Abstract
Free full text

Two groups of rhinoviruses revealed by a panel of antiviral compounds present sequence divergence and differential pathogenicity.
Abstract
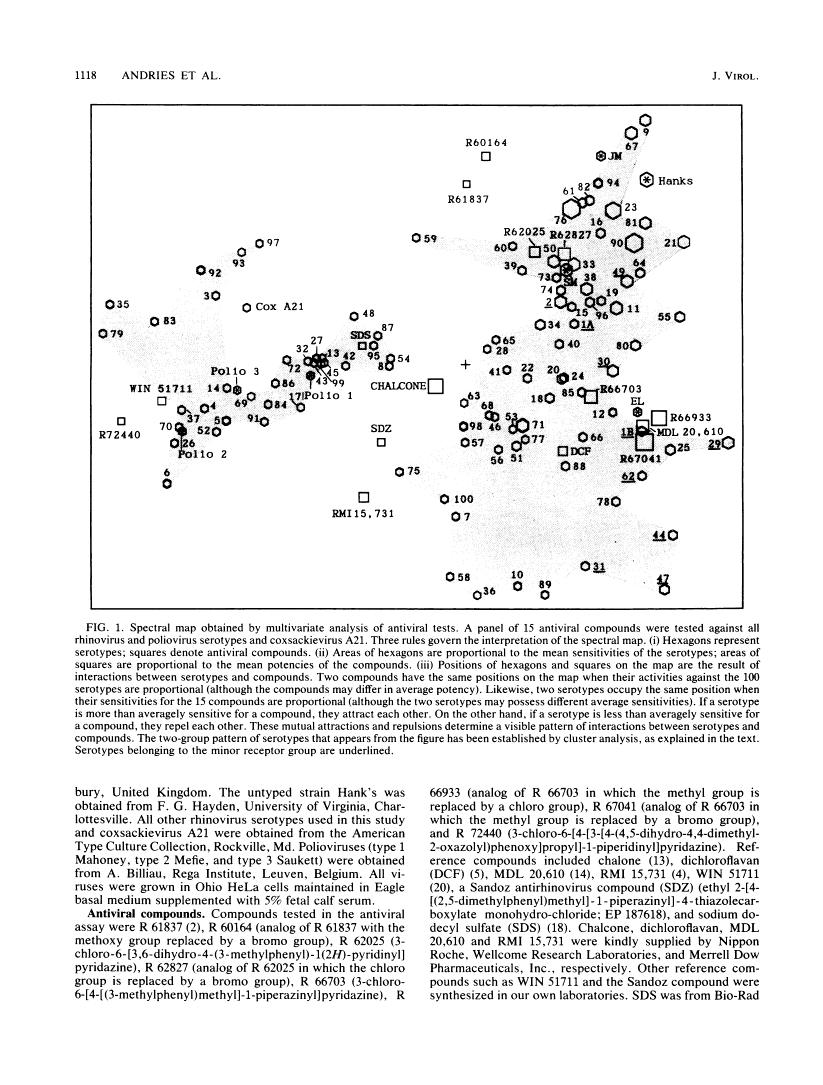
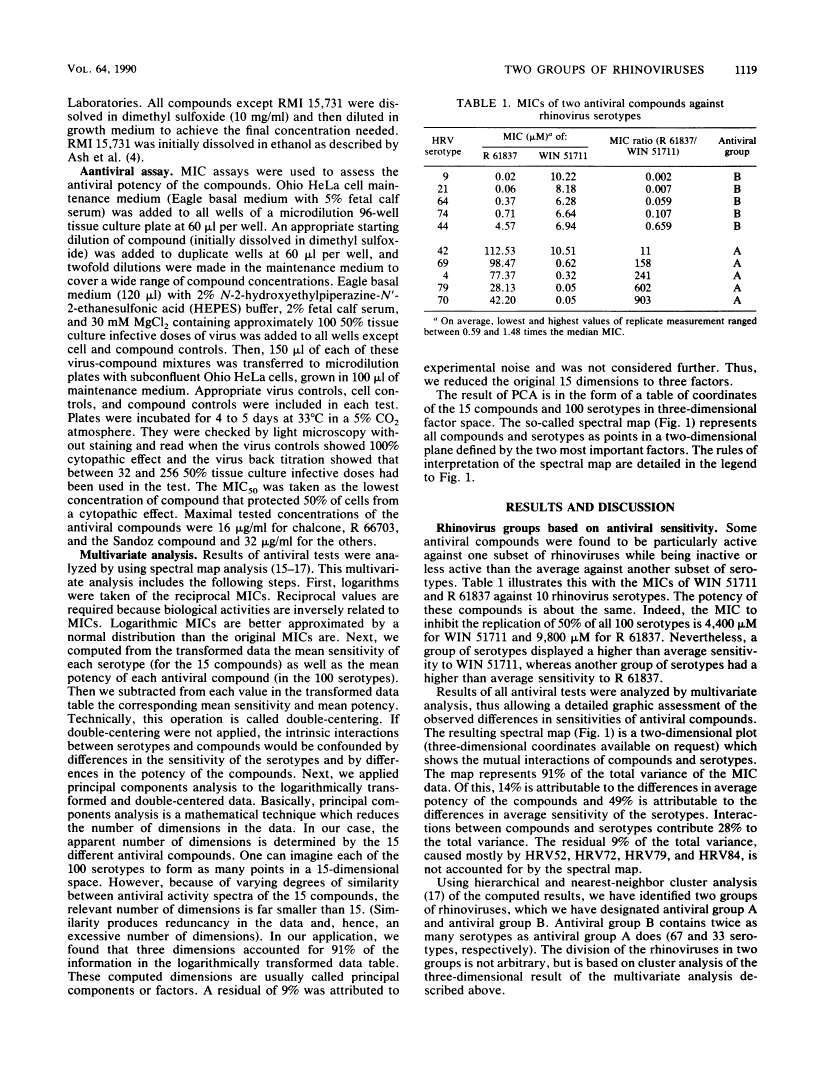
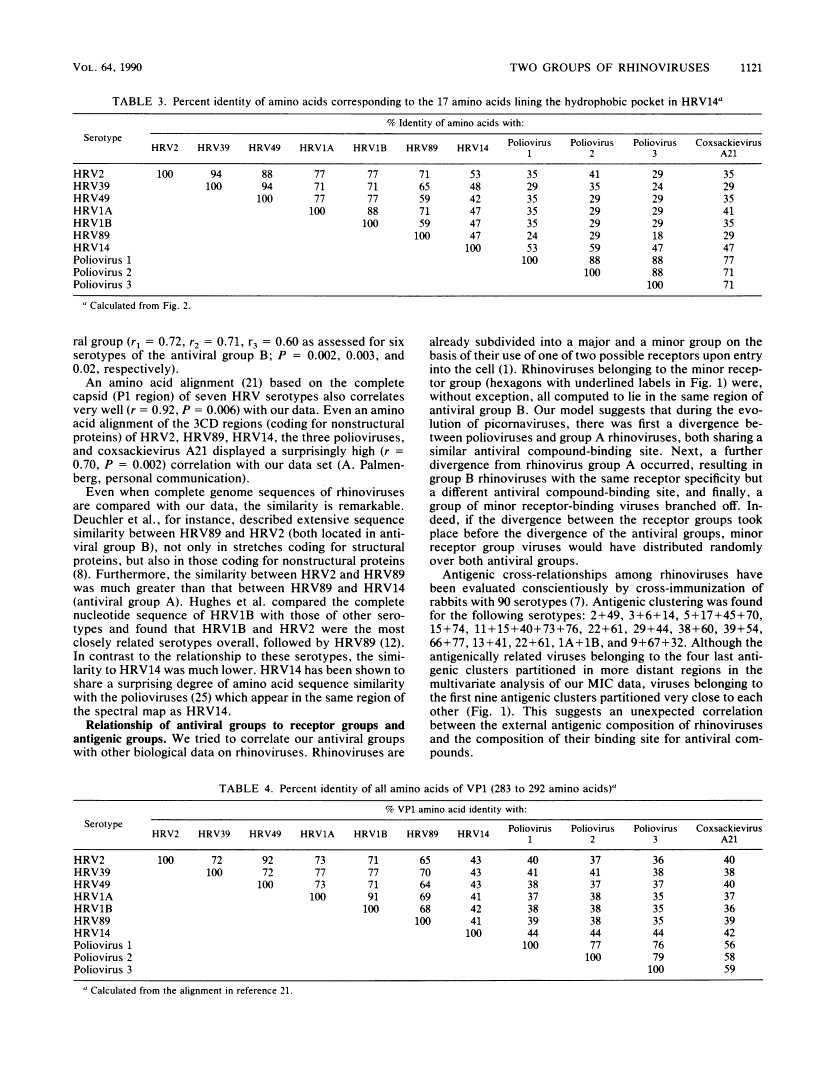
A variety of chemically different compounds inhibit the replication of several serotypes of rhinoviruses (common-cold viruses). We noticed that one of these antiviral compounds, WIN 51711, had an antiviral spectrum clearly distinctive from a consensus spectrum or other capsid-binding compounds, although all of them were shown to share the same binding site. A systematic evaluation of all known rhinovirus capsid-binding compounds against all serotyped rhinoviruses was therefore initiated. Multivariate analysis of the results revealed the existence of two groups of rhinoviruses, which we will call antiviral groups A and B. The differential sensitivity of members of these groups to antiviral compounds suggests the existence of a dimorphic binding site. The antiviral groups turned out to be a reflection of a divergence of rhinovirus serotypes on a much broader level. Similarities in antiviral spectra were highly correlated with sequence similarities, not only of amino acids lining the antiviral compound-binding-site, but also of amino acids of the whole VP1 protein. Furthermore, analysis of epidemiological data indicated that group B rhinoviruses produced more than twice as many clinical infections per serotype than group A rhinoviruses did. Rhinoviruses belonging to the minor receptor group were without exception all computed to lie in the same region of antiviral group B.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.3M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G, Colonno RJ. Many rhinovirus serotypes share the same cellular receptor. J Virol. 1984 Aug;51(2):340–345. [Europe PMC free article] [Abstract] [Google Scholar]
- Andries K, Dewindt B, De Brabander M, Stokbroekx R, Janssen PA. In vitro activity of R 61837, a new antirhinovirus compound. Arch Virol. 1988;101(3-4):155–167. [Europe PMC free article] [Abstract] [Google Scholar]
- Andries K, Dewindt B, Snoeks J, Willebrords R. Lack of quantitative correlation between inhibition of replication of rhinoviruses by an antiviral drug and their stabilization. Arch Virol. 1989;106(1-2):51–61. [Abstract] [Google Scholar]
- Ash RJ, Parker RA, Hagan AC, Mayer GD. RMI 15,731 (1-[5-tetradecyloxy-2-furanyl]-ethanone), a new antirhinovirus compound. Antimicrob Agents Chemother. 1979 Sep;16(3):301–305. [Europe PMC free article] [Abstract] [Google Scholar]
- Bauer DJ, Selway JW, Batchelor JF, Tisdale M, Caldwell IC, Young DA. 4',6-Dichloroflavan (BW683C), a new anti-rhinovirus compound. Nature. 1981 Jul 23;292(5821):369–370. [Europe PMC free article] [Abstract] [Google Scholar]
- Colonno RJ, Condra JH, Mizutani S, Callahan PL, Davies ME, Murcko MA. Evidence for the direct involvement of the rhinovirus canyon in receptor binding. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5449–5453. [Europe PMC free article] [Abstract] [Google Scholar]
- Cooney MK, Fox JP, Kenny GE. Antigenic groupings of 90 rhinovirus serotypes. Infect Immun. 1982 Aug;37(2):642–647. [Europe PMC free article] [Abstract] [Google Scholar]
- Duechler M, Skern T, Sommergruber W, Neubauer C, Gruendler P, Fogy I, Blaas D, Kuechler E. Evolutionary relationships within the human rhinovirus genus: comparison of serotypes 89, 2, and 14. Proc Natl Acad Sci U S A. 1987 May;84(9):2605–2609. [Europe PMC free article] [Abstract] [Google Scholar]
- Fox JP, Cooney MK, Hall CE, Foy HM. Rhinoviruses in Seattle families, 1975-1979. Am J Epidemiol. 1985 Nov;122(5):830–846. [Abstract] [Google Scholar]
- Hamparian VV, Colonno RJ, Cooney MK, Dick EC, Gwaltney JM, Jr, Hughes JH, Jordan WS, Jr, Kapikian AZ, Mogabgab WJ, Monto A, et al. A collaborative report: rhinoviruses--extension of the numbering system from 89 to 100. Virology. 1987 Jul;159(1):191–192. [Abstract] [Google Scholar]
- Hogle JM, Chow M, Filman DJ. Three-dimensional structure of poliovirus at 2.9 A resolution. Science. 1985 Sep 27;229(4720):1358–1365. [Abstract] [Google Scholar]
- Hughes PJ, North C, Jellis CH, Minor PD, Stanway G. The nucleotide sequence of human rhinovirus 1B: molecular relationships within the rhinovirus genus. J Gen Virol. 1988 Jan;69(Pt 1):49–58. [Abstract] [Google Scholar]
- Ishitsuka H, Ninomiya YT, Ohsawa C, Fujiu M, Suhara Y. Direct and specific inactivation of rhinovirus by chalcone Ro 09-0410. Antimicrob Agents Chemother. 1982 Oct;22(4):617–621. [Europe PMC free article] [Abstract] [Google Scholar]
- Kenny MT, Dulworth JK, Bargar TM, Torney HL, Graham MC, Manelli AM. In vitro antiviral activity of the 6-substituted 2-(3',4'-dichlorophenoxy)-2H-pyrano[2,3-b]pyridines MDL 20,610, MDL 20,646, and MDL 20,957. Antimicrob Agents Chemother. 1986 Sep;30(3):516–518. [Europe PMC free article] [Abstract] [Google Scholar]
- Lewi PJ. Spectral mapping, a technique for classifying biological activity profiles of chemical compounds. Arzneimittelforschung. 1976;26(7):1295–1300. [Abstract] [Google Scholar]
- Lonberg-Holm K, Noble-Harvey J. Comparison of in vitro and cell-mediated alteration of a human Rhinovirus and its inhibition by sodium dodecyl sulfate. J Virol. 1973 Oct;12(4):819–826. [Europe PMC free article] [Abstract] [Google Scholar]
- Monto AS, Bryan ER, Ohmit S. Rhinovirus infections in Tecumseh, Michigan: frequency of illness and number of serotypes. J Infect Dis. 1987 Jul;156(1):43–49. [Abstract] [Google Scholar]
- Otto MJ, Fox MP, Fancher MJ, Kuhrt MF, Diana GD, McKinlay MA. In vitro activity of WIN 51711, a new broad-spectrum antipicornavirus drug. Antimicrob Agents Chemother. 1985 Jun;27(6):883–886. [Europe PMC free article] [Abstract] [Google Scholar]
- Rossmann MG. The evolution of RNA viruses. Bioessays. 1987 Sep;7(3):99–103. [Abstract] [Google Scholar]
- Rossmann MG, Arnold E, Erickson JW, Frankenberger EA, Griffith JP, Hecht HJ, Johnson JE, Kamer G, Luo M, Mosser AG, et al. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. [Abstract] [Google Scholar]
- Smith TJ, Kremer MJ, Luo M, Vriend G, Arnold E, Kamer G, Rossmann MG, McKinlay MA, Diana GD, Otto MJ. The site of attachment in human rhinovirus 14 for antiviral agents that inhibit uncoating. Science. 1986 Sep 19;233(4770):1286–1293. [Abstract] [Google Scholar]
- Stanway G, Hughes PJ, Mountford RC, Minor PD, Almond JW. The complete nucleotide sequence of a common cold virus: human rhinovirus 14. Nucleic Acids Res. 1984 Oct 25;12(20):7859–7875. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jvi.64.3.1117-1123.1990
Read article for free, from open access legal sources, via Unpaywall:
https://jvi.asm.org/content/jvi/64/3/1117.full.pdf
Free after 4 months at jvi.asm.org
http://jvi.asm.org/cgi/reprint/64/3/1117
Free to read at jvi.asm.org
http://jvi.asm.org/cgi/content/abstract/64/3/1117
Citations & impact
Impact metrics
Article citations
Recommendations for the nomenclature of enteroviruses and rhinoviruses.
Arch Virol, 165(3):793-797, 01 Mar 2020
Cited by: 78 articles | PMID: 31980941 | PMCID: PMC7024059
Development of real-time fluorescent reverse transcription loop-mediated isothermal amplification assays for rhinovirus detection.
J Med Virol, 91(7):1232-1238, 22 Feb 2019
Cited by: 8 articles | PMID: 30735248 | PMCID: PMC7166982
Rhinovirus - From bench to bedside.
J Formos Med Assoc, 116(7):496-504, 08 May 2017
Cited by: 46 articles | PMID: 28495415
Review
3-Phenylalkyl-2H-chromenes and -chromans as novel rhinovirus infection inhibitors.
Bioorg Med Chem, 25(7):2074-2083, 10 Feb 2017
Cited by: 4 articles | PMID: 28242170
Prevalence and molecular characterization of human rhinovirus in stool samples of individuals with and without acute gastroenteritis.
J Med Virol, 89(5):801-808, 11 Oct 2016
Cited by: 2 articles | PMID: 27678016
Go to all (93) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Human rhinovirus 3 at 3.0 A resolution.
Structure, 4(10):1205-1220, 01 Oct 1996
Cited by: 57 articles | PMID: 8939746
VP1 sequencing of all human rhinovirus serotypes: insights into genus phylogeny and susceptibility to antiviral capsid-binding compounds.
J Virol, 78(7):3663-3674, 01 Apr 2004
Cited by: 132 articles | PMID: 15016887 | PMCID: PMC371056
A comparative test of fifteen compounds against all known human rhinovirus serotypes as a basis for a more rational screening program.
Antiviral Res, 16(3):213-225, 01 Oct 1991
Cited by: 28 articles | PMID: 1666824
Selective human enterovirus and rhinovirus inhibitors: An overview of capsid-binding and protease-inhibiting molecules.
Med Res Rev, 24(4):449-474, 01 Jul 2004
Cited by: 26 articles | PMID: 15170592 | PMCID: PMC7168432
Review Free full text in Europe PMC