristoyl-acyl carrier protein (R-3-OHC14-ACP)-dependent N-acylation of UDP-3-O-(R-3-hydroxymyristoyl)-α-D-glucosamine [UDP-3-O-(R-3-OHC14)-GlcN] (9) (Fig. 1A). Although essential for growth and an excellent target for the design of new antibiotics (9), LpxD is one of the least characterized enzymes in the pathway. Kelly and Raetz identified the function of LpxD and reported a quantitative assay, which demonstrated high selectivity of E. coli LpxD (EcLpxD) for the presence of the R-3-OH moiety and fourteen carbons in the acyl-ACP donor substrate (9). Apparent KM values in cell-free extracts for UDP-3-O-(R-3-OHC14)-GlcN and R-3-OHC14-ACP were reported as 1.3 and 1.9 μM respectively (9). No other functional studies of LpxD catalysis have appeared.
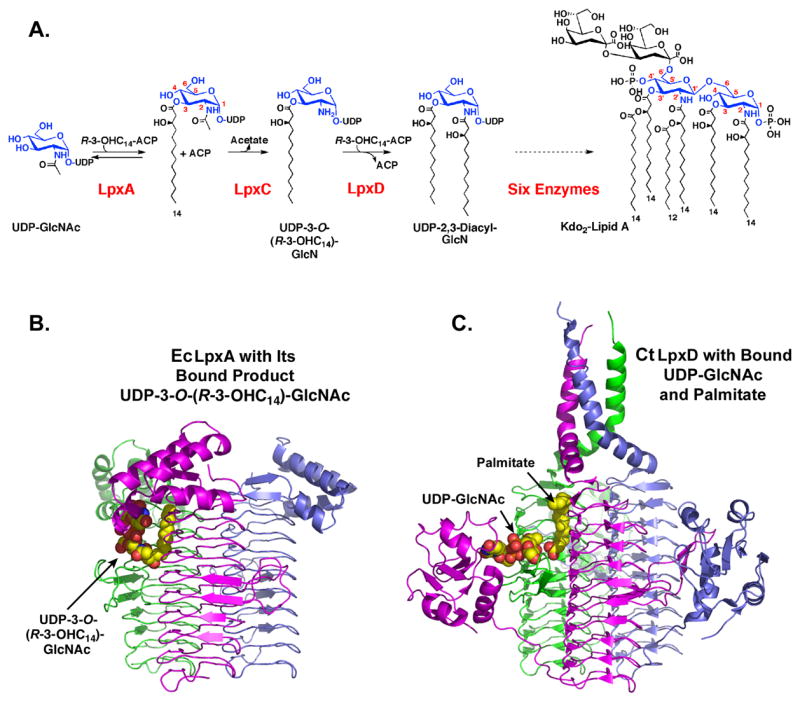
Panel A. LpxD is the third enzyme in the lipid A pathway. E. coli LpxD catalyzes the R-3-OHC14-ACP-dependent N-acylation of UDP-3-O-(R-3-OHC14)-GlcN to generate UDP-2,3-diacylglucosamine and ACP (9). Six additional enzymes are required to make Kdo2-lipid A, the minimal LPS substructure required for growth in most Gram-negative bacteria (3). Panel B. Crystal structure at 1.75. Å of E. coli LpxA with its bound product UDP-3-O-(R-3-hydroxymyristoyl)-α-D-N-acetylglucosamine (17). Panel C. Crystal structure of LpxD with bound UDP-GlcNAc and free fatty acid, modeled as palmitate (Complex I) (14). Each subunit of the LpxA or LpxD homotrimer is colored green, magenta or slate. In the bound ligands, carbon is yellow, nitrogen blue, oxygen red and phosphorus orange.
Based on its sequence, LpxD belongs to a large family of trimeric acyl- and acetyl-transferases that are characterized by the presence of a left-handed parallel β helix (LβH) domain (10–13). This unusual secondary structure was first identified in E. coli LpxA (Fig. 1B) (10). Recently, several crystal structures of Chlamydia trachomatis LpxD (CtLpxD) were reported by Buetow et al (14). Complex I, determined at 2.2 Å (14), was crystallized in the presence of 25 mM UDP-GlcNAc; it also contained extraneous bound palmitate molecules (Fig. 1C). Complex II (not shown in Fig. 1) was solved at 3.1 Å resolution (14). This sample was prepared in the presence of 100 mM UDP-GlcNAc and likewise contained bound free fatty acids (14). All CtLpxD structures revealed the same LβH fold and trimeric architecture seen with LpxA (Figs. 1B and 1C) (14). Although no assays were reported, Buetow et al. (14) proposed a mechanism for LpxD involving nucleophilic attack by the N-atom of UDP-3-O-(R-3-OHC14)-GlcN on the thioester carbonyl moiety of acyl-ACP. Given their general locations within the active site region, both H247 and H284 of CtLpxD were proposed to activate the N-atom of the acceptor UDP-3-O-(R-3-OHC14)-GlcN and/or stabilize the oxyanion intermediate (14). In fact, all the enzymes in the LβH acyltransferase class contain at least one essential histidine residue thought to function as the general base (13). In LpxA the H125A mutation abolishes activity (15), consistent with the fact that Nε2 of H125 is within hydrogen bonding distance of the 3-OH group of bound UDP-GlcNAc or UDP-3-O-(R-3-hydroxymyristoyl)-GlcNAc (16, 17).
Many acyltransferases in the LβH family are thought to operate by sequential mechanisms. For example, serine acetyltransferase has an ordered sequential mechanism in which acetyl-CoA binds prior to L-serine, and O-acetylserine dissociates before coenzyme A (18). Galactoside acetyltransferase (19), maltose acetyltransferase (20), and LpxA (21) likewise display sequential mechanisms, but in the case of LpxA the binding order is unknown (21). Glycerol-3-phosphate acyltransferase of squash chloroplasts, a soluble non-LβH acyltransferase, displays an ordered sequential mechanism in which the acyl-ACP donor binds first (22).
We now present the first steady-state kinetic analysis of EcLpxD. We have purified untagged EcLpxD and an active N-terminally His6-tagged LpxD variant to near homogeneity. Based on the steady state kinetics and the effects of several inhibitors, we suggest a compulsory ordered mechanism in which R-3-OHC14-ACP binds prior to UDP-3-O-(R-3-OHC14)-GlcN, and UDP-2,3-diacylglucosamine dissociates prior to ACP (Scheme 1). Direct binding studies with the physiological LpxD substrates, R-3-OHC14-ACP and UDP-3-O-(R-3-OHC14)-GlcN (Fig. 1A), provide independent support for this order of events. Site-directed mutagenesis and kinetic analyses of selected mutants support a chemical mechanism in which EcLpxD H239 (CtLpxD H247) is the catalytic base, and EcLpxD H276 (CtLpxD H284) participates in substrate binding. This conclusion was not clear from the crystal structures of CtLpxD Complexes I and II (14), which were determined in the presence of bound UDP-GlcNAc and free fatty acids (Fig. 1C), but not with the physiological CtLpxD ligands (Fig. 1A).
Materials and Methods
Reagents and Materials
[α-32P]-UTP was purchased from NEN DuPont, Boston, MA, and oligonucleotides were purchased from M.W.G. Biotech, High Point, NC. R-3-hydroxylauroyl-methylphosphopantetheine was synthesized by Avanti Polar Lipids, Alabaster, AL. All other chemicals were purchased from Sigma Chemical Co., St. Louis, MO or as prepared as described below. G-50 spin columns were purchased from GE Healthcare Piscataway, NJ, and C18 Sep-Pak columns were purchased from Waters, Milford, MA. Substrates and reagents, such as R-3-OHC14-ACP, R/S-3-hydroxylauroyl-ACP (9), [α-32P]-UDP-GlcNAc (9), and [α-32P]-UDP-3-O-(R-3-OHC14)-GlcN (9, 23), were prepared by minor modifications of published methods (see Supporting Information).
The LpxD Assay
The LpxD assay was carried out as previously reported (9). Unless otherwise noted, the 10 μl assay mixture contained 40 mM Hepes, pH 7.5, 0.02 mg/ml BSA, 0.1 nM pure wild-type E. coli LpxD (or up to 10 nM mutant LpxD), [α-32P]-UDP-3-O-(R-3-OHC14)-GlcN (4 μM, 0.005–0.04 μCi/μl) and 6 μM R-3-OHC14-ACP. The concentration of UDP-3-O-(R-3-OHC14)-GlcN was determined using the UDP extinction coefficient of 9900 M−1 cm−1 (24). The components were equilibrated at 30 °C, and the reaction was started by addition of enzyme. Time points were taken by spotting 1 μl portions of the reaction mixture onto a silica thin layer chromatography plate. After drying under a cold air stream, plates were developed with the solvent chloroform/methanol/water/acetic acid (25:15:4:2, v/v/v/v). After removal of the solvent, the plates were exposed overnight to a Molecular Dynamics PhosphorImager Screen. Percent of the [α-32P]-UDP-3-O-(R-3-OHC14)-GlcN converted to [α-32P]-UDP-2,3-diacylglucosamine was quantified with ImageQuant Software.
Cloning, Expression, and Purification of EcLpxD
Both untagged EcLpxD and His6-EcLpxD were prepared and purified to homogeneity (see Supporting Information). Cells were grown on LB broth or LB agar (25), supplemented with antibiotics as indicated, and protein concentrations were determined using the bicinchoninic acid method (26).
Site-directed Mutagenesis
LpxD point mutants were constructed using pNH6LpxD as the template, and the mutants were over expressed in the same manner as described above for the untagged protein. The various His6-tagged LpxDs were purified from cell free extracts derived from 125 ml cultures. The LpxD derivatives were purified over a 1 ml Ni-NTA column (Qiagen) washed with 40 mM potassium phosphate, pH 8.0, containing 250 mM NaCl and 20 mM imidizole. The desired LpxDs were eluted from the column in 40 mM potassium phosphate, pH 8.0, containing 250 mM NaCl and 150 mM imidizole. PD-10 columns (GE-Healthcare) were used to exchange the LpxDs eluted from the Ni-NTA columns into 10 mM potassium phosphate, pH 7.0, containing 20% glycerol and 200 mM NaCl. Proteins were concentrated to 1–10 mg/ml and stored at −80 °C. The specific activity and Michaelis-Menten constants of the purified N-terminally His6-tagged LpxD were the same as for the pure untagged protein (data not shown). However, a C-terminally His6 tagged LpxD construct was 50-time less active than the native protein, when assayed in cell-free extracts, even though protein expression, as judged by SDS-PAGE analysis, was not diminished (data not shown).
Bi-Substrate Kinetics
Bi-substrate kinetic analysis was performed by titrating one substrate from 0.5 – 8 μM, while holding the other substrate constant at 0.5, 1, 2, 4, or 8 μM. Equations for a ping-pong (eq. 1) or a sequential (eq. 2) reaction mechanism were fit globally (GraFit software2) using a least-squares non-linear approach:
A and B are the concentrations of substrates A and B respectively, KMa = KM for substrate A, KMb = KM for substrate B, and Kia = dissociation constant for substrate A. Vm is the maximal velocity.
Inhibition Kinetics
To determine inhibition patterns for ACP, UDP-2-N-(R-3-OHC14)-GlcN and R-3-hydroxylauroyl-methylphosphopantetheine, one substrate was varied from approximately 5-fold below to 5-fold above the KM, while holding the other substrate constant at about 2-fold above KM at fixed concentrations of the inhibitor (0 to 5-fold above the Ki). Non-linear least squares fits (GraFit software2) of the equations for competitive (eq. 3), uncompetitive (eq. 4), or noncompetitive (eq. 5) inhibition were used to evaluate the data:
Kic is the inhibition constant for a competitive inhibitor with respect to substrate S, and Kiu is the inhibition constant for an uncompetitive inhibitor with respect to substrate S. Kin is the inhibition constant for a noncompetitive inhibitor with respect to substrate S. Data were also evaluated by fitting to a mixed inhibition model (where the first Kin in eq. 5 does not equal the second), but this approach did not yield the lowest chi2.
Binding Assays
A modified procedure of Penefsky was used to determine dissociation constants for each substrate (27, 28). Either [α-32P]-UDP-3-O-(R-3-OHC14)-GlcN (24 nM) or non-radioactive R-3-OHC14-ACP (5 nM) was incubated with pure LpxD (0 to 160 μM as indicated) in 20 μl for 15 min at 30 °C in 40 mM Hepes, pH 7.5. EDTA (1 mM) was included in the incubations containing R-3-OHC14-ACP to prevent interactions with residual divalent cations. At concentrations of 1 mM and below, EDTA has no effect on LpxD activity (data not shown). G-50 spin columns (GE Healthcare) were supplied in 10 mM Tris-Cl (pH 8.0) and 1 mM EDTA, and they were equilibrated by centrifugation at 2000 × g for 1 min. The binding mixture was then applied, and the column was centrifuged again for 1 min. For the [α-32P]-UDP-3-O-(R-3-OHC14)-GlcN binding assays, the counts in the run-through, a measure of the binding of [α-32P]-UDP-3-O-(R-3-OHC14)-GlcN to LpxD, were quantified and compared to the total counts loaded onto the column. Approximately 5% of the total counts of [α-32P]-UDP-3-O-(R-3-OHC14)-GlcN loaded on the columns emerged in the run-through in the absence of LpxD. The fraction of the [α-32P]-UDP-3-O-(R-3-OHC14)-GlcN bound to LpxD at various concentrations of LpxD was then determined using eq. 6.
Because the R-3-OHC14-ACP substrate was not radiolabeled, the amount of R-3-OHC14-ACP bound to LpxD ([EL] in eq. 6) in the absence of acceptor substrate was determined by diluting 5 μl of the run through into a 10 μl assay mixture containing 5 nM [α-32P]-UDP-3-O-(R-3-OHC14)-GlcN (approximately two-fold excess of the maximum concentration of R-3-OHC14-ACP) and ≥ 1 μM LpxD (single turnover conditions). The concentration of the [α-32P]-UDP-2,3-diacylglucosamine product formed under these conditions was directly proportional to the concentration of R-3-OHC14-ACP in the run through. Concentrations were normalized to a control assay mixture that contained 2.5 nM R-3-OHC14-ACP. Under these conditions, the LpxD reaction goes to completion within seconds, and no reverse reaction occurs (data not shown). About 5–10% of the total R-3-OHC14-ACP loaded onto the G-50 spin column emerges in the run-through in the absence of LpxD, and this background was subtracted. Equation 7 was fit to the data to determine dissociation constants for both ligands (29).
Steady-State Kinetic Constants of Wild-type and Mutant EcLpxD
To determine the apparent kcat and KM for wild-type and mutant LpxDs, one substrate was varied from approximately 5-fold below to 5-fold above the KM while holding the other substrate constant at 2-fold above KM. R-3-OHC14-ACP curves were used to determine kcat in all cases. To determine kcat and KM, eq. 8 was fit to the data using KaleidaGraph (Synergy Software, Reading PA):
Inhibition by Divalent Cations
To study the inhibition of EcLpxD by Ca2+, Mg2+, Na+, or K+ ions, the chloride salt of each cation was titrated into the LpxD assay system from 0 to 10 mM. Inhibition data were fit to eq. 9, using KaleidaGraph:
In this case vi is the inhibited velocity, v0 the velocity without added inhibitor, [I] the concentration of the cation, IC50 the concentration of I when vi/v0 = 0.5, and h the Hill Coefficient, which was always in the range of 0.7 – 1.3.
Results
Purification of Untagged EcLpxD
EcLpxD activity was over-expressed approximately 1000-fold relative to a vector control (based on specific activity measurements) by inducing the lpxD gene cloned behind the T7 promoter of the pDC015-1 plasmid harbored in E. coli Rosetta (DE3)/pLysS. Purification to greater than 95 % homogeneity of the untagged protein was achieved by a combination of dye affinity (Green-19), anion exchange (Q-Sepharose) and gel filtration (Sephadex-G200) chromatography. Supporting Fig. 1 shows the analysis by SDS-PAGE of samples from each step of the purification. In a typical preparation derived from an induced 4-liter culture, a 3.4-fold purification (Table 1) was required to achieve near homogeneity (~15 mg pure protein per liter), as judged by SDS/PAGE. LpxD eluted as a single symmetrical peak from a Sephadex-G200 column at an elution volume consistent with a mass of 108 kDa, indicating that the enzyme is a homotrimer in aqueous solution. Electrospray-ionization/time-of-flight mass spectrometry gave a subunit molecular weight of 35,880 Da, in good agreement with the expected molecular weight of 35,881 Da for LpxD (untagged LpxD with the P2A substitution).
Table 1
Purification of E. coli LpxD.
| Step | Total Volume (ml) | Total Protein (mg) | Total Units (nmol/min) | Specific activity (nmol/min/mg) | Fold Purification | Yield (%) |
|---|---|---|---|---|---|---|
| Crude Extract | 250 | 692.5 | 1,828,200 | 2,640 | 1 | 100 |
| Green 19 | 139 | 150.1 | 1,059,800 | 7,060 | 2.7 | 58 |
| Q-Sepharose | 10 | 80.3 | 629,600 | 7,840 | 3.0 | 34 |
| Gel Filtration | 5.4 | 64.3 | 571,900 | 8,900 | 3.4 | 31 |
Steady State Kinetics of EcLpxD
LpxD catalyzes the transfer of the R-3-hydroxymyristoyl chain from R-3-OHC14-ACP to UDP-3-O-(R-3-OHC14)-GlcN to make UDP-2,3-diacylglucosamine and ACP (Fig. 1). Like LpxA and LpxC, LpxD does not require the presence of a detergent for catalytic activity because the critical micelle concentrations of its substrates are likely to be > 100 μM (well above their KMs) based on studies with other monoacylated phospholipids (30).
To determine the order of substrate binding and product release, we first determined whether LpxD catalysis conforms to a ping-pong or a sequential mechanism (31). A priori, it seemed unlikely that LpxD would function through an acyl-enzyme intermediate, because no conserved cysteine or serine residues are found in aligning diverse LpxD sequences. Initial velocity patterns for each substrate (varied from 5-fold below to 5-fold above the KM) were determined at fixed concentrations (0.5 μM, 1 μM, 2 μM, 4 μM, and 8 μM) of the other substrate. A ping-pong (eq. 1) or a sequential mechanism (eq. 2) was fit to the data. The global fit of the sequential mechanism (Fig. 2) was much better than of the ping-pong mechanism (data not shown). In the double reciprocal plots (Fig. 2), the initial velocity patterns intersect to the left of the y-axis, clearly supporting a sequential mechanism with Vm = 0.11 ± 0.007 μM/min, KMa = 3.2 ± 0.6 μM, KMb = 1.3 ± 0.2 μM, and Kia = 4.3 ± 2.6 μM, where substrate A is R-3-OHC14-ACP and substrate B is UDP-3-O-(R-3-OHC14)-GlcN, as shown in Scheme 1. A sequential mechanism implies that both substrates must bind to the enzyme in a ternary complex before catalysis can occur, but it does not distinguish ordered from random substrate binding and product dissociation.

The plot displays the Lineweaver-Burke representation of the initial velocities as a function of substrate concentrations. R-3-OHC14-ACP concentrations were varied from 5-fold below to 5-fold above the KM as shown with the UDP-3-O-(R-3-OHC14)-GlcN concentrations held constant at 0.5 μM (open circles), 1 μM (closed circles), 2 μM (open squares), 4 μM (closed squares), or 8 μM (open triangles). Non-linear fits (displaying the lowest chi2 values) were derived by least squares fitting using GraFit Software2.
Inhibition of EcLpxD by ACP and UDP-2-N-(R-3-OHC14)-GlcN
Product and substrate analog inhibition patterns were examined to determine the order of substrate binding and product dissociation (31). Initial velocity patterns were then assigned to a competitive (eq. 3), uncompetitive (eq. 4) or noncompetitive (eq. 5) mode of inhibition, based on the model that yielded the best least-squares fit. In all cases, two or more experiments were performed to determine the mode of inhibition (data not shown). The representative plots display the double-reciprocal (Lineweaver-Burke) representations of the best non-linear fits derived from least squares fitting.
As shown in Figs. 3A and 3B, ACP is a competitive inhibitor with respect to R-3-OHC14-ACP with a Kic of 48 ± 10 μM and a noncompetitive inhibitor with respect to UDP-3-O-(R-3-OHC14)-GlcN with a Kin of 139 ± 20 μM. The data are consistent with Scheme 1, an ordered mechanism in which R-3-OHC14-ACP (A) binds to LpxD (E), followed by UDP-3-O-(R-3-OHC14)-GlcN (B). After catalysis, UDP-2,3-diacylglucosamine (P) dissociates before ACP (Q) to reform the free enzyme (E). In this model ACP should be competitive with respect to R-3-OHC14-ACP because both bind to the free form of the enzyme. Furthermore, ACP should be noncompetitive with respect to UDP-3-O-(R-3-OHC14)-GlcN because each binds to a different form of the enzyme (Scheme 1).
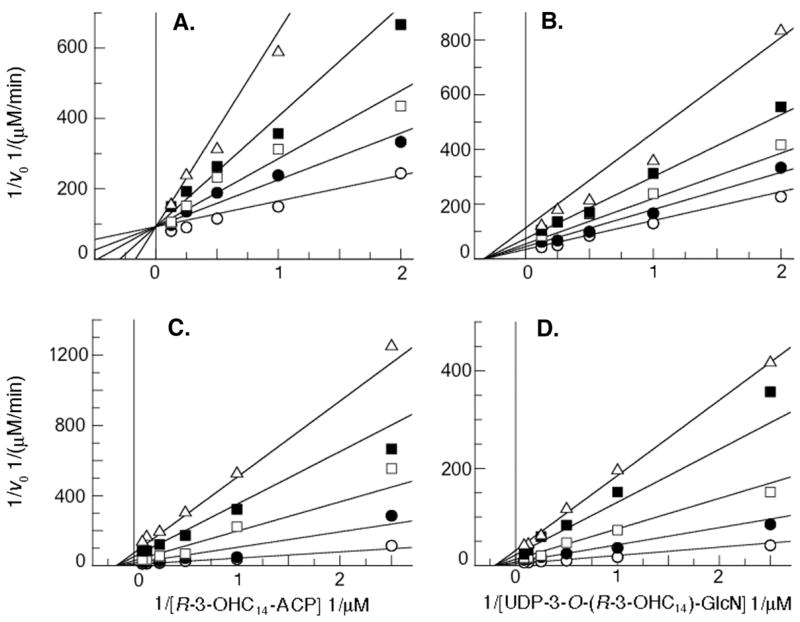
The plots display the Lineweaver-Burke representations of initial velocities as a function of substrate concentrations at increasing constant concentrations of each inhibitor. Substrate concentrations were varied as shown from 5-fold below to 5-fold above KM with the second substrate concentration held constant at 2-fold above its KM. Non-linear fits (displaying the lowest chi2 values) were derived by least squares fitting using GraFit Software2. ACP concentrations were held constant at 0 μM (open circles), 40 μM (closed circles), 80 μM (open squares), 160 μM (closed squares) or 360 μM (open triangles). UDP-2-N-(R-3-OHC14)-GlcN concentrations were held constant at 0 μM (open circles), 10 μM (closed circles), 25 μM (open squares), 50 μM (closed squares) or 75 μM (open triangles). Panel A. ACP is competitive with respect to R-3-OHC14-ACP. Panel B. ACP is noncompetitive with respect to UDP-3-O-(R-3-OHC14)-GlcN. Panel C. UDP-2-N-(R-3-OHC14)-GlcN is noncompetitive with respect to R-3-OHC14-ACP (chi2 for a competitive fit: 2.9×10−5; chi2 for a non-competitive fit: 1.0×10−5). Panel D. UDP-2-N-(R-3-OHC14)-GlcN is noncompetitive with respect to UDP-3-O-(R-3-OHC14)-GlcN (chi2 for a competitive fit: 5.8×10−5; chi2 for a non-competitive fit: 3.9×10−5).
Previous studies have demonstrated that the critical micelle concentration of the LpxD product UDP-2,3-diacylglucosamine is ≤ 20 μM (32). In order to avoid issues related to micelle formation, inhibition by UDP-2,3-diacylglucosamine was not investigated. Instead, UDP-2,3-diacylglucosamine was O-deacylated with mild base at the GlcN 3-position to form the product substructure UDP-2-N-(R-3-OHC14)-GlcN, which should have a CMC > 100 μM. As shown in Fig. 3C and 3D, UDP-2-N-(R-3-OHC14)-GlcN is a noncompetitive inhibitor with respect to both substrates with a Kin of 6.0 ± 1.1 μM against R-3-OHC14-ACP and a Kin of 9.4 ± 0.8 μM against UDP-3-O-(R-3-OHC14)-GlcN. This is consistent with Scheme 1 in which UDP-2,3-diacylglucosamine would not be expected to bind to the free form or the R-3-OHC14-ACP bound form of LpxD. Although the possibility that UDP-2,3-diacylglucosamine has some affinity for the free form of LpxD cannot be completely excluded, the data clearly show that UDP-2-N-(R-3-OHC14)-GlcN is not a competitive inhibitor with respect to either substrate.
Inhibition of EcLpxD by R-3-hydroxylauroyl-methylphosphopantetheine
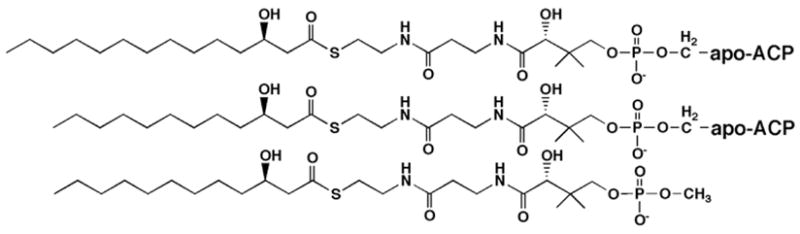
R-3-hydroxylauroyl-methylphosphopantetheine was synthesized (Table 2) because it represents a well-defined substructure of R-3-OHC14-ACP. The phosphopantetheine moiety is covalently attached to Ser-36 of ACP. We found that R-3-hydroxylauroyl-methylphosphopantetheine is a very slow substrate for LpxD (Table 2). The specific activity, measured at either 10 μM (Table 2) or 1 mM (not shown) R-3-hydroxylauroyl-methylphosphopantetheine as the acyl donor, is >100-fold lower than with 10 μM R-3-OHC14-ACP. The slow reactivity of R-3-hydroxylauroyl-methylphosphopantetheine as a substrate does not interfere with its use for the analysis of inhibition kinetics.
Table 2
LpxD Reactivity and Structures of Various Acyl Donors.
| Acyl Donor (10 μM) | S.A. (nmol/min)/mg |
|---|---|
| R-3-hydroxymyristoyl-ACP | 15.1 × 103 |
| R-3-hydroxylauroyl-ACP* | 1.46 × 103 |
| R-3-hydroxylauroyl-methylphosphopantetheine | 0.010 × 103 |
 | |
Surprisingly, R-3-hydroxylauroyl-methylphosphopantetheine is not a competitive inhibitor with respect to R-3-OHC14-ACP (Fig. 4). Instead, it is a competitive inhibitor with respect to UDP-3-O-(R-3-OHC14)-GlcN with an apparent Kic of 390 ± 70 μM and an uncompetitive inhibitor with respect to R-3-OHC14-ACP with an apparent Kiu of 690 ± 100 μM (Fig. 4). Taking into account substrate concentrations, these inhibition constants are converted to a Kic of 298 ± 53 qM and a Kiu of 394 ± 62 qM based on the KMs calculated from Fig. 2. These patterns suggest that R-3-hydroxylauroyl-methylphosphopantetheine actually binds to the same form of the enzyme as does the substrate UDP-3-O-(R-3-OHC14)-GlcN. R-3-hydroxylauroyl-methylphosphopantetheine does contain an R-3-hydroxyacyl chain and phosphate group like UDP-3-O-(R-3-OHC14)-GlcN; so perhaps, these two compounds can bind to the same form of the enzyme because the acyl chain and the phosphate group provide most of the binding energy. The Kic (for UDP-3-O-(R-3-OHC14)-GlcN) and Kiu (for R-3-OHC14-ACP) are within error of each other, consistent with the idea that both inhibition constants arise from the same R-3-hydroxylauroyl-methylphosphopantetheine binding process on the R-3-OHC14-ACP-bound form of LpxD (Scheme 1). The fact that R-3-hydroxylauroyl-methylphosphopantetheine does not predominately bind to the free form of LpxD further suggests that the phosphopantetheine moiety contributes relatively little to the binding. This in turn suggests that the phosphopantetheine moiety of R-3-OHC14-ACP likewise contributes relatively little to the interaction between free LpxD and R-3-OHC14-ACP.
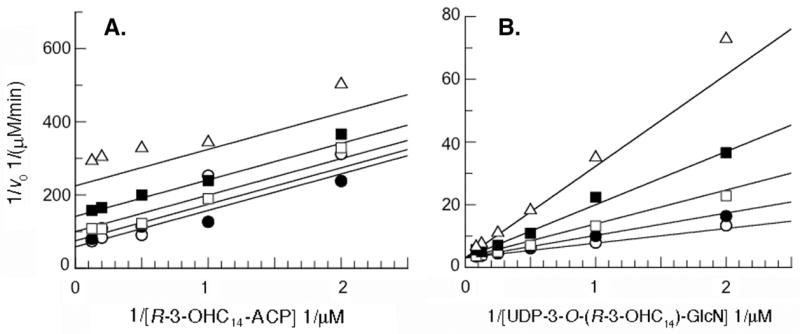
The plots display the Lineweaver-Burke representations of the initial velocities as a function of substrate concentrations at increasing constant concentrations of the synthetic inhibitor R-3-hydroxylauroyl-methylphosphopantetheine. Substrate concentrations were varied as shown from 5-fold below to 5-fold above KM, while holding the other substrate at a constant concentration at approximately 2-fold above KM. Non-linear fits (displaying the lowest chi2 values) were derived by least squares fitting using GraFit Software2. R-3-hydroxylauroyl-methylphosphopantetheine concentrations were held constant 0 mM (open circles), 0.2 mM (closed circles), 0.5 mM (open squares), 1 mM (closed squares) or 2 mM (open triangles). Panel A. R-3-hydroxylauroyl-methylphosphopantetheine is an uncompetitive inhibitor with respect to R-3-OHC14-ACP. Panel B. R-3-hydroxylauroyl-methylphosphopantetheine is a competitive inhibitor with respect to UDP-3-O-(R-3-OHC14)-GlcN.
According to Scheme 1, if R-3-hydroxylauroyl-methylphosphopantetheine is a competitive inhibitor with respect to UDP-3-O-(R-3-OHC14)-GlcN, it should be an uncompetitive inhibitor with respect to R-3-OHC14-ACP. This is clearly the case (Fig. 4), indicating that the preferred form of LpxD that R-3-hydroxylauroyl-methylphosphopantetheine interacts with is the R-3-OHC14-ACP bound form. As noted above, however, R-3-hydroxylauroyl-methylphosphopantetheine can also function as a poor substrate in the absence of R-3-OHC14-ACP, suggesting that R-3-hydroxylauroyl-methylphosphopantetheine does in fact have a low affinity for the free form of EcLpxD, or perhaps, that EcLpxD employs a different kinetic mechanism when R-3-hydroxylauroyl-methylphosphopantetheine is the acyl donor.
Direct Evaluation of Substrate Binding by EcLpxD
To provide further evidence for Scheme 1, binding constants for each substrate to free EcLpxD were estimated using spin column separations (27, 28). In this procedure, the ligand is first incubated with excess enzyme, and then unbound ligand is separated from bound ligand by rapid centrifugation over a small Sephadex G-50 column. Molecules larger than 30 kDa (including the bound ligand) emerge in the run-though, whereas molecules smaller than 30 kDa are retained by the column.
The fraction of the [α-32P]-UDP-3-O-(R-3-OHC14)-GlcN bound to excess EcLpxD ([EL]/[L]total) was determined by liquid scintillation counting and evaluated using eq. 6. Because the R-3-OHC14-ACP ligand was not radiolabeled, the concentration of R-3-OHC14-ACP bound to LpxD was determined indirectly by diluting a portion of the spin column run through into an assay mixture that contained a two-fold molar excess of [α-32P]-UDP-3-O-(R-3-OHC14)-GlcN and ≥ 1 μM LpxD (single turnover conditions). The amount of [α-32P]-UDP-2,3-diacylglucosamine product formed under these conditions was proportional to the concentration of R-3-OHC14-ACP (i.e. that bound to LpxD) in the run-through. The resulting data was fit to eq. 7 to determine the affinity of each substrate for the free form of LpxD. Fig. 5 shows the binding isotherms for each substrate. [α-32P]-UDP-3-O-(R-3-OHC14)-GlcN shows very little affinity for the free form of EcLpxD (Kd > 160 μM), whereas R-3-OHC14-ACP shows a high affinity for free EcLpxD (Kd = 59 ± 8 nM). These findings independently support Scheme 1, in which R-3-OHC14-ACP binds to the free form of LpxD and UDP-3-O-(R-3-OHC14)-GlcN binds to the R-3-OHC14-ACP-bound form.
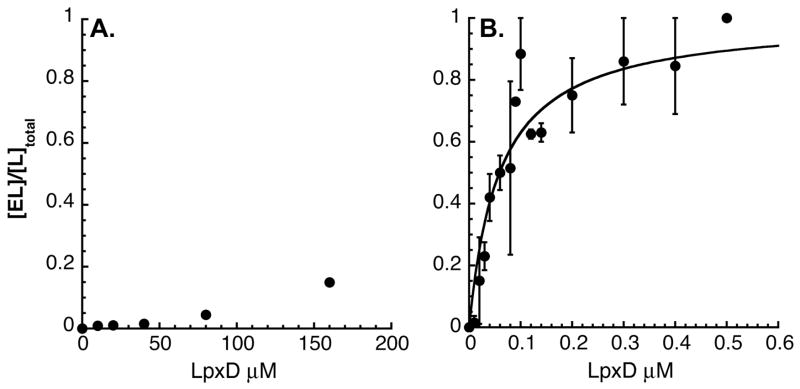
Panel A. Pure LpxD (0 to 160 μM) was incubated with 24 nM [α-32P]-UDP-3-O-(R-3-OHC14)-GlcN. Panel B. Pure LpxD (0 to 0.5 μM) was incubated with 5 nM R-3-OHC14-ACP for 10 min. Free ligand was rapidly separated from bound ligand by centrifugation over a small Sephadex G-50 column (28). The fraction of ligand bound to enzyme (determined as described in Material and Methods section) is plotted as a function of total enzyme concentration. Eq. 7 was fit to the data and the Kd was estimated as >160 μM for UDP-3-O-(R-3-OHC14)-GlcN and as 59 ± 8 nM for R-3-OHC14-ACP.
Role of Acyl Carrier Protein in Binding
Table 2 shows the EcLpxD specific activities with the acyl donor substrates R-3-OHC14-ACP, R/S-3-hydroxylauroyl-ACP, or R-3-hydroxylauroyl-methylphosphopantetheine (each at 10 μM of the R-form, assuming a 1:1 ratio of R:S for the R/S-3-hydroxylauroyl-ACP) and UDP-3-O-(R-3-OHC14)-GlcN (6 μM) as the acceptor. The assumption was also made that the S-form does not function as an acyl donor or inhibit the reaction. Removal of two-carbons from the acyl chain reduces the LpxD specific activity by about 10-fold. The additional loss of the acyl carrier protein portion of the substrate further reduces activity over 100-fold (Table 2). The results show that the full-length 14-carbon acyl chain and the ACP protein itself are very important for efficient LpxD catalysis, presumably by providing optimal binding energy.
Divalent cation inhibition of EcLpxD activity provides independent evidence for the importance of the protein portion of R-3-OHC14-ACP in LpxD catalysis. Divalent cations are known to bind key acidic side chains of E. coli ACP, which are often important for productive interaction of ACP with other proteins (33–36). Divalent cations such as Ca2+ and Mg2+ might inhibit LpxD activity by disrupting salt bridges between basic residues on EcLpxD and acidic residues on R-3-OHC14-ACP. Various divalent and monovalent cations were therefore tested as inhibitors in the range of 0 to 100 mM with substrate concentrations held at 6 μM R-3-OHC14-ACP and 4 μM UDP-3-O-(R-3-OHC14)-GlcN. As shown in Fig. 6A, Ca2+ inhibits LpxD with an IC50 of 0.42 ± 0.06 mM and Mg2+ with an IC50 of 1.41 ± 0.2 mM. Na+ (Fig. 6A) and K+ ions (not shown) do not inhibit LpxD activity. These findings are consistent with reports that ACP has a high affinity for both Ca2+ and Mg2+, but only a weak affinity for Na+ and K+ (37, 38). Fig. 6B shows that Ca2+ inhibition is overcome by the addition of excess R-3-OHC14-ACP, consistent with the idea that Ca2+ is interacting with acidic sites on R-3-OHC14-ACP, thereby rendering it less capable of interacting with LpxD.
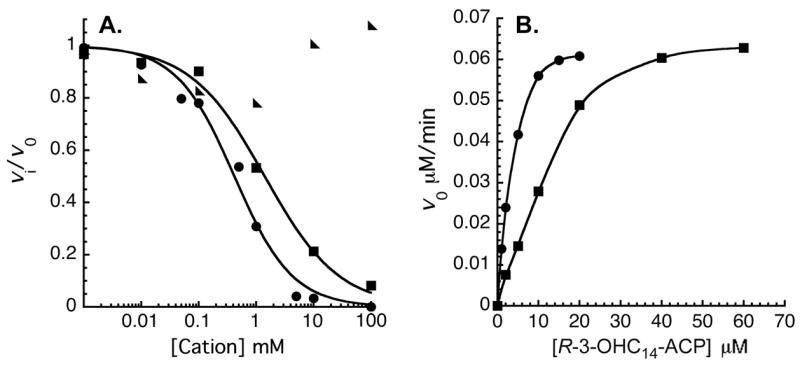
Panel A. CaCl2 (circles), MgCl2 (squares), or NaCl (triangles) concentrations were varied from 0 to 100 mM, as indicated. To determine the IC50 for the divalent cations, eq. 9 was fit to the data. Panel B. Ca2+ inhibition is overcome by increasing the R-3-OHC14-ACP concentration. The initial velocities as a function of R-3-OHC14-ACP concentrations are shown with 0 mM (circles) or 1 mM CaCl2 (squares) in the assay system, as indicated.
To confirm that the protein portion of R-3-OHC14-ACP is required for Ca2+ inhibition, we measured the specific activity of LpxD with either 10 μM R-3-OHC14-ACP or 10 μM R-3-hydroxylauroyl-methylphosphopantetheine in the presence or absence of 10 mM Ca2+. With R-3-OHC14-ACP, the specific activity in the absence of Ca2+ was 7.14×103 nmol/min/mg, whereas in the presence of Ca2+ it was 1.14×103 nmol/min/mg. In contrast, with R-3-hydroxylauroyl-methylphosphopantetheine as the acyl donor, the specific activity in the absence of Ca2+ was 3.0 nmol/min/mg versus 3.3 nmol/min/mg the presence of Ca2+, consistent with the idea that Ca2+ interacts the protein portion of R-3-OHC14-ACP.
Approach to the Site-Directed Mutagenesis of EcLpxD
Residues for site-directed mutagenesis (Tables 3 and and4)4) were chosen by sequence alignment (39, 40) or were selected based on the CtLpxD crystal structure (14). The CtLpxD residues and atoms listed in Table 3 are potentially close enough to the bound UDP-GlcNAc ligand (3.3 Å or less) to engage in polar interactions (14). Table 4 shows the residues that were subjected to mutagenesis in EcLpxD. Group I residues (Table 4) correspond to those near the UDP-GlcNAc binding site in CtLpxD Complexes I and II (Table 3) (14). Group II residues (Table 4) correspond to those near the carboxyl group of the bound palmitic acid seen in CtLpxD Complex I and II (14). Group III residues are other conserved basic side chains (14) that might be involved in acyl-ACP binding.
Table 3
Differences in UDP-GlcNAc Binding between Chains A and B of CtLpxD Complex I versus CtLpxD Complex II.
| Ct LpxD Residue and Heteroatom near UDP- GlcNAc | Corresponding EcLpxD Residue§ | Possible H-bond or Polar Partner on UDP-GlcNAc | Distance in Å Complex I | Distance in Å Complex II |
|---|---|---|---|---|
| Chain A | ||||
| F190 O | GlcNAc O4 | 2.67* | 8.14* | |
| Y192 N | GlcNAc O6 | 3.15 | 11.3 | |
| H247 NE2** | H239 | GlcNAc O3 | 3.07 | 6.71 |
| H247 NE2 | H239 | GlcNAc O7‡ | 5.01 | 2.61 |
| H247 NE2 | H239 | GlcNAc N2 | 4.50 | 4.60 |
| Q248 OE1 | N240 | Ribose O3 | 3.03 | 2.92 |
| Q248 NE2 | N240 | Ribose O3 | 3.30 | 3.66 |
| G265 O | GlcNAc O7‡ | 2.35 | 5.74 | |
| S266 OG | S258 | Ribose O3 | 2.78 | 3.93 |
| H284 NE2 | H276 | PO alpha 1 | 3.11 | 2.70 |
| H284 NE2 | H276 | PO beta 2 | 3.34 | 2.53 |
| H284 NE2 | H276 | GlcNAc O7‡ | 3.21 | 5.42 |
| H284 NE2 | H276 | GlcNAc N2 | 3.19 | 4.69 |
| Chain B | ||||
| E32 OE2 | Ribose O3 | 3.40 | 2.62 | |
| I33 N | Uracil O2 | 2.82 | 2.80 | |
| E34 OE1 | Q32 | Ribose O2 | 4.50 | 3.01 |
| F43 O | F41 | Uracil N3 | 2.93 | 2.68 |
| D45 N | Uracil O4 | 2.82 | 2.78 | |
| N46 N | N44 | Uracil O4 | 3.50 | 3.26 |
| N46 ND2 | N44 | GlcNAc O6 | 8.77 | 3.09 |
| N46 ND2 | N44 | PO beta 1 | 3.84 | 3.23 |
| Y49 OH | Y47 | PO beta 2 | 3.08 | 2.40 |
Distance measurements were made using PyMol (DeLano Scientific, San Carlos, CA) on pdb file 2iu8 for Complex I and 2iu9 for Complex II (14).
Table 4
Steady-State Kinetic Parameters for Selected EcLpxD Mutants.
| EcLpxD Protein | kcat (s−1) | KM, UDP-3-O-(R-3-OHC14)-GlcN (μM) | KM,R-3-hydroxymyristoyl-ACP (μM) |
|---|---|---|---|
| Wild type | 23 ± 1 | 2.5 ± 0.3 | 3.2 ± 0.8 |
| Group I | |||
| F41A | 4.1 ± 0.9 | 73 ± 30 | 3.5 ± 0.5 |
| Y47A | 18 ± 3 | 7.8 ± 3 | 5 ± 1 |
| H239A | 0.032 ± 0.001 | 0.84 ± 0.1 | 3.4 ± 1 |
| H276A | 0.73 ± 0.3 | 6.3 ± 0.9 | 1.7 ± 0.4 |
| Group II | |||
| D232A | 1.9 ± 0.1 | 67 ± 10 | 47 ± 7 |
| N233A | 1.4 ± 0.1 | 28 ± 7 | 13 ± 4 |
| Q236A | 12 ± 1 | 4.2 ± 0.9 | 13 ± 3 |
| Group III | |||
| K46A | 17 ± 1 | 7.1 ± 1 | 12 ± 5 |
| K194A | 5.7 ± 0.2 | 5.6 ± 0.5 | 3.8 ± 0.9 |
| R293A | 8.9 ± 0.7 | 3.6 ± 1.0 | 74 ± 0.3 |
Two Modes of UDP-GlcNAc Binding in CtLpxD
Given the superior 2.2 Å resolution of Complex I, which contains a single bound UDP-GlcNAc between chains A and B (pdb code: 2iu8) (14), and the good electron density of the UDP-GlcNAc ligand situated between chains A and B in the 3.1 Å Complex II (pdb code: 2iu9) (14), we focused on these two sites (Table 3). All protein heteroatoms within 3.3 Å of plausible H-bonding or polar partners on the UDP-GlcNAc in Complex I or II (or both) were evaluated (Table 3).
The distances between key atoms of the UDP moiety of the bound UDP-GlcNAc and various CtLpxD side chains are quite similar in Complexes I and II (Table 3, black numbers). However, there are large differences between these two complexes in the distances between key side chains of CtLpxD and the heteroatoms of the GlcNAc moiety. Discrepancies greater than 1.5 Å are highlighted in red (Table 3). These anomalies are easily explained when the UDP-GlcNAc ligand of Complex I is overlaid onto its counterpart in Complex II (Fig. 7, sticks). The UDP moieties of the two complexes display similar conformations, accounting for the relatively consistent inter-atomic distances (Table 3). However, the GlcNAc group is flipped in Complex I versus Complex II (Fig. 7, sticks). The positions of the surrounding LpxD side chains are very similar in both complexes (Fig. 7, magenta sticks and lines versus green sticks and lines). Why the GlcNAc moiety is oriented differently in Complex I versus II is uncertain and was not discussed by Buetow et al. (14).
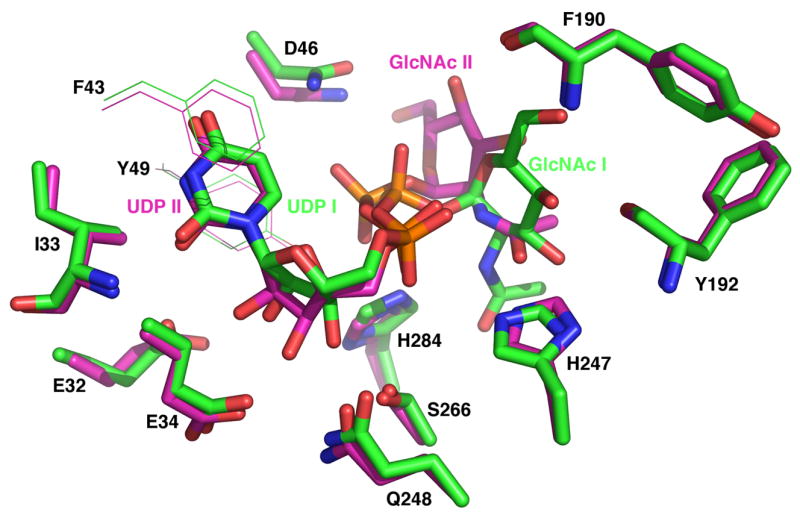
The UDP-GlcNAc carbons of Complex I are colored green, whereas those of Complex II are colored magenta (14). The positions of the CtLpxD side chains (green sticks for Complex I and magenta sticks for Complex II) are very similar in both complexes (14), but the GlcNAc moiety of the bound UDP-GlcNAc is displaced by over 90°, accounting for the dramatic differences in some of the inter-atomic distances listed in Table 3. The side chains of the two aromatic residues that flank the uracil moiety (14) are shown in lines for clarity.
Site Directed Mutagenesis of EcLpxD Group I Residues
As shown in Table 4, the conserved EcLpxD residue H239 is critically important for enzymatic activity. It likely functions as the general base to activate the amine group of the acceptor substrate during catalysis (Scheme 2). When H239 is mutated to alanine, the kcat of EcLpxD is reduced about 1000-fold with very little effect on the KM of either substrate (Table 4). Conserved in all LpxD sequences, H239 of EcLpxD (equivalent to H247 of CtLpxD) (14) also aligns with H125 in E. coli LpxA (15, 17); the Nε2 atom of the latter is the general base in LpxA, being situated within H-bonding distance of the GlcNAc 3-OH group in the LpxA structure and functioning to activate it for attack on the thioester carbonyl moiety of acyl-ACP (16, 17). Our suggestion that H239 of EcLpxD is the catalytic base is, however, inconsistent with the CtLpxD structure (Table 3) (14). The Nε2 of H247 of CtLpxD is 4.5 and 4.6 Å away from the N2 atom of the GlcNAc moiety in Complex I and II respectively (Table 3), too far to activate the N2 atom of the substrate (14). Buetow et al. [Fig 1 in (14)] therefore propose that CtLpxD H247 (corresponding to EcLpxD H239) and CtLpxD H284 (corresponding to EcLpxD H276) stabilize the oxyanion intermediate.
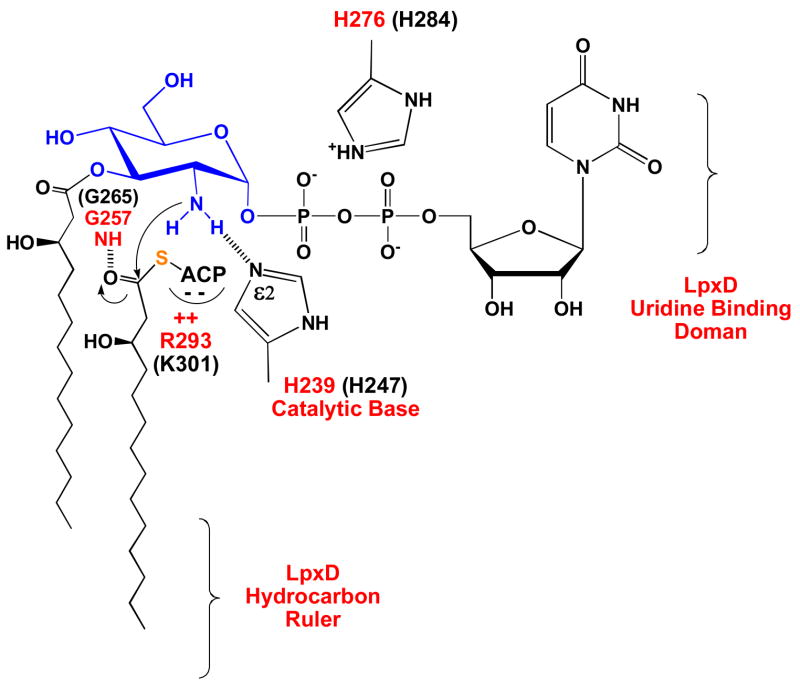
After the ternary complex is formed, the Nε2 atom of H239 activates the amine group of UDP-3-O-(R-3-OHC14)-GlcN (blue), which then attacks the carbonyl moiety of R-3-OHC14-ACP. R293 of EcLpxD may contribute to binding of the acyl-ACP donor substrate by providing ionic interactions. The tetrahedral intermediate is presumably stabilized by the oxyanion hole at G257 of EcLpxD. H276 functions mainly to bind the diphosphate moiety of the acceptor substrate. EcLpxD residue numbers are in red and corresponding CtLpxD residue numbers are in black.
Based on our analysis, H276 of EcLpxD (H284 of CtLpxD) may function in acceptor substrate binding but not in catalysis. We see a modest (30-fold) reduction in kcat in the H276A mutant (Table 4), suggesting that H276 is not likely to be involved in stabilization of the oxyanion intermediate as proposed by Buetow et al. (14). In both Complex I and II, however, CtLpxD H284 is well positioned to participate in binding the diphosphate moiety of the acceptor substrate (Table 3 and Fig. 7) (14).
In the CtLpxD structure several additional residues were implicated in binding UDP-GlcNAc (14). In Complex I and II, F43 and Y49 create π-stacking interactions with the uracil base, and the backbone carbonyl oxygen of F43 provides an important H-bond acceptor (Table 3). The corresponding EcLpxD F41A variant displays a 30-fold increase in KM, UDP-3-O-(R-3-OHC14)-GlcN and a 5-fold decrease in kcat, consistent with its position in the crystal structure (14). However, the EcLpxD substitution Y47A (Table 4) has only a small effect on KM, UDP-3-O-(R-3-OHC14)-GlcN (a 3-fold increase) with little effect on kcat or KM, R-3-OHC14-ACP.
E34 and N46 in CtLpxD Complex I are proposed to hydrogen bond the 2′-hydroxyl group of the ribose ring and the beta phosphate group, respectively, of UDP-GlcNAc (Table 3), whereas Q248 hydrogen bonds the ribose 3′-hydroxyl group (14). In our analysis of EcLpxD, the corresponding residues (Table 3) contribute little to the binding of the native EcLpxD substrates. The corresponding EcLpxD substitutions, Q32A, N44A and N240A, caused less than a 2-fold reduction in specific activity, when assayed at substrate concentrations at 2-fold above KM with the purified proteins (data not shown).
Site-Directed Mutagenesis of EcLpxD Group II Residues
The CtLpxD structure revealed the presence of an extraneous fatty acid in each subunit (14), situated in a hydrophobic groove within the LβH domains of the protein. Aliphatic residues line the walls of this groove. In addition, three polar CtLpxD residues, D240, N241, and Q244 (corresponding to D232, N233, and Q236 in EcLpxD, respectively), are close to the carboxylate moiety of the bound fatty acid. The EcLpxD mutations D232A and N233A caused a 10-fold reduction in kcat and a striking increase in the KM for both substrates, suggesting they are somehow involved in binding of the physiological substrates, whereas the Q236A mutation had little effect on activity (Table 4).
Site Directed Mutagenesis of EcLpxD Group III Residues
As noted above (Fig. 6 and Table 2), acidic side chains on R-3-OHC14-ACP may be important for its binding to LpxD. Consequently, LpxD may contain cognate basic residues that interact with R-3-OHC14-ACP. The CtLpxD structure reveals a basic cleft near the active site around the highly conserved K48 residue (K46 in EcLpxD) (14). As shown in Table 4, however, the K46A substitution in EcLpxD caused only a 3-fold increase in KM, R-3-OHC14-ACP and had no effect on kcat. Mutation of another basic residue close to the active site, K194 (H202 in CtLpxD), to alanine likewise had little effect on activity (Table 4).
Although not considered in the analysis of the crystal structure (14), we noticed another conserved basic residue in the general vicinity of the active site, R293, which has a dramatic effect on catalysis when mutated to alanine (Table 4). The KM, R-3-OHC14-ACP for R293A increases 23-fold compared to wild-type with little effect on kcat (Table 4), suggesting a role for R293 in R-3-OHC14-ACP binding (Scheme 2).
Discussion
LpxD catalyzes the third step of lipid A biosynthesis in E. coli, the R-3-OHC14-ACP dependent N-acylation of UDP-3-O-(R-3-OHC14)-GlcN (Fig. 1A) (9, 41). A crystal structure of C. trachomatis LpxD has recently appeared (14), demonstrating that LpxD resembles LpxA (10, 17, 42, 43) in its homotrimeric architecture and β-helical fold (Figs. 1B and 1C). However, little is known about the chemical mechanism of LpxD or how it recognizes its physiological substrates (Fig. 1A versus Fig. 1C). Because lipid A is required for the growth of most Gram-negative bacteria (3), LpxD inhibitors should be useful as antibiotics, but to date, no inhibitors with antibacterial activity have been identified. A possible advantage of LpxD inhibitors over the many available LpxC inhibitors (1, 3, 4) might be the intra-cellular accumulation of the intermediate UDP-3-O-(R-3-OHC14)-GlcN (Fig. 1A), a detergent-like molecule that could enhance cell killing.
As a prelude to the search for antibacterial LpxD inhibitors, EcLpxD has now been subjected to kinetic studies and site directed mutagenesis (9). Our kinetic data suggest a predominantly compulsory-ordered mechanism for EcLpxD in which R-3-OHC14-ACP binds prior to UDP-3-O-(R-3-OHC14)-GlcN and UDP-2,3-diacylglucosamine dissociates prior to ACP (Scheme 1). The following results support this conclusion: a) ACP is a competitive inhibitor with respect to R-3-OHC14-ACP and a noncompetitive inhibitor with respect to UDP-3-O-(R-3-OHC14)-GlcN (Fig. 3); b) UDP-2-N-(R-3-OHC14)-GlcN is a noncompetitive inhibitor with respect to both substrates (Fig. 3); c) R-3-hydroxylauroyl-methylphosphopantetheine is an uncompetitive inhibitor with respect to R-3-OHC14-ACP and a competitive inhibitor with respect to UDP-3-O-(R-3-OHC14)-GlcN (Fig. 4); and d) R-3-OHC14-ACP displays a high affinity for free LpxD, whereas UDP-3-O-(R-3-OHC14)-GlcN does not (Fig. 5). Our data are consistent with results obtained for related enzymes, such as serine acetyltransferase, galactoside acetyltransferase, and sn-glycerol-3-phosphate acyltransferase, which likewise proceed through analogous ordered mechanisms (13).
Based on multiple sequence alignments, H239 of EcLpxD is equivalent to H247 of CtLpxD, and both align with H125 of LpxA (Fig. 8A), the catalytic base as determined by structural studies and mutagenesis of LpxA (15–17). As shown by the 1000-fold reduction in kcat of the H239A mutant (Table 4), H239 of EcLpxD is now directly implicated as the catalytic base. However, the corresponding H247 residue in the CtLpxD structure 14) is not properly positioned either in Complex I or in Complex II (Table 3 and Fig. 7) to hydrogen-bond the N-2 atom of the GlcN moiety of UDP-GlcNAc, nor is it likely to stabilize the oxyanion intermediate (see below), as suggested by Buetow et al. (14).

Panel A. EcLpxA H125 is positioned to hydrogen bond the O-3 atom of the GlcNAc moiety, whereas the backbone N atom of G143 (one turn of the β-helix distal to H125) is positioned to stabilize the putative oxyanion intermediate, given its proximity to the ester carbonyl in the bound UDP-3-O-(R-3-hydroxymyristoyl)-GlcNAc product seen in the crystal structure of the EcLpxA/UDP-3-O-(R-3-hydroxymyristoyl)-GlcNAc complex (17). Dashes indicate the inter-atomic distances in Å. Panel B. The conserved H247 residue of CtLpxD, which aligns with H125 LpxA in sequence comparisons, is proposed to hydrogen bond the N-2 GlcN atom of the physiological LpxD substrate (see Scheme 2). Shown here is the positioning of the conserved CtLpxD H247 side chain relative to the N-atom of the bound non-physiological ligand, UDP-GlcNAc, as seen in Complex I (14). The Nε2 atom of H247 is too far removed to engage in a hydrogen bond with N-2 atom of the GlcNAc moiety (4.5 Å). However, given the uncertainties in the GlcNAc conformation (Fig. 7), the possible differences in the binding of the physiological LpxD acceptor substrate versus UDP-GlcNAc, and the profound loss of activity caused by the H239A mutation in EcLpxD (Table 4), H247 is nevertheless proposed to be the general base. CtLpxD also contains the conserved G265 residue (one turn of the β-helix distal to H247), the backbone NH moiety of which could function as the oxyanion hole, exactly like that of G143 in EcLpxA. The color scheme is the same as in Fig. 1C.
Our data reveal only a 30-fold reduction in the kcat of the EcLpxD H276A mutant (Table 4), which argues against the direct participation of this residue (or of CtLpxD H284) in any aspect of catalysis, such as the proposed stabilization of the oxyanion intermediate (14). The alternative that CtLpxD H284 is important for substrate binding is, however, compatible with its positioning relative to the diphosphate group of UDP-GlcNAc, which is similar in both Complex I and II (Fig. 7) (14). The reasons for the dramatic differences in the orientations of the GlcNAc moieties in the two complexes (Fig. 7) are not known (14).
As noted, Buetow et al. suggested that both H247 and H284 of CtLpxD could stabilize the oxyanion intermediate formed during LpxD catalysis [Fig. 1 in (14)]. This possibility is consistent with the structure of Complex II for H247 or with the structure of Complex I for H284 (Table 3), but not with both structures for both histidines, given the differences in the positioning of the GlcNAc residue in the two complexes (Table 3 and Fig. 7) (14). Furthermore, because most oxyanion “holes” in serine proteases (44), protein N-myristoyl transferases (45) and lipases (46) feature backbone NH groups, we favor the alternative mechanistic scenario, shown in Scheme 2 and Fig. 8. As noted above, we propose that EcH239 (CtH247) is the catalytic base, whereas EcH276 (CtH284) is involved in substrate binding (Table 4). We suggest that the backbone NH group of the absolutely conserved EcLpxD G257 residue (G265 in CtLpxD), located one turn of the β-helix distal to EcLpxD H239 (H247 in CtLpxD), stabilizes the oxyanion intermediate (Scheme 2 and Fig. 8B). The equivalent residue (G143 in E. coli LpxA) is likewise present in all LpxA sequences, and its function as the oxyanion hole can be inferred directly from structural studies of the LpxA/3-O-(R-3-hydroxymyristoyl)-GlcNAc product complex (Fig. 8A) in which the G143 backbone NH group of LpxA is appropriately positioned (17).
Previous studies have suggested that the catalytic histidine residues of the acyltransferases of the LβH family may be hydrogen bonded to (and/or be oriented by) an adjacent negatively charged atom (13). The side chain of D126 serves this purpose for H125 in E. coli LpxA (15, 17), whereas a backbone threonine carbonyl group plays this role in the P. aeuroginosa xenobiotic acetyltransferase (12) and an aspartate side chain is utilized in E. coli serine acetyl transferase (47). Given the conservation of D126 in most LpxA sequences, the homologous EcLpxD N240 residue seemed to be a possible candidate for interaction with EcLpxD H239. However, the N240A mutation had no effect on EcLpxD activity (data not shown). Furthermore, Q248 (the residue corresponding to N240 in CtLpxD) is too far removed (>4.5 Å) to interact with H247 in CtLpxD (14), and based on the structure, the Q248 side chain is instead hydrogen bonded the ribose 3-OH group of UDP-GlcNAc (14) (Table 3).
From the C. trachomatis LpxD structure solved with bound UDP-GlcNAc (14), several additional residues were deemed to be important in substrate binding. Unlike the GlcNAc moieties, the UDP-groups of the bound UDP-GlcNAc ligands were quite similar in both Complex I and II (Fig. 7). F43 and Y49 (F41 and Y47 in EcLpxD) form π-stacking interactions with uracil moiety as part of a novel uridine-binding domain (14). Our mutagenesis data support the idea that F41 (F43 in CtLpxD) indeed plays an important role in nucleotide binding (Table 4). Two other side chains in CtLpxD, E34 and N46, may be involved in hydrogen bonding to the 2-OH group of the ribose ring and the PO β1 atom, respectively, albeit only in Complex II (Table 3). The corresponding E. coli mutants (Q32A and N44A) show less than a 2-fold effect on activity, suggesting that these residues contribute little to the binding of the nucleotide substrate. Alternatively, E. coli and C. trachomatis LpxD may engage their ligands differently, or the physiological LpxD substrates may interact with CtLpxD in a different manner than do UDP-GlcNAc and free fatty acids. In future structural studies of LpxD, it will be desirable to use physiological substrates as bound ligands (Fig. 1A) in order to derive stronger mechanistic conclusions.
It is unclear whether the free fatty acids observed in the CtLpxD crystal structure (Fig. 1C) occupy the acyl chain binding site of the acyl-ACP donor substrate or of the UDP-3-O-(acyl)-glucosamine acceptor. In this regard, it is worth noting that EcLpxD and CtLpxD must contain hydrocarbon rulers to ensure the incorporation of the proper N-linked hydroxyacyl chain. In the case of EcLpxD, this ruler is highly selective for 14 carbons (9), whereas CtLpxD presumably prefers 20 carbons (14). However, in E. coli LpxD there is much less acyl chain selectivity with regard to the acceptor substrate. If the E. coli lpxA gene is inactivated by mutation and replaced with Pseudomonas lpxA, which encodes a C10-specific acyltransferase (48, 49), the cells are viable, but a hybrid lipid A structure is synthesized in which the 3-O-linked hydroxyacyl chains are mostly hydroxydecanoate. However, the N-linked hydroxyacyl chains are still predominantly hydroxymyristate, as in wild-type cells (48). This result demonstrates that LpxD efficiently utilizes an acceptor substrate containing a C10 hydroxyacyl chain in living cells. As noted by Kelly et al. (9), however, UDP-GlcN (lacking an acyl chain) is a very poor substrate for LpxD, indicating the presence on LpxD of an important hydrophobic pocket for the 3-O-linked acyl chain on the acceptor substrate. This pocket appears to be less selective than acyl chain binding pocket of the acyl-ACP donor substrate.
ACP is small acidic protein, present in a dynamic equilibrium of two or more conformers (50). This equilibrium is governed in part by the concentration of divalent cations, which bind to acidic residues on ACP (37, 38). Divalent calcium and magnesium cations have high affinities for these sites, with an average Kd of about 80 μM (38). These divalent cation-binding sites lie along helix II of ACP. Various binding, mutagenesis, and docking studies have implicated helix II of ACP to be the “recognition helix” that facilitates interaction with various enzymes (33–36). The recognition helix contains two acidic sub-sites: Site A, composed of residues E30, D35, D38 and E41, and Site B, composed of E47, D51, E53, and D56 (51).
Three observations suggest that the divalent cation-binding sites on ACP are important for interaction with LpxD. First, divalent cations inhibit LpxD activity (Fig. 6A). Furthermore, divalent cation inhibition is overcome by increasing the concentration of R-3-OHC14-ACP (Fig. 6B). Lastly, removal of the protein portion of ACP (for instance when R-3-hydroxylauroyl-methylphosphopantetheine is used as the acyl donor) reduces activity over 100-fold but eliminates divalent cation inhibition (Table 2).
Although we suspect that the acidic sites on ACP interact with LpxD, we still do not understand how LpxD recognizes ACP. The CtLpxD structure revealed a conserved basic residue at K48 (K46 in EcLpxD) that might be part of a basic cleft for ACP binding (14). However, the corresponding K46A mutant of EcLpxD displays only a 3-fold increase on the KM, R-3-OHC14-ACP with no effect on kcat. This finding suggests that K46/K48 (EcLpxD/CtLpxD) plays at best a minor role in the interaction of LpxD with ACP. In contrast, we have found that R293 (K301 in CtLpxD), a conserved basic residue that was not considered in the structural analysis (14), plays a significant role in the binding of R-3-OHC14-ACP (Table 4 and Scheme 2). Given the distance of K301 from the active site of CtLpxD (approximately 18 Å), it may be that R293/K301 (EcLpxD/CtLpxD) interacts with the Site A residues on ACP. Structural studies of LpxD/acyl-ACP complexes will be required to address these issues.
Supplementary Material
2
Supporting information available
Methods for the preparation of substrates and reagents, including R-3-OHC14-ACP, R/S-3-hydroxylauroyl-ACP, [α-32P]-UDP-GlcNAc, and [α-32P]-UDP-3-O-(R-3-OHC14)-GlcN, are described. The purification of untagged EcLpxD and His6-EcLpxD, including criteria used to assess purity, are presented. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We would like to thank Dr. Dale Christensen for construction of pDC015-1 and Dr. Ziquiang Guan for help with the mass spectrometry of LpxD. This work was supported by NIH Grant GM-51310 to C. R. H. Raetz.
This research was supported by NIH Grant GM-51310 to C. R. H. Raetz.
Footnotes
1The abbreviations are: ACP, acyl carrier protein; Bis-Tris, 2,2-bis(hydroxymethyl)-2,2′,2″-nitrilotriethanol; CMC, critical micelle concentration; EcLpxD, Escherichia coli LpxD; CtLpxD, Chlamydia trachomatis LpxD; Hepes, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; LβH, left-handed parallel β helix; LPS, lipopolysaccharide; R-3-OHC14-ACP, R-3-hydroxymyristoyl-acyl carrier protein; SDS-PAGE, sodium dodecylsulfate polyacrylamide gel electrophoresis; UDP-3-O-(R-3-OHC14)-GlcN, UDP-3-O-(R-3-hydroxymyristoyl)-α-D-glucosamine; UDP-2-N-(R-3-OHC14)-GlcN, UDP-2-N-(R-3-hydroxymyristoyl)-α-D-glucosamine.
2Leatherbarrow, R. J. (2001) GraFit Version 5, Erithacus Software, Ltd., Horley, U.K.
References
 -D-glucosamine N-acyltransferase: the third step of endotoxin biosynthesis. J Biol Chem. 1993;268:19866–19874. [Abstract] [Google Scholar]
-D-glucosamine N-acyltransferase: the third step of endotoxin biosynthesis. J Biol Chem. 1993;268:19866–19874. [Abstract] [Google Scholar]Full text links
Read article at publisher's site: https://doi.org/10.1021/bi800240r
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2435086?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1021/bi800240r
Article citations
Fatty acids of Helicobacter pylori lipoproteins CagT and Lpp20.
Microbiol Spectr, 12(5):e0047024, 19 Mar 2024
Cited by: 0 articles | PMID: 38501821 | PMCID: PMC11064636
Suppressors of lapC Mutation Identify New Regulators of LpxC, Which Mediates the First Committed Step in Lipopolysaccharide Biosynthesis.
Int J Mol Sci, 24(20):15174, 14 Oct 2023
Cited by: 0 articles | PMID: 37894855 | PMCID: PMC10607373
Making a chink in their armor: Current and next-generation antimicrobial strategies against the bacterial cell envelope.
Adv Microb Physiol, 83:221-307, 27 Jun 2023
Cited by: 0 articles | PMID: 37507160 | PMCID: PMC10517717
The Effect of Mutation in Lipopolysaccharide Biosynthesis on Bacterial Fitness.
Cells, 11(20):3249, 16 Oct 2022
Cited by: 0 articles | PMID: 36291117 | PMCID: PMC9600226
Checkpoints That Regulate Balanced Biosynthesis of Lipopolysaccharide and Its Essentiality in Escherichia coli.
Int J Mol Sci, 23(1):189, 24 Dec 2021
Cited by: 8 articles | PMID: 35008618 | PMCID: PMC8745692
Review Free full text in Europe PMC
Go to all (42) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Protein structures in PDBe (2)
-
(2 citations)
PDBe - 2iu9View structure
-
(2 citations)
PDBe - 2iu8View structure
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Crystal structure and acyl chain selectivity of Escherichia coli LpxD, the N-acyltransferase of lipid A biosynthesis.
Biochemistry, 48(36):8672-8683, 01 Sep 2009
Cited by: 41 articles | PMID: 19655786 | PMCID: PMC2748855
Structural basis for the sugar nucleotide and acyl-chain selectivity of Leptospira interrogans LpxA.
Biochemistry, 48(26):6191-6201, 01 Jul 2009
Cited by: 19 articles | PMID: 19456129 | PMCID: PMC2710806
Biosynthesis of lipid A in Escherichia coli: identification of UDP-3-O-[(R)-3-hydroxymyristoyl]-alpha-D-glucosamine as a precursor of UDP-N2,O3-bis[(R)-3-hydroxymyristoyl]-alpha-D-glucosamine.
Biochemistry, 27(6):1908-1917, 01 Mar 1988
Cited by: 44 articles | PMID: 3288280
Acyltransferases in bacteria.
Microbiol Mol Biol Rev, 77(2):277-321, 01 Jun 2013
Cited by: 76 articles | PMID: 23699259 | PMCID: PMC3668668
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIGMS NIH HHS (3)
Grant ID: R01 GM051310-14
Grant ID: GM-51310
Grant ID: R01 GM051310






