Abstract
Free full text

Inhibition of rhinovirus attachment by neutralizing monoclonal antibodies and their Fab fragments.
Abstract
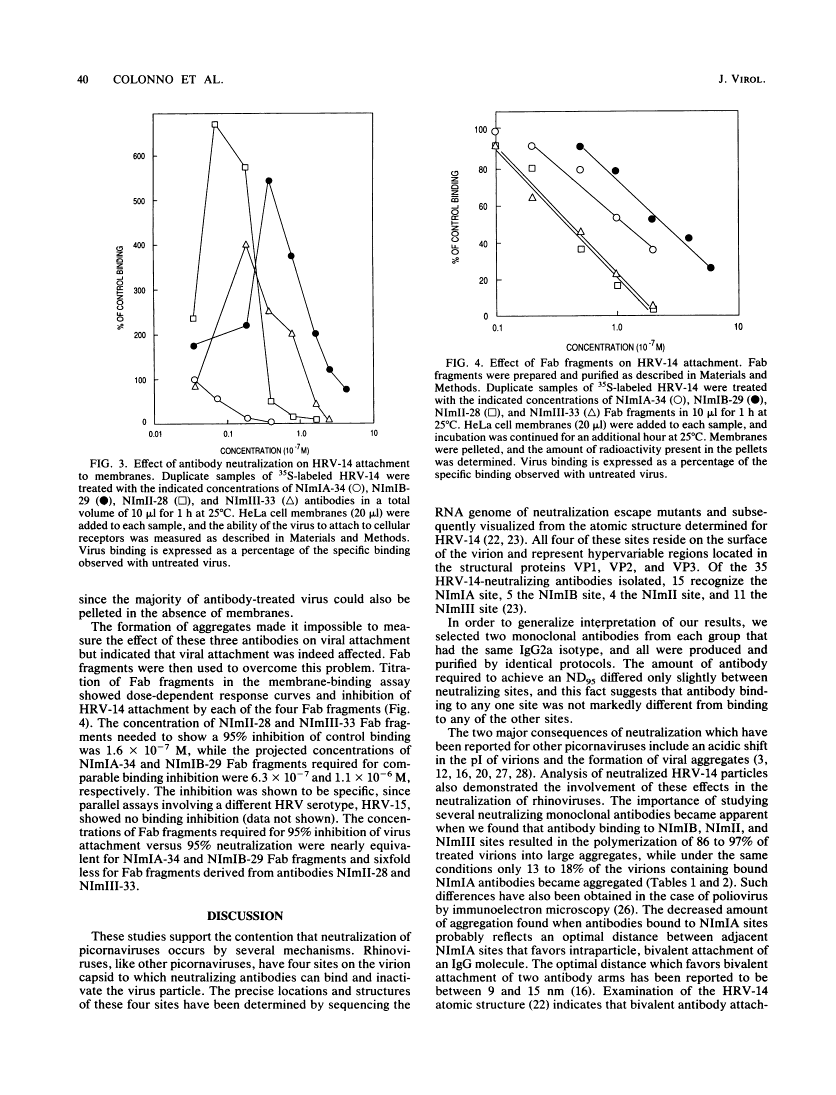
Previous molecular and immunological studies have mapped four neutralization sites on human rhinovirus type 14 (B. Sherry, A. G. Mosser, R. J. Colonno, and R. R. Rueckert, J. Virol. 57:246-257, 1986). Eight monoclonal antibodies, one pair for each of the four target sites and all belonging to a single isotype, immunoglobulin G2a, were studied under conditions which resulted in 95% neutralization of infectious viral particles. All eight antibodies shifted the isoelectric point of virions from 6.7 to much more acidic forms, ranging from pI 1.8 to 3.2. In addition, antibodies targeted against three of the four neutralization sites caused significant aggregation of virions under the neutralization conditions employed. Aggregation could be reversed by digesting virus-antibody complexes with papain. Following papain digestion, the acidic pIs of three of the neutralized virus preparations returned to neutral and infectivity was restored. Membrane-binding assays with virus neutralized with a nonaggregating antibody showed a dose-related inhibition of virus attachment to cellular receptors. Purified Fab fragments at a 13- to 61-fold-higher concentration than intact antibodies caused a comparable isoelectric shift, neutralized virions in the absence of aggregation, and interfered with attachment of virions to host cell receptors in a membrane-binding assay. These findings suggest that neutralizing antibodies interfere with the attachment of rhinoviruses to cellular receptors and that bivalent attachment of antibody is not a prerequisite for neutralization.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.5M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
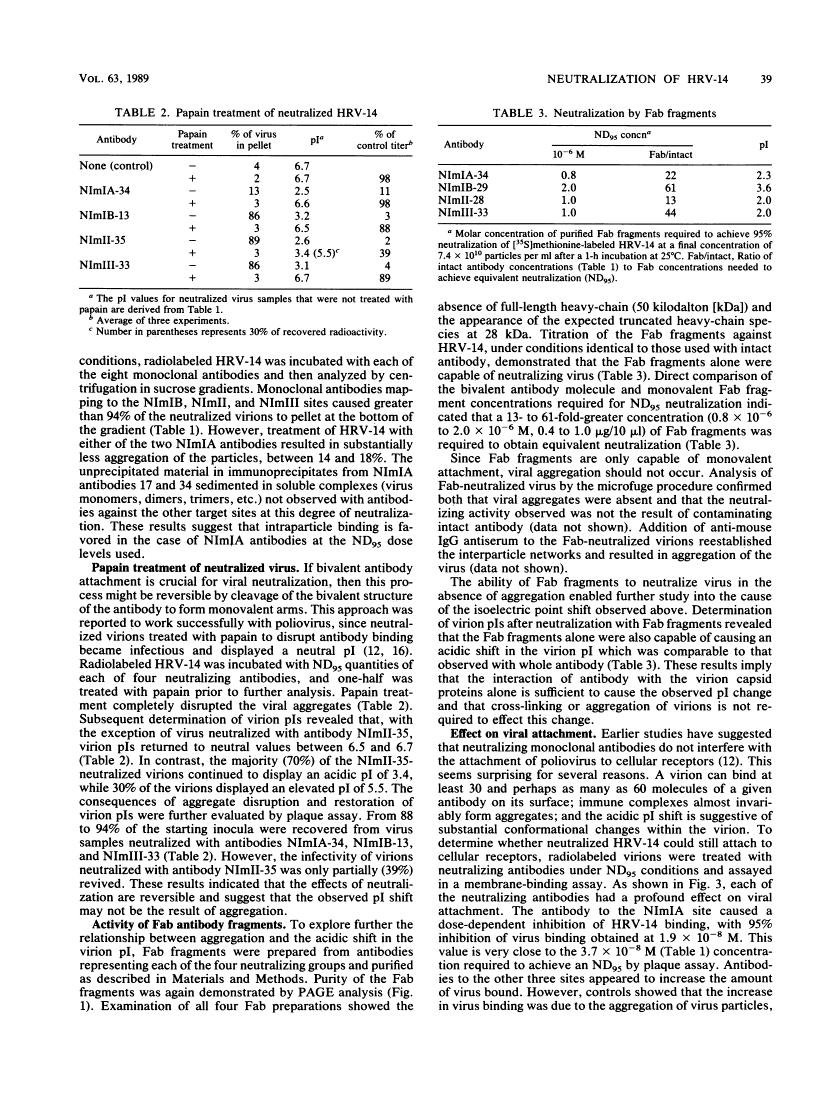
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G, Colonno RJ. Many rhinovirus serotypes share the same cellular receptor. J Virol. 1984 Aug;51(2):340–345. [Europe PMC free article] [Abstract] [Google Scholar]
- Badger J, Minor I, Kremer MJ, Oliveira MA, Smith TJ, Griffith JP, Guerin DM, Krishnaswamy S, Luo M, Rossmann MG, et al. Structural analysis of a series of antiviral agents complexed with human rhinovirus 14. Proc Natl Acad Sci U S A. 1988 May;85(10):3304–3308. [Europe PMC free article] [Abstract] [Google Scholar]
- Brioen P, Dekegel D, Boeyé A. Neutralization of poliovirus by antibody-mediated polymerization. Virology. 1983 Jun;127(2):463–468. [Abstract] [Google Scholar]
- Brioen P, Rombaut B, Boeyé A. Hit-and-run neutralization of poliovirus. J Gen Virol. 1985 Nov;66(Pt 11):2495–2499. [Abstract] [Google Scholar]
- Carthew P. The surface nature of proteins of a bovine enterovirus, before and after neutralization. J Gen Virol. 1976 Jul;32(1):17–23. [Abstract] [Google Scholar]
- Colonno RJ. Cell surface receptors for picornaviruses. Bioessays. 1986 Dec;5(6):270–274. [Abstract] [Google Scholar]
- Colonno RJ, Callahan PL, Long WJ. Isolation of a monoclonal antibody that blocks attachment of the major group of human rhinoviruses. J Virol. 1986 Jan;57(1):7–12. [Europe PMC free article] [Abstract] [Google Scholar]
- Colonno RJ, Condra JH, Mizutani S, Callahan PL, Davies ME, Murcko MA. Evidence for the direct involvement of the rhinovirus canyon in receptor binding. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5449–5453. [Europe PMC free article] [Abstract] [Google Scholar]
- Dimmock NJ. Mechanisms of neutralization of animal viruses. J Gen Virol. 1984 Jun;65(Pt 6):1015–1022. [Abstract] [Google Scholar]
- Emini EA, Kao SY, Lewis AJ, Crainic R, Wimmer E. Functional basis of poliovirus neutralization determined with monospecific neutralizing antibodies. J Virol. 1983 May;46(2):466–474. [Europe PMC free article] [Abstract] [Google Scholar]
- Emini EA, Ostapchuk P, Wimmer E. Bivalent attachment of antibody onto poliovirus leads to conformational alteration and neutralization. J Virol. 1983 Nov;48(2):547–550. [Europe PMC free article] [Abstract] [Google Scholar]
- GREENWOOD FC, HUNTER WM, GLOVER JS. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. [Europe PMC free article] [Abstract] [Google Scholar]
- Hamparian VV, Colonno RJ, Cooney MK, Dick EC, Gwaltney JM, Jr, Hughes JH, Jordan WS, Jr, Kapikian AZ, Mogabgab WJ, Monto A, et al. A collaborative report: rhinoviruses--extension of the numbering system from 89 to 100. Virology. 1987 Jul;159(1):191–192. [Abstract] [Google Scholar]
- Icenogle J, Shiwen H, Duke G, Gilbert S, Rueckert R, Anderegg J. Neutralization of poliovirus by a monoclonal antibody: kinetics and stoichiometry. Virology. 1983 Jun;127(2):412–425. [Abstract] [Google Scholar]
- Keller R. The stability of neutralization of poliovirus by native antibody and enzymatically derived fragments. J Immunol. 1966 Jan;96(1):96–106. [Abstract] [Google Scholar]
- Keller R. Studies on the mechanism of the enzymatic reactivation of antibody-neutralized poliovirus. J Immunol. 1968 May;100(5):1071–1099. [Abstract] [Google Scholar]
- Kirschenbaum DM. Molar absorptivity and A1 percent 1cm values for proteins at selected wavelengths of the ultraviolet and visible regions. IX. Anal Biochem. 1973 Nov;56(1):237–263. [Abstract] [Google Scholar]
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [Abstract] [Google Scholar]
- Mandel B. Neutralization of poliovirus: a hypothesis to explain the mechanism and the one-hit character of the neutralization reaction. Virology. 1976 Feb;69(2):500–510. [Abstract] [Google Scholar]
- Rossmann MG, Arnold E, Erickson JW, Frankenberger EA, Griffith JP, Hecht HJ, Johnson JE, Kamer G, Luo M, Mosser AG, et al. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. [Abstract] [Google Scholar]
- Sherry B, Mosser AG, Colonno RJ, Rueckert RR. Use of monoclonal antibodies to identify four neutralization immunogens on a common cold picornavirus, human rhinovirus 14. J Virol. 1986 Jan;57(1):246–257. [Europe PMC free article] [Abstract] [Google Scholar]
- Sherry B, Rueckert R. Evidence for at least two dominant neutralization antigens on human rhinovirus 14. J Virol. 1985 Jan;53(1):137–143. [Europe PMC free article] [Abstract] [Google Scholar]
- Stanway G, Hughes PJ, Mountford RC, Minor PD, Almond JW. The complete nucleotide sequence of a common cold virus: human rhinovirus 14. Nucleic Acids Res. 1984 Oct 25;12(20):7859–7875. [Europe PMC free article] [Abstract] [Google Scholar]
- Taniguchi K, Urasawa S. Different virus-precipitating activities of neutralizing monoclonal antibodies that recognize distinct sites of poliovirus particles. Arch Virol. 1987;92(1-2):27–40. [Abstract] [Google Scholar]
- Thomas AA, Brioen P, Boeyé A. A monoclonal antibody that neutralizes poliovirus by cross-linking virions. J Virol. 1985 Apr;54(1):7–13. [Europe PMC free article] [Abstract] [Google Scholar]
- Thomas AA, Vrijsen R, Boeyé A. Relationship between poliovirus neutralization and aggregation. J Virol. 1986 Aug;59(2):479–485. [Europe PMC free article] [Abstract] [Google Scholar]
- Tomassini JE, Colonno RJ. Isolation of a receptor protein involved in attachment of human rhinoviruses. J Virol. 1986 May;58(2):290–295. [Europe PMC free article] [Abstract] [Google Scholar]
- Wetz K, Willingmann P, Zeichhardt H, Habermehl KO. Neutralization of poliovirus by polyclonal antibodies requires binding of a single IgG molecule per virion. Arch Virol. 1986;91(3-4):207–220. [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jvi.63.1.36-42.1989
Read article for free, from open access legal sources, via Unpaywall:
https://jvi.asm.org/content/jvi/63/1/36.full.pdf
Free after 4 months at jvi.asm.org
http://jvi.asm.org/cgi/reprint/63/1/36
Free to read at jvi.asm.org
http://jvi.asm.org/cgi/content/abstract/63/1/36
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/jvi.63.1.36-42.1989
Article citations
Mechanisms of Rhinovirus Neutralisation by Antibodies.
Viruses, 13(3):360, 25 Feb 2021
Cited by: 8 articles | PMID: 33668934 | PMCID: PMC7996599
Review Free full text in Europe PMC
The Dynamic Life of Virus Capsids.
Viruses, 12(6):E618, 05 Jun 2020
Cited by: 16 articles | PMID: 32516952 | PMCID: PMC7354500
Review Free full text in Europe PMC
Deconstructing the Antiviral Neutralizing-Antibody Response: Implications for Vaccine Development and Immunity.
Microbiol Mol Biol Rev, 80(4):989-1010, 26 Oct 2016
Cited by: 68 articles | PMID: 27784796 | PMCID: PMC5116878
Review Free full text in Europe PMC
Short Communication: Virion Aggregation by Neutralizing and Nonneutralizing Antibodies to the HIV-1 Envelope Glycoprotein.
AIDS Res Hum Retroviruses, 31(11):1160-1165, 20 Jul 2015
Cited by: 9 articles | PMID: 26086186 | PMCID: PMC4651053
Aggregate complexes of HIV-1 induced by multimeric antibodies.
Retrovirology, 11:78, 02 Oct 2014
Cited by: 22 articles | PMID: 25274446 | PMCID: PMC4193994
Go to all (31) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Effects of specific monoclonal antibodies on La Crosse virus neutralization: aggregation, inactivation by Fab fragments, and inhibition of attachment to baby hamster kidney cells.
Virology, 180(2):591-601, 01 Feb 1991
Cited by: 4 articles | PMID: 1703370
Efficient neutralization of foot-and-mouth disease virus by monovalent antibody binding.
J Virol, 71(12):9813-9816, 01 Dec 1997
Cited by: 16 articles | PMID: 9371652 | PMCID: PMC230296
Structural Insights into Reovirus σ1 Interactions with Two Neutralizing Antibodies.
J Virol, 91(4):e01621-16, 31 Jan 2017
Cited by: 17 articles | PMID: 27928010 | PMCID: PMC5286903
Neutralization of animal virus infectivity by antibody.
Arch Virol, 152(6):1047-1059, 15 Feb 2007
Cited by: 71 articles | PMID: 17516034
Review