Abstract
Free full text

Isolation of a receptor protein involved in attachment of human rhinoviruses.
Abstract
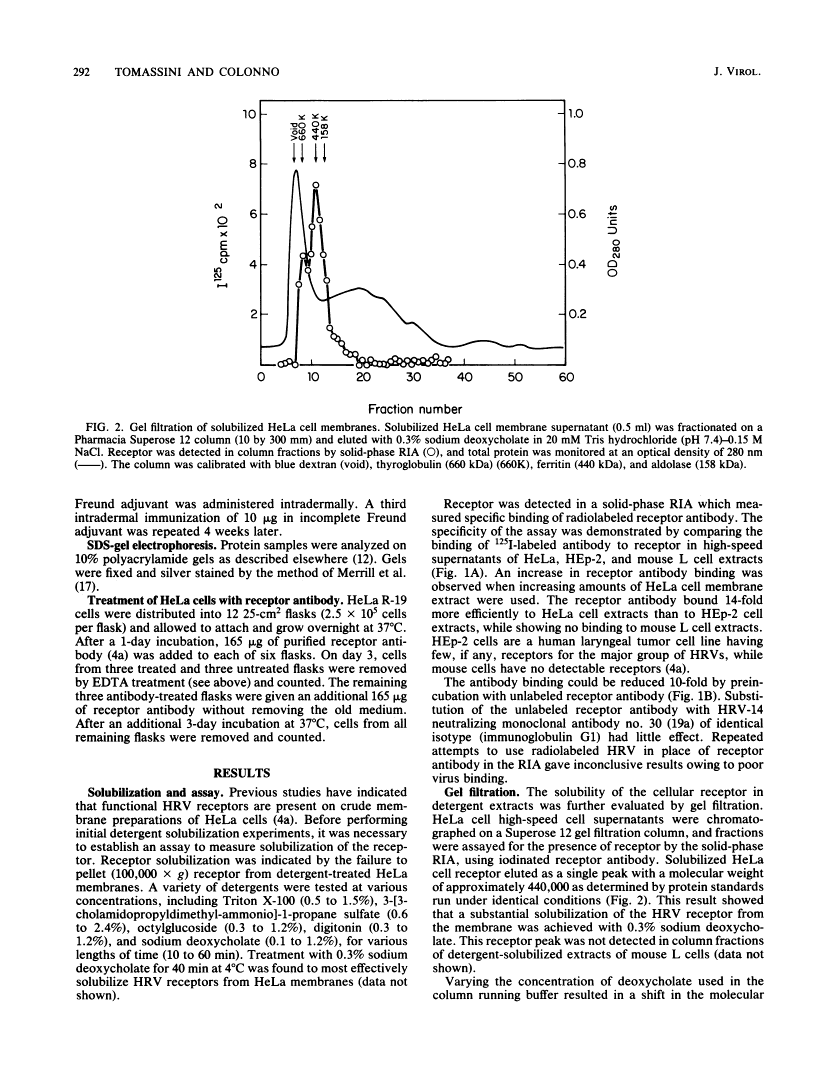
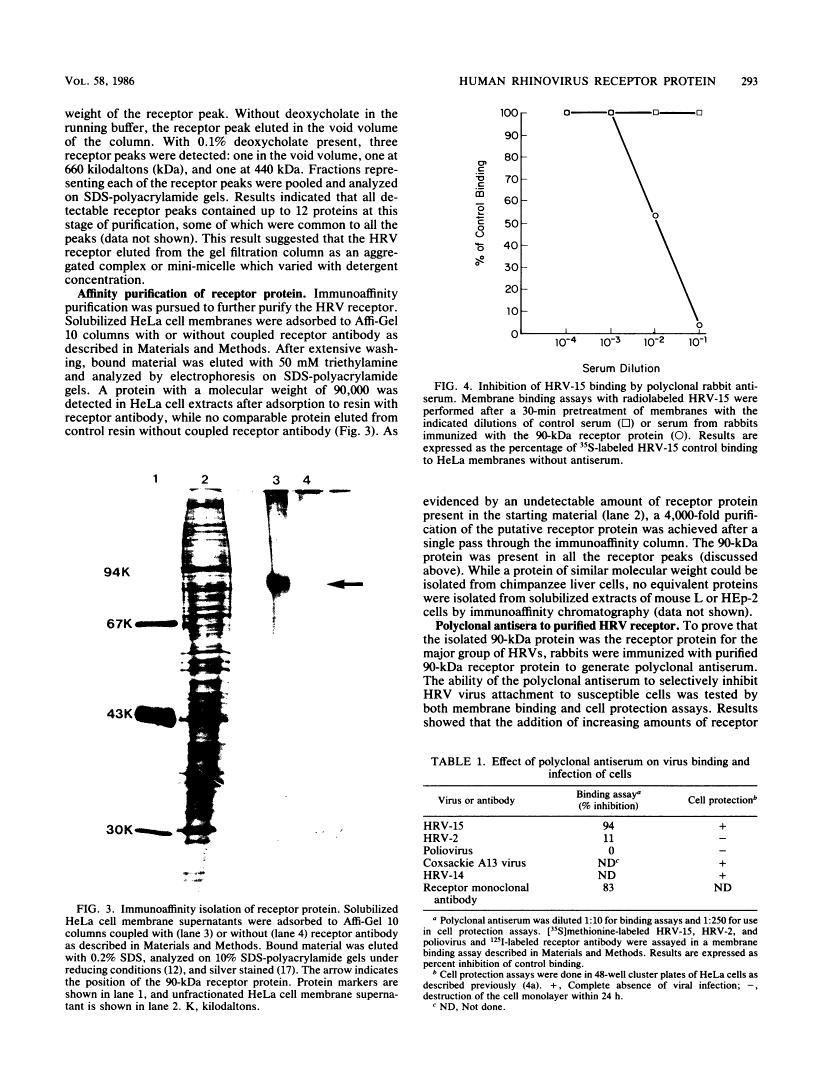
Human rhinoviruses can be classified into major and minor groups on the basis of receptor specificity. Recently, a mouse monoclonal antibody was isolated which selectively blocked the attachment of the major group of human rhinoviruses to cells. Using this monoclonal antibody, the cellular receptor for the major group of human rhinoviruses was isolated. A radioimmunoassay was developed by using the receptor antibody to specifically detect rhinovirus receptor during isolation. Solubilized receptor from detergent-treated HeLa cell membrane extracts eluted from gel filtration columns with an apparent molecular weight of 440,000. A cellular receptor protein, which had a molecular weight of 90,000 when analyzed on sodium dodecyl sulfate-polyacrylamide gels, was purified from solubilized extracts on an immunoaffinity column containing receptor antibody. Polyclonal rabbit antiserum, resulting from immunization with the isolated receptor protein, specifically blocked the attachment of the major group of human rhinoviruses and indicated that the 90-kilodalton protein plays a functional role in attachment. Prolonged exposure of HeLa cell monolayers with the receptor antibody showed no inhibition of cell growth and division.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G, Colonno RJ. Many rhinovirus serotypes share the same cellular receptor. J Virol. 1984 Aug;51(2):340–345. [Europe PMC free article] [Abstract] [Google Scholar]
- Callahan PL, Mizutani S, Colonno RJ. Molecular cloning and complete sequence determination of RNA genome of human rhinovirus type 14. Proc Natl Acad Sci U S A. 1985 Feb;82(3):732–736. [Europe PMC free article] [Abstract] [Google Scholar]
- Campbell BA, Cords CE. Monoclonal antibodies that inhibit attachment of group B coxsackieviruses. J Virol. 1983 Nov;48(2):561–564. [Europe PMC free article] [Abstract] [Google Scholar]
- Co MS, Gaulton GN, Fields BN, Greene MI. Isolation and biochemical characterization of the mammalian reovirus type 3 cell-surface receptor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1494–1498. [Europe PMC free article] [Abstract] [Google Scholar]
- Colonno RJ, Callahan PL, Long WJ. Isolation of a monoclonal antibody that blocks attachment of the major group of human rhinoviruses. J Virol. 1986 Jan;57(1):7–12. [Europe PMC free article] [Abstract] [Google Scholar]
- Dorsett PH, G8nsberg HS. Characterization of type 5 adenovirus fiber protein. J Virol. 1975 Jan;15(1):208–216. [Europe PMC free article] [Abstract] [Google Scholar]
- Fields BN, Greene MI. Genetic and molecular mechanisms of viral pathogenesis: implications for prevention and treatment. Nature. 1982 Nov 4;300(5887):19–23. [Abstract] [Google Scholar]
- Gwaltney JM., Jr Rhinoviruses. Yale J Biol Med. 1975 Mar;48(1):17–45. [Europe PMC free article] [Abstract] [Google Scholar]
- Krah DL, Crowell RL. A solid-phase assay of solubilized HeLa cell membrane receptors for binding group B coxsackieviruses and polioviruses. Virology. 1982 Apr 15;118(1):148–156. [Abstract] [Google Scholar]
- Krah DL, Crowell RL. Properties of the deoxycholate-solubilized HeLa cell plasma membrane receptor for binding group B coxsackieviruses. J Virol. 1985 Mar;53(3):867–870. [Europe PMC free article] [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Lee PW, Hayes EC, Joklik WK. Protein sigma 1 is the reovirus cell attachment protein. Virology. 1981 Jan 15;108(1):156–163. [Abstract] [Google Scholar]
- Lonberg-Holm K, Crowell RL, Philipson L. Unrelated animal viruses share receptors. Nature. 1976 Feb 26;259(5545):679–681. [Abstract] [Google Scholar]
- Mapoles JE, Krah DL, Crowell RL. Purification of a HeLa cell receptor protein for group B coxsackieviruses. J Virol. 1985 Sep;55(3):560–566. [Europe PMC free article] [Abstract] [Google Scholar]
- Merril CR, Goldman D, Sedman SA, Ebert MH. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. [Abstract] [Google Scholar]
- Minor PD, Pipkin PA, Hockley D, Schild GC, Almond JW. Monoclonal antibodies which block cellular receptors of poliovirus. Virus Res. 1984;1(3):203–212. [Abstract] [Google Scholar]
- Rossmann MG, Arnold E, Erickson JW, Frankenberger EA, Griffith JP, Hecht HJ, Johnson JE, Kamer G, Luo M, Mosser AG, et al. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. [Abstract] [Google Scholar]
- Sherry B, Mosser AG, Colonno RJ, Rueckert RR. Use of monoclonal antibodies to identify four neutralization immunogens on a common cold picornavirus, human rhinovirus 14. J Virol. 1986 Jan;57(1):246–257. [Europe PMC free article] [Abstract] [Google Scholar]
- Sherry B, Rueckert R. Evidence for at least two dominant neutralization antigens on human rhinovirus 14. J Virol. 1985 Jan;53(1):137–143. [Europe PMC free article] [Abstract] [Google Scholar]
- Stanway G, Hughes PJ, Mountford RC, Minor PD, Almond JW. The complete nucleotide sequence of a common cold virus: human rhinovirus 14. Nucleic Acids Res. 1984 Oct 25;12(20):7859–7875. [Europe PMC free article] [Abstract] [Google Scholar]
- Svensson U, Persson R, Everitt E. Virus-receptor interaction in the adenovirus system I. Identification of virion attachment proteins of the HeLa cell plasma membrane. J Virol. 1981 Apr;38(1):70–81. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jvi.58.2.290-295.1986
Read article for free, from open access legal sources, via Unpaywall:
https://jvi.asm.org/content/jvi/58/2/290.full.pdf
Free to read at jvi.asm.org
http://jvi.asm.org/cgi/content/abstract/58/2/290
Free after 4 months at jvi.asm.org
http://jvi.asm.org/cgi/reprint/58/2/290
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/jvi.58.2.290-295.1986
Article citations
Infection and propagation of human rhinovirus C in human airway epithelial cells.
J Virol, 86(24):13524-13532, 03 Oct 2012
Cited by: 53 articles | PMID: 23035218 | PMCID: PMC3503113
Engineering of single Ig superfamily domain of intercellular adhesion molecule 1 (ICAM-1) for native fold and function.
J Biol Chem, 285(21):15906-15915, 19 Mar 2010
Cited by: 12 articles | PMID: 20304924 | PMCID: PMC2871458
The minor receptor group of human rhinovirus (HRV) includes HRV23 and HRV25, but the presence of a lysine in the VP1 HI loop is not sufficient for receptor binding.
J Virol, 79(12):7389-7395, 01 Jun 2005
Cited by: 51 articles | PMID: 15919894 | PMCID: PMC1143622
Biochemical characterization of rhinovirus RNA-dependent RNA polymerase.
Antiviral Res, 56(2):99-114, 01 Nov 2002
Cited by: 41 articles | PMID: 12367717
Viral evolution toward change in receptor usage: adaptation of a major group human rhinovirus to grow in ICAM-1-negative cells.
J Virol, 75(19):9312-9319, 01 Oct 2001
Cited by: 24 articles | PMID: 11533194 | PMCID: PMC114499
Go to all (40) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Characteristics of the minor group receptor of human rhinoviruses.
Virology, 163(1):19-25, 01 Mar 1988
Cited by: 16 articles | PMID: 2831654
Isolation of a monoclonal antibody that blocks attachment of the major group of human rhinoviruses.
J Virol, 57(1):7-12, 01 Jan 1986
Cited by: 85 articles | PMID: 3001366 | PMCID: PMC252692
Soluble LDL minireceptors. Minimal structure requirements for recognition of minor group human rhinovirus.
J Biol Chem, 273(50):33835-33840, 01 Dec 1998
Cited by: 17 articles | PMID: 9837974
Characterization of human rhinoviruses displaced by an anti-receptor monoclonal antibody.
J Virol, 62(7):2300-2306, 01 Jul 1988
Cited by: 6 articles | PMID: 2836613 | PMCID: PMC253381