Abstract
Free full text

Recombination between Sindbis virus RNAs.
Abstract
The genome (49S RNA) of Sindbis virus is a positive-strand RNA of 11.7 kb that consists of two domains. The 5' two-thirds of the RNA codes for the proteins required for replication and transcription of the RNA. The 3' one-third codes for the structural proteins. The latter are translated from a 26S subgenomic RNA identical in sequence to the 3' one-third of the genome. The 26S RNA is transcribed by initiation from an internal promoter that spans the junction between the nonstructural and structural genes. We have used Sindbis virus RNAs transcribed from cloned cDNAs to demonstrate recombination between Sindbis virus RNAs in cultured cells. Several different combinations of deleted or mutationally altered RNAs gave rise to infectious recombinants. In 7 of 10 different crosses, the infectious recombinant RNAs were larger than wild-type 49S RNA. We sequenced the recombinant RNAs in the region spanning the junction between the nonstructural and structural protein genes from five different crosses. In three of the crosses, this is the only region within which recombination could have taken place to produce an infectious 49S RNA. Recombination also occurred in this region in the other two crosses. The recombinant RNAs were distinct from wild-type RNA and from each other. All contained sequence insertions derived from the parental RNAs. One contained a deletion and a rearrangement, and one also contained a stretch of 11 nucleotides not found in the Sindbis virus genome. When each of the parental RNAs contained a functional subgenomic RNA promoter, both promoters were present and functional in the recombinant RNA. Those recombinants with large sequence insertions showed evidence of evolution toward the wild-type single-junction RNA.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.8M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
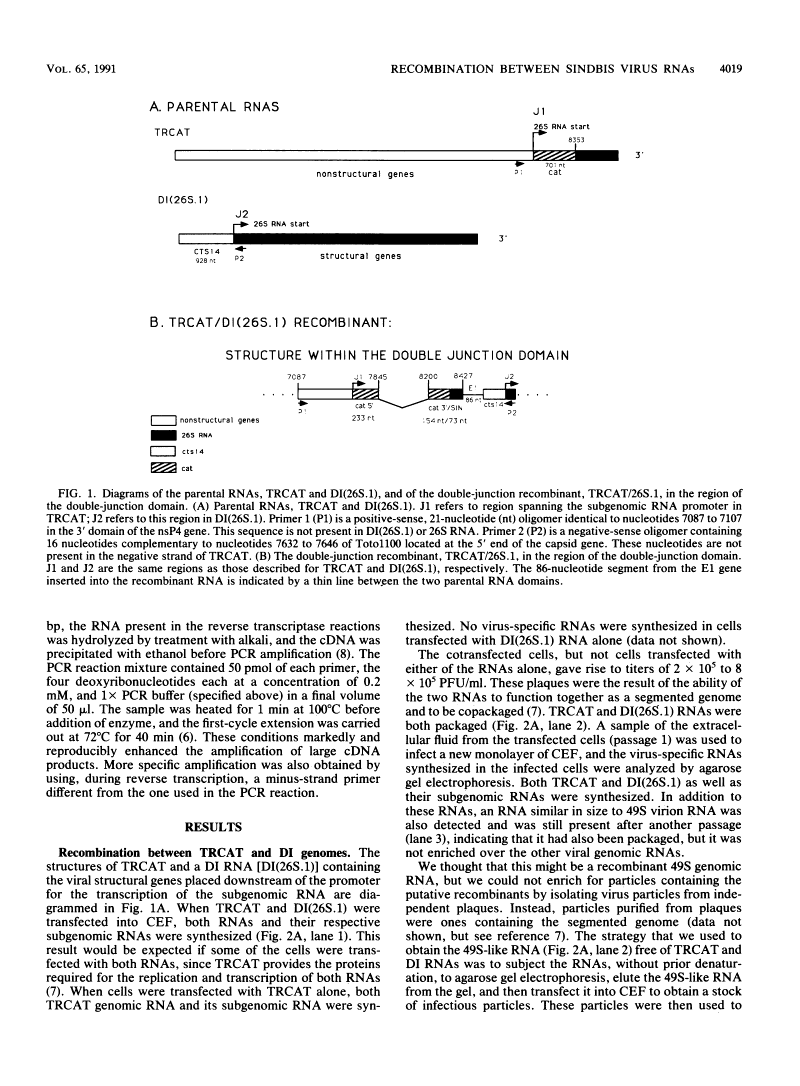
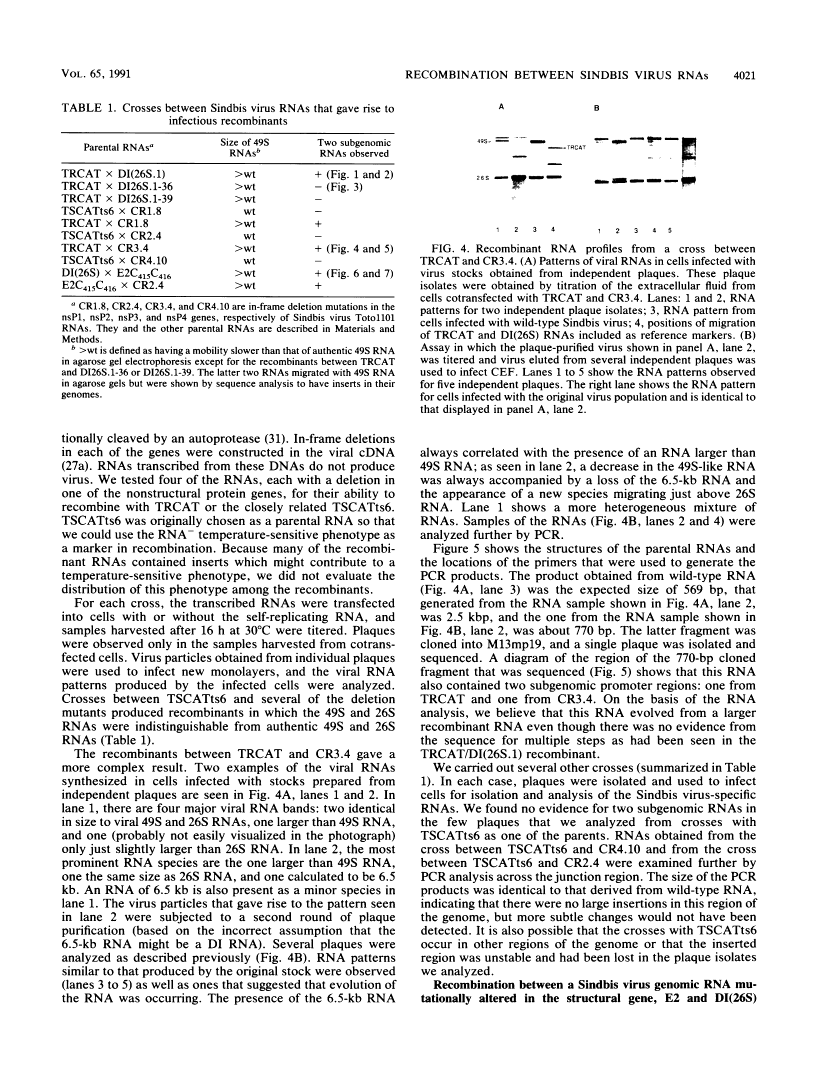
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P, Strauss EG, Rice CM, Strauss JH, Haseloff J, Zimmern D. Sindbis virus proteins nsP1 and nsP2 contain homology to nonstructural proteins from several RNA plant viruses. J Virol. 1985 Feb;53(2):536–542. [Europe PMC free article] [Abstract] [Google Scholar]
- Allison R, Thompson C, Ahlquist P. Regeneration of a functional RNA virus genome by recombination between deletion mutants and requirement for cowpea chlorotic mottle virus 3a and coat genes for systemic infection. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1820–1824. [Europe PMC free article] [Abstract] [Google Scholar]
- Baric RS, Fu K, Schaad MC, Stohlman SA. Establishing a genetic recombination map for murine coronavirus strain A59 complementation groups. Virology. 1990 Aug;177(2):646–656. [Europe PMC free article] [Abstract] [Google Scholar]
- Bujarski JJ, Kaesberg P. Genetic recombination between RNA components of a multipartite plant virus. Nature. 321(6069):528–531. [Europe PMC free article] [Abstract] [Google Scholar]
- Burge BW, Pfefferkorn ER. Complementation between temperature-sensitive mutants of Sindbis virus. Virology. 1966 Oct;30(2):214–223. [Abstract] [Google Scholar]
- Frohman MA, Dush MK, Martin GR. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. [Europe PMC free article] [Abstract] [Google Scholar]
- Geigenmüller-Gnirke U, Weiss B, Wright R, Schlesinger S. Complementation between Sindbis viral RNAs produces infectious particles with a bipartite genome. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3253–3257. [Europe PMC free article] [Abstract] [Google Scholar]
- Grakoui A, Levis R, Raju R, Huang HV, Rice CM. A cis-acting mutation in the Sindbis virus junction region which affects subgenomic RNA synthesis. J Virol. 1989 Dec;63(12):5216–5227. [Europe PMC free article] [Abstract] [Google Scholar]
- Hahn CS, Lustig S, Strauss EG, Strauss JH. Western equine encephalitis virus is a recombinant virus. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5997–6001. [Europe PMC free article] [Abstract] [Google Scholar]
- HIRST GK. Genetic recombination with Newcastle disease virus, polioviruses, and influenza. Cold Spring Harb Symp Quant Biol. 1962;27:303–309. [Abstract] [Google Scholar]
- Keck JG, Stohlman SA, Soe LH, Makino S, Lai MM. Multiple recombination sites at the 5'-end of murine coronavirus RNA. Virology. 1987 Feb;156(2):331–341. [Europe PMC free article] [Abstract] [Google Scholar]
- Khatchikian D, Orlich M, Rott R. Increased viral pathogenicity after insertion of a 28S ribosomal RNA sequence into the haemagglutinin gene of an influenza virus. Nature. 1989 Jul 13;340(6229):156–157. [Abstract] [Google Scholar]
- King AM, McCahon D, Slade WR, Newman JW. Recombination in RNA. Cell. 1982 Jul;29(3):921–928. [Europe PMC free article] [Abstract] [Google Scholar]
- Kirkegaard K, Baltimore D. The mechanism of RNA recombination in poliovirus. Cell. 1986 Nov 7;47(3):433–443. [Europe PMC free article] [Abstract] [Google Scholar]
- Lai MM, Baric RS, Makino S, Keck JG, Egbert J, Leibowitz JL, Stohlman SA. Recombination between nonsegmented RNA genomes of murine coronaviruses. J Virol. 1985 Nov;56(2):449–456. [Europe PMC free article] [Abstract] [Google Scholar]
- Lehtovaara P, Söderlund H, Keränen S, Pettersson RF, Käriäinen L. Extreme ends of the genome are conserved and rearranged in the defective interfering RNAs of Semliki Forest virus. J Mol Biol. 1982 Apr 25;156(4):731–748. [Abstract] [Google Scholar]
- Levis R, Schlesinger S, Huang HV. Promoter for Sindbis virus RNA-dependent subgenomic RNA transcription. J Virol. 1990 Apr;64(4):1726–1733. [Europe PMC free article] [Abstract] [Google Scholar]
- Levis R, Weiss BG, Tsiang M, Huang H, Schlesinger S. Deletion mapping of Sindbis virus DI RNAs derived from cDNAs defines the sequences essential for replication and packaging. Cell. 1986 Jan 17;44(1):137–145. [Abstract] [Google Scholar]
- Makino S, Keck JG, Stohlman SA, Lai MM. High-frequency RNA recombination of murine coronaviruses. J Virol. 1986 Mar;57(3):729–737. [Europe PMC free article] [Abstract] [Google Scholar]
- Meyers G, Tautz N, Dubovi EJ, Thiel HJ. Viral cytopathogenicity correlated with integration of ubiquitin-coding sequences. Virology. 1991 Feb;180(2):602–616. [Europe PMC free article] [Abstract] [Google Scholar]
- Monroe SS, Schlesinger S. RNAs from two independently isolated defective interfering particles of Sindbis virus contain a cellular tRNA sequence at their 5' ends. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3279–3283. [Europe PMC free article] [Abstract] [Google Scholar]
- Monroe SS, Schlesinger S. Common and distinct regions of defective-interfering RNAs of Sindbis virus. J Virol. 1984 Mar;49(3):865–872. [Europe PMC free article] [Abstract] [Google Scholar]
- Ou JH, Rice CM, Dalgarno L, Strauss EG, Strauss JH. Sequence studies of several alphavirus genomic RNAs in the region containing the start of the subgenomic RNA. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5235–5239. [Europe PMC free article] [Abstract] [Google Scholar]
- Raju R, Huang HV. Analysis of Sindbis virus promoter recognition in vivo, using novel vectors with two subgenomic mRNA promoters. J Virol. 1991 May;65(5):2501–2510. [Europe PMC free article] [Abstract] [Google Scholar]
- Rice CM, Levis R, Strauss JH, Huang HV. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987 Dec;61(12):3809–3819. [Europe PMC free article] [Abstract] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. [Europe PMC free article] [Abstract] [Google Scholar]
- Strauss EG, Rice CM, Strauss JH. Complete nucleotide sequence of the genomic RNA of Sindbis virus. Virology. 1984 Feb;133(1):92–110. [Abstract] [Google Scholar]
- Strauss JH, Strauss EG. Evolution of RNA viruses. Annu Rev Microbiol. 1988;42:657–683. [Abstract] [Google Scholar]
- Tsiang M, Monroe SS, Schlesinger S. Studies of defective interfering RNAs of Sindbis virus with and without tRNAAsp sequences at their 5' termini. J Virol. 1985 Apr;54(1):38–44. [Europe PMC free article] [Abstract] [Google Scholar]
- Weiss B, Nitschko H, Ghattas I, Wright R, Schlesinger S. Evidence for specificity in the encapsidation of Sindbis virus RNAs. J Virol. 1989 Dec;63(12):5310–5318. [Europe PMC free article] [Abstract] [Google Scholar]
- Xiong C, Levis R, Shen P, Schlesinger S, Rice CM, Huang HV. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science. 1989 Mar 3;243(4895):1188–1191. [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jvi.65.8.4017-4025.1991
Read article for free, from open access legal sources, via Unpaywall:
https://jvi.asm.org/content/jvi/65/8/4017.full.pdf
Free to read at jvi.asm.org
http://jvi.asm.org/cgi/content/abstract/65/8/4017
Free after 4 months at jvi.asm.org
http://jvi.asm.org/cgi/reprint/65/8/4017
Citations & impact
Impact metrics
Article citations
Characterization of Variant RNAs Encapsidated during Bromovirus Infection by High-Throughput Sequencing.
Pathogens, 13(1):96, 22 Jan 2024
Cited by: 0 articles | PMID: 38276169 | PMCID: PMC10819421
Alphavirus-based replicons demonstrate different interactions with host cells and can be optimized to increase protein expression.
J Virol, 97(11):e0122523, 25 Oct 2023
Cited by: 1 article | PMID: 37877718 | PMCID: PMC10688356
Rise of the RNA machines - self-amplification in mRNA vaccine design.
Trends Biotechnol, 41(11):1417-1429, 14 Jun 2023
Cited by: 16 articles | PMID: 37328401 | PMCID: PMC10266560
Review Free full text in Europe PMC
RNA recombination at Chikungunya virus 3'UTR as an evolutionary mechanism that provides adaptability.
PLoS Pathog, 15(4):e1007706, 15 Apr 2019
Cited by: 27 articles | PMID: 30986247 | PMCID: PMC6502353
Mechanisms and consequences of positive-strand RNA virus recombination.
J Gen Virol, 99(10):1345-1356, 29 Aug 2018
Cited by: 49 articles | PMID: 30156526
Review
Go to all (81) article citations
Other citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Recombination between Sindbis virus RNAs.
Arch Virol Suppl, 9:213-220, 01 Jan 1994
Cited by: 9 articles | PMID: 7518272
A cis-acting mutation in the Sindbis virus junction region which affects subgenomic RNA synthesis.
J Virol, 63(12):5216-5227, 01 Dec 1989
Cited by: 42 articles | PMID: 2685355 | PMCID: PMC251186
Promoter for Sindbis virus RNA-dependent subgenomic RNA transcription.
J Virol, 64(4):1726-1733, 01 Apr 1990
Cited by: 115 articles | PMID: 2319651 | PMCID: PMC249310
Genesis of Sindbis virus by in vivo recombination of nonreplicative RNA precursors.
J Virol, 69(12):7391-7401, 01 Dec 1995
Cited by: 62 articles | PMID: 7494243 | PMCID: PMC189675