Abstract
Free full text

The UNI3 Gene Is Required for Assembly of Basal Bodies of Chlamydomonas and Encodes δ-Tubulin, a New Member of the Tubulin Superfamily
Abstract
We have cloned the UNI3 gene in Chlamydomonas and find that it encodes a new member of the tubulin superfamily. Although Uni3p shares significant sequence identity with α-, β-, and γ-tubulins, there is a region of Uni3p that has no similarity to tubulins or other known proteins. Mutant uni3–1 cells assemble zero, one, or two flagella. Pedigree analysis suggests that flagellar number in uni3–1 cells is a function of the age of the cell. The uniflagellate uni3–1 cells show a positional phenotype; the basal body opposite the eyespot templates the single flagellum. A percentage of uni3–1 cells also fail to orient the cleavage furrow properly, and basal bodies have been implicated in the placement of cleavage furrows in Chlamydomonas. Finally when uni3–1 cells are observed by electron microscopy, doublet rather than triplet microtubules are observed at the proximal end of the basal bodies. We propose that the Uni3 tubulin is involved in both the function and cell cycle-dependent maturation of basal bodies/centrioles.
INTRODUCTION
Eukaryotic cells use microtubules in diverse ways: microtubules are required for chromosome segregation, for organization and movement of vesicles and organelles in the cytoplasm, and for flagellar beating. α- and β-tubulin heterodimers assemble into polymers that give rise to the microtubules of the cytoskeleton, spindle, and flagella. Assembly and establishment of microtubule patterns are under the control of the microtubule organizing center (MTOC), which is an organelle that regulates the spatial and temporal distribution of microtubules. In animal and algal cells, the MTOC consists of a pair of centrioles surrounded by pericentriolar material. Microtubules are polar, and the “plus” end, which has a faster assembly rate, is found distal to the MTOC. γ-Tubulin is the third and most recently identified member of the tubulin superfamily (Oakley and Oakley, 1989). γ-Tubulin is found at the “minus” end of the microtubules, generally localizes to the pericentriolar material, and is a part of structures that may serve as initiators for microtubule polymerization. In Xenopus, these structures contain α-, β-, and γ-tubulin and at least five other polypeptides (Zheng et al., 1995). γ-Tubulin has also been postulated to convert the basal body contributed by the sperm during fertilization into a centriole in Xenopus (Félix et al., 1994; Stearns and Kirschner, 1994). The conversion of basal bodies into centrioles also occurs in each cell cycle in many green algae before mitosis (Ringo, 1967; Moestrup, 1982).
The centrioles assemble conservatively but segregate semiconservatively. At the end of cell division, each daughter cell receives a new and an old centriole (Vorobjev and Chentsov, 1982; Melkonian et al., 1987; Beech et al., 1988; Holmes and Dutcher, 1989). In cells injected with biotinylated tubulin, Kochanski and Borisy (1990) demonstrated that most, if not all, of the labeled tubulin was found in the new centriole. The two centrioles and the pericentriolar material of the MTOC are morphologically, immunologically, and functionally distinguishable. In many mammalian and algal cells, there are morphological differences between the structures around the two centrioles (Reider and Borisy, 1982, Vorobjev and Chentsov, 1982; Melkonian et al., 1987; Beech et al., 1988; Wetherbee et al., 1988; Paintrand et al., 1992) that are correlated with the age of the centrioles. In mammalian cells, the pericentriolar material is concentrated around the older or parental centrioles (Reider and Borisy, 1982; Vorobjev and Chentsov, 1982). As might be expected from a functional point of view, daughter centrioles mature into parental centrioles near the beginning of mitosis and acquire pericentriolar material (Vorobjev and Chentsov, 1982). Furthermore, the grandparental centriole has additional structures and a primary cilium (Vorobjev and Chentsov, 1982). Basal bodies also show differences depending on their age. In several green algae, grandparental basal bodies nucelate elaborate and longer flagella. Parental basal bodies nucleate flagella that are longer, but not as elaborate. Daughter basal bodies nucleate short flagella (Beech et al., 1987; Melkonian et al., 1987). Lange and Gull (1995) identified a monoclonal antibody that distinguishes between the two centrioles in mammalian cells. An epitope is recognized on the older of the two centrioles, and the acquisition of this epitope requires one cell cycle to achieve. In Chlamydomonas reinhardtii, there are functional differences between the two flagella that are templated by the basal bodies. The eyespot, a structure used for phototaxis, is associated with a specialized microtubule bundle and the new basal body (Foster and Smyth, 1981; Holmes and Dutcher, 1989). In addition, the two flagella templated by the parent and daughter basal bodies respond differently to phototactic signals (Rüffer and Nultsch, 1987; Horst and Witman, 1993; King and Dutcher, 1997).
The basal bodies of Chlamydomonas are affected differently in strains with the uni1 mutation (Huang et al., 1982). Although the postmitotic assembly of the two flagella is normally coordinately controlled, the assembly of the two flagella becomes uncoordinated in uni1 strains. In the uni1 mutant strains, two basal bodies are assembled, but only one basal body assembles a flagellum (Huang et al., 1982; Holmes and Dutcher, 1989). The assembly-competent basal body is always found in a specific orientation with respect to the asymmetrically assembled eyespot and is referred to as the trans basal body. Subsequently, it was shown that the trans basal body is the older or parental basal body (Holmes and Dutcher, 1989, 1992). Analysis of uni1 strains suggested that the basal bodies play a role in providing positional information (Huang et al., 1982).
We have identified two new mutations in Chlamydomonas that produce a uniflagellate phenotype. An increased percentage of the cells assemble a single flagellum. These mutations map to two previously undescribed loci, which are designated UNI2 and UNI3. The uni3–1 mutation was generated using insertional mutagenesis (Tam and Lefebvre, 1993) and has a molecular tag associated with the mutant phenotype. The UNI3 gene has been cloned and encodes the first representative of a new member of the tubulin superfamily.
MATERIALS AND METHODS
Cell Culture and Genetic Analysis
Culture conditions and media were as described previously (Lux and Dutcher, 1991); standard matings and matings with bld2–1 cells were as described by Harris (1989) and Dutcher (1995a), respectively. Diploid strains were selected as described by King and Dutcher (1997) using the linked nit2–1 and ac17 mutations. The sr1 and pf16–2 strains were obtained from the Chlamydomonas Genetics Center (Duke University, Durham, NC). Transfers of cells in the pedigree experiments were performed with a braking pipette with kind instruction from Dr. David Prescott. Strains referred to as uni1, uni2, uni3, and uni4 by Huang et al. (1982) are alleles at the UNI1 locus and are designated as uni1–1-uni1–4 (Dutcher, 1986).
Light and Electron Microscopy
Flagellar number counts were performed using phase optics and a 40× objective with cells at densities of 5 × 105 to 2 × 106 cells/ml to ensure that the cells were not approaching stationary phase. Eyespots were visualized using differential interference contrast (DIC) or brightfield light microscopy (Holmes and Dutcher, 1989). The cells used for obtaining longitudinal images were processed for electron microscopy as described in Porter et al. (1992). Images were examined at a magnification of 21,000× with a CM10 microscope (Philips Electronic Instruments, Mahwah, NJ) operating at 80 kV. The cells used for cross-sectional and tangential images were processed for electron microscopy as described by Goodenough and Weiss (1978).
Southern Blot Analysis and Library Screen
Chlamydomonas DNA was isolated as described by Johnson and Dutcher (1991), except that the DNA was precipitated with polyethylene glycol an additional time. Upon resuspension of the DNA pellet with Tris-EDTA, the salt concentration was adjusted to 0.8 M NaCl, and polyethylene glycol (PEG)-4000 was added to a final concentration of 7.5%. Each sample was incubated on ice for >30 min, centrifuged at 14,000 rpm for 10 min, and resuspended in water or Tris-EDTA. This extra precipitation step increased the purity of the sample and made subsequent enzyme digestions significantly more reliable. Hybridization conditions for Southern blots and library filters were described elsewhere (Johnson and Dutcher, 1991). All probes were labeled using the Multiprime DNA Labeling System (Amersham, Arlington Heights, IL).
Plasmids and Phage DNA
pARG7.8, a pBR329-based plasmid containing the Chlamydomonas argininosuccinate lyase gene (ARG7) (Debuchy et al., 1989) was used in the construction of pγΔ-1, which was used for transformation of arg7–8 cells. pMN56, a pUC119-based plasmid containing the nitrate reductase gene (NIT1) was used for cotransformation/rescue experiments of uni3–1::ARG7 NIT2 nit1–1 cells with λ-phage or genomic subclones (Fernández et al., 1989; Nelson and Lefebvre, 1995). Plasmid and λ-phage DNA preparations were performed using Qiagen (Chatsworth, CA) DNA purification kits. Plasmids pCU-1 and pCU-2 were constructed in the vector pUC18. pCU-1 has a 9.5-kb insert after a digestion of λ-CU-2 with SalI. pCU-2 has a 5.68-kb insert made by digesting pCU-1 with XbaI and EcoNI.
Chlamydomonas Transformations
For all transformations we used the glass bead method (Kindle, 1990). Briefly, logarithmically growing 1L cultures were harvested by centrifugation, incubated with autolysin (Harris, 1989; Dutcher, 1995b) for 20 min at room temperature to remove the cell wall, recentrifuged, and resuspended in selective medium: 525 μl of cells, 500 μl of acid-washed glass beads (710–1, 180 nm in diameter, Sigma Chemical, St. Louis, MO), 175 μl of 20% PEG-4000, and 2 μg of EcoRI linearized pΔγ-1 DNA were vortexed at maximum speed for 30 s. Cells were allowed to recover for 2 h to overnight at 25°C and plated onto selective medium that contained ammonium nitrate and no arginine. Cotransformation experiments used 2 μg of EcoRI-linearized pMN56, and 0.5, 1.0, or 2.0 μg of each genomic λ phage. Cells were plated onto medium with sodium nitrate as the sole nitrogen source and with 0.5% top agarose to improve the transformation efficiency (Gumpel et al., 1994). The transforming DNA, pΔγ-1, which was used to generate the uni3–1 allele, contained short stretches of γ-tubulin. Although most integration events in Chlamydomonas are nonhomologous, it is possible that these γ-tubulin sequences may have provided homology with either α-tubulin or the Uni3 to promote integration in this region of the genome.
Construction of the Size-fractionated Library of uni3–1 DNA
uni3–1 (20 μg) DNA was digested with BamHI and AvaI (Boehringer Mannheim, Indianapolis, IN) and fractionated on a 1% low-melting temperature agarose gel (SeaKem, Rockland, ME). Four fractions that included regions slightly above and slightly below the 2.3-kb band of the λ DNA molecular weight marker were excised and purified using β-agarase (Boehringer Mannheim). One-tenth volume of each fraction was electrophoresed on a 1% agarose gel, transferred to a Zetabind filter (Amersham), and hybridized with a single-copy γ-tubulin probe. The band from the endogenous γ-tubulin gene was significantly smaller than the aforementioned 2.3-kb band and therefore did not pose a contamination problem. The fraction with the most intense signal was ligated to the pBR329 vector and transformed into DH5αF′ electrocompetent cells. A colony containing the hybrid γ-tubulin and flanking genomic DNA sequences was isolated using standard colony hybridization techniques and the single-copy γ-tubulin sequences as the hybridization probe (Sambrook et al., 1989).
Isolation of Genomic and cDNA Clones
Genomic clones were isolated from two independent libraries of MboI partially digested arg7–8 and wild-type DNA cloned into the BamHI cloning site of λDASHII (Stratagene, La Jolla, CA) and λEMBL3, respectively (kind gifts of Anthony Palombella and Dr. David Johnson). Screening with a 870-bp fragment that contained flanking genomic DNA from the cloned BamHI-AvaI DNA fragment yielded phage λCU-1 and λCU-2. The remainder of the walk came from screens using single-copy sequences at the ends of λCU-2 and λCU-3 as hybridization probes.
A cDNA library made from light-grown vegetative NO− cells (a gift from Dr. Jeff P. Woessner, Washington University, St. Louis, MO) was screened with the genomic clone, and no positive clones were isolated from 500,000 plaques. cDNA libraries made from light and dark grown cells (a gift from Dr. Andy Wang, Iowa State University, Ames, IA) was screened, and no positives were isolated in 500,000 plaques. A Novagen custom cDNA library of mRNAs isolated from nitrogen-starved cells cloned into the λEXlox vector (a gift from Dr. Rogene Schnell, University of Minnesota, St. Paul, MN) was screened with a 449-bp UNI3 reverse transcriptase (RT)-PCR fragment (see following section). In 2.5 × 106 plaques screened, a single positive plaque was detected. The 1.75- kb cDNA clone, named Exlox53, is missing 133 bp of coding sequence and the 5′ untranslated region.
Reverse Transcription PCR
Poly-A+ RNA was enriched from 10 μg of total RNA isolated from wild-type cells using an mRNA isolation kit (Promega, Madison, WI). The primers used for PCR amplification were designed from DNA sequences obtained from the rescuing genomic clone whose predicted amino acid sequence showed similarity to known tubulins using BLAST searches. The primers varied in length from 18 to 37 bases. u12 and u14 (37 and 36 bases, respectively) each contained a 9-base linker with an EcoRI site to facilitate cloning of PCR fragments. The sequence from the genomic clones is underlined below. These primers amplified a 449-bp fragment that was used as a probe to screen the λEXlox cDNA library. A cDNA probe was generated because attempts to screen other libraries and Northern blots with genomic probes failed to detect a UNI3-specific signal. u24 and u26 (both 18 bases long) were used to extend the cDNA sequences beyond the 5′ end of the 1.75-kb cDNA clone, Exlox53. The 384- bp fragment amplified by the primer combination contained 133 bp of coding sequence and 90 bp of 5′ untranslated sequences not found in Exlox53. The sequence of both RT-PCR fragments was identical to the corresponding positions in the genomic clone.
All PCR reactions were performed using Taq DNA polymerase from Boehringer Mannheim in a Perkin Elmer-Cetus (Norwalk, CT) 480 DNA thermal cycler. mRNA (500 ng) primed with 250 ng of random hexamers (Life Technologies/Bethesda Research Laboratories, Gaithersburg, MD) was reverse transcribed with avian myeloblastosis virus-RT (Promega). One microliter of a 1:10 dilution of the first strand synthesis reaction was used in a 100 μl reaction with 25 pmol of each primer. PCR parameters were as follows: one cycle at 97°C for 10 min and 80°C for 5–10 min before the addition of Taq polymerase; 10 cycles at 95°C for 1 min, 53°C for 2 min, and 72°C for 3 min; 30 cycles at 95°C for 45 s, 57°C for 1 min, and 72°C for 2 min; and one final cycle at 95°C for 45 s, 57°C for 1 min, and 72°C for 10 min. The conditions for the RT-PCR with primers u24 and u26 were similar, except that the annealing temperatures were deceased by 1°C in the first 10 cycles, and by 2°C in the middle 30 cycles and in the final cycle.
u12 (antisense): 5′CCGGAATTCcagcgcctcgttctccagcagcaccagc;
u14 (sense): 5′CCGGAATTCggcgctccgtgctcatcgacatggagc; u24 (antisense): 5′CCTTGTGCCCAGTTGTTG; u26 (sense): 5′AGCACGCTCTGTTACTAG.
DNA Sequencing
Double-stranded DNA was made using a combined alkaline lysis-PEG precipitation method and sequenced by the DNA Sequencing Facility of Iowa State University. The cDNA clone, Exlox53, and the 384- and 449-bp RT-PCR fragments were sequenced on both strands. Sequence data were compiled using GCG Sequence Analysis Software (Madison, WI). GenBank database searches for sequence homologies were initially performed using the BLASTN program (Altschul et al., 1990).
Isolation of the Genomic DNA for γ-Tubulin
The genomic DNA was isolated using degenerate PCR with primers designed to match regions of identity among the known γ-tubulins. These included Aspergillus nidulans, Schizosaccharomyces pombe, Drosophila melanogaster, Xenopus laevis, and Homo sapiens. Underlined sequences indicate the region that hybridize to γ-tubulin, and the remainder contain an EcoRI site to facilitate cloning. I indicates an inosine residue, Y indicates a pyrimidine (C or T), R indicates a purine (A or G), and N indicates any base.
g1 (sense): 5′CCGGAATTCTAYCCNGGITAYATGGAAY; g2 (antisense): 5′CCGGAATTCACYTTRTTRAAIARRTG
Primers g1 and g2 generated a 773-bp PCR product that was used to screen a λEMBL3 library (constructed by Dr. David Johnson in our laboratory) using procedures described above for the cloning of the UNI3 gene. The clone was sequenced by the Iowa State University Sequencing Facility.
Phylogenetic Analysis of Tubulins
An alignment of 59 tubulins obtained from the SwissProt database was made by PileUp (Devereux et al., 1984), CLUSTAL-W (Thompson et al., 1994), or Pfam (Sonnhammer et al., 1997). The tree shown in Figure Figure77 was inferred by Fitch-Margoliash analysis using a Dayhoff PAM250 substitution matrix with PROTDIST, NEIGHBOR, and FITCH programs from the PHYLIP package, version 3.5 (Felsenstein, 1996) (J. Felsenstein [1993], PHYLIP [phylogenetic inference package], http://evolution.genetics.washington.edu.phylip/). Unweighted parsimony trees were inferred using the PROTPARS program from PHYLIP package (J. Felsenstein [1993], PHYLIP [phylogenetic inference package], http://evolution.genetics.washington.edu.phylip/). Trees generated with and without the middle sequences were qualitatively similar.
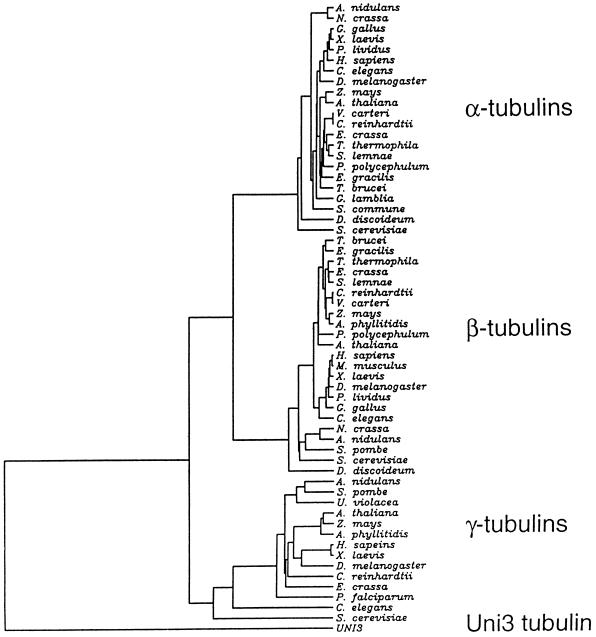
Phylogenetic tree of α-, β-, and γ-tubulins from different organisms compared with Uni3p using the method of Fitch-Margoliash. Only the horizontal distances are important for determining distances. In organisms with multiple tubulin genes, the following proteins were used in the tree: TBB1 and TBA5 from Gallus gallus; TBB2 from X. laevis; TBA1 from Paracentrotus lividus; TBB1 and TBA1 from D. melanogaster; TBB1, TBA1, and TBG1 from Zea mays; TBB1, TBA1, and TBG1 from Arabidopsis thaliana; TBB1 and TBA1 from Volvox carteri; TBB1 and TBAE from Physarum polycephalum; TBA2 from Stylonychia lemnae; TBA1 from Anemia phyllitidis, TBA2 from Mus musculus; TBA2 from Neurospora crassa; TBA1 from A. nidulans; and TBA1 from S. cerevisiae.
RESULTS
Phenotypic Analysis of uni2–1 and uni3–1
To investigate how differences are generated between the two centrioles/basal bodies and how these differences are used to generate positional and functional differences in the cell, we screened for mutant strains that assembled only a single flagellum. Cells with a single flagellum spin in place rather than swim. This phenotype provides a rapid visual screen.
The uni2–1 mutation was isolated after exposing cells to a brief heat shock (42°C for 40 min) and identifying strains with motility defects. The frequency of motility-defective strains was 1 in 2,000, and the uni2–1 mutation was one of ten mutations isolated. The uni3–1 mutation was isolated after insertional mutagenesis (Tam and Lefebvre, 1993); transformed strains were selected by virtue of arginine prototrophy conferred by integration of the pγΔ-1 plasmid (see below; Debuchy et al., 1989). A screen among about 1,000 transformants produced 20 strains with motility defects. Other strains are described elsewhere (King and Dutcher, 1997; Myster et al., 1997; Ehler and Dutcher, manuscript submitted).
From light microscopic counts of flagellar number, each Uni− strain had a significant number of cells with a single flagellum (uniflagellate cells) as well as aflagellate and biflagellate cells (Table (Table1).1). We examined the uni2–1 and uni3–1 cells for the positioning of the flagellum; 96% and 93% of the uniflagellate cells in uni2–1 and uni3–1 strains, respectively, have a trans flagellum (n = 500). In all three known Uni− strains, the trans flagellum is the predominant class among the uniflagellate cells.
Table 1
Flagellar number and cleavage furrow placement phenotypes of the unimutant strains
| Percentage of cells with
| ||||
|---|---|---|---|---|
| Flagella
| Cleavage Defect | |||
| Two | One | No | ||
| Wild-type | 90 | 2 | 8 | 1.8 |
| uni1-1 | 5 | 93 | 2 | 2.0 |
| uni1-3 | 3 | 90 | 7 | 1.5 |
| uni2-1 | 23 | 22 | 55 | 3.2 |
| uni3-1 | 26 | 25 | 49 | 15.0 |
| uni1-1 uni2-1 | 0 | 0 | 100 | 2.5 |
| uni1-1 uni3-1 | 0 | 0 | 100 | 35.0 |
| uni1-3 uni3-1 | 0 | 0 | 100 | 39.2 |
| uni2-1 uni3-1 | 0 | 0 | 100 | 23.6 |
Five hundred cells were counted to obtain the percentages for flagellar number and for cleavage defects. Some of the cleavage-defective cells in the single-mutant strains had more than two flagella and were not included in the cells counted for flagellar number.
Genetic analysis showed that both the uni2–1 and uni3–1 mutations segregated 2+:2− for the flagellar phenotype, and that the two mutations were not linked to each other or to the uni1–1 mutation (Huang et al., 1982; Holmes et al., 1991). In addition, arginine prototrophy conferred by the transforming DNA in the uni3–1 strain cosegregated with the flagellar phenotype in 130 tetrads, which indicates that transforming DNA and the flagellar number phenotype are tightly linked. Both mutations were recessive to the wild-type allele and complemented mutations at the other two UNI loci. Each mutation appears to define a newly identified locus based on mapping to known mutations. The uni2–1 mutation maps to linkage group IX near sr1 (112:0:0) and pf16–2 (110:0:2). The uni3–1 mutation maps to linkage group III between ac17 (235:0:2) and nit2–1 (128:0:16). One of the genes for α-tubulin (TUA1) also maps to this region of linkage group III (Ranum et al., 1988); the tua1–1 allele confers resistance to the antimicrotubule herbicide, oryzalin (James et al., 1993). The uni3–1 and tua1–1 alleles are tightly linked (115:0:0) but complement each other in diploid strains. Another mutation, sup-cs-1, has a cold-sensitive lethal phenotype and maps to this region. It is tightly linked to uni3–1 (67:0:0), and these mutations complement each other for the lethal and flagellar assembly defects. This complementation data and data below suggest that these mutations fall into different genes.
The uni2–1 and uni3–1 strains have phenotypes different from each other and from uni1–1 cells when examined by electron microscopy. The basal body can be divided into two parts. It consists of the triplet microtubular cylinder of the proximal portion, and the distal portion where triplet microtubules become doublet microtubules (Ringo, 1967; Figure Figure1A).1A). The transition zone between the basal body and flagellum is marked by osmophilic-staining H-shaped material in longitudinal sections of the basal bodies. In uni1–1 strains, the daughter basal body lacks a transition zone, and the parental basal body has abnormal material present in the transition zone (Huang et al., 1982). In the uni2–1 strain, some basal bodies appeared normal. In most sections only one of the two basal bodies appeared abnormal. Based on three sections that had both basal bodies and an eyespot for orientation, the daughter basal body was abnormal. We observed aberrant material in the transition zone as was observed in uni1–1 sections. We also observed very short cone-shaped flagella that were never observed in wild-type or uni1–1 strains. In the uni3–1 strain, all basal bodies examined showed abnormalities. In wild-type cells, triplet microtubules are found near the proximal end, and the center of the basal body has a cartwheel-like structure (see Figure Figure1,1, A–D). In multiple sections from the proximal region of the basal body, triplet microtubules were not observed. Only doublet microtubules were found (Figure (Figure1,1, E and F). Figure Figure1E1E is likely to be a section from a region similar to the one diagrammed in Figure Figure1B.1B. Figure Figure1F1F is likely to be section that includes both regions similar to the ones diagrammed in Figure Figure1,1, C and D. Thus, the uni3–1 mutation has an effect on the assembly of triplet microtubules in the Chlamydomonas basal body. The outermost or C tubule of the triplets is missing. In longitudinal section from uni3–1 cells, we observed the accumulation of osmophilic material in the barrel of the basal body.
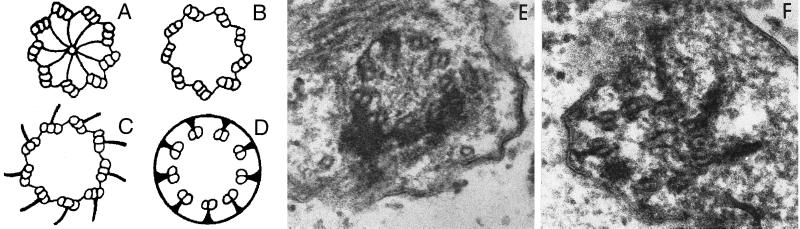
Diagram and electron micrographs of uni3–1 basal bodies. (A–D) Diagram of the cross-sectional images of a Chlamydomonas basal body based on Ringo (1967). Diagram A is the most proximal region and shows the classical cartwheel and diagram C is the most distal region of the basal body with triplet microtubules. Shown in diagram D is the appearance of doublet microtubules, which are attached to the plasma membrane. Panel E is a cross-sectional image that is likely to correspond to diagram B. Panel F is a tangential section that is represented by diagram C on the right side and diagram D on the left side. Final magnification in E and F is 125,000 ×.
The uni3–1 strain showed a cell division defect similar to the one observed in bld2–1 cells, which fail to assemble basal bodies/centrioles. bld2–1 cells also have defects in the coordination of the placement of the cleavage furrow and spindle. In ~75% of bld2–1 cells, the spindle and cleavage furrow were mispositioned with respect to the pyrenoid, a starch-containing structure in the chloroplast, and unequally sized daughter cells were produced with misplaced eyespots (Ehler et al., 1995). In uni3–1 cells, we observed an increase in the number of cells that had misplaced eyespots in newly divided pairs of cells and unequal sizes between the pairs of sister cells. The magnitude of the cleavage defect was quantified by counting the number of cells with multiple nuclei (Table (Table1).1). Neither uni1–1 nor uni2–1 cells have cleavage furrow placement defects (Table (Table1).1). Thus, the Uni3 gene product is likely to be needed for assembly of triplet microtubules and is needed, directly or indirectly, for flagellar assembly and cleavage furrow placement.
To determine whether UNI1, UNI2, and UNI3 act in the same or different pathways, we examined double mutant strains. In all combinations, we observed that the phenotypes of the double mutant strains were different than the phenotypes of the parental strains. All three double-mutant combinations were completely aflagellate (Table (Table1).1). In addition, the double-mutant combinations with uni3–1 showed an enhanced defect in cleavage furrow placement (Table (Table1).1). It seems likely that these genes act in at least two different pathways. We also examined double-mutant strains with the bld2–1 mutation. The uni- bld2–1 double-mutant strains all resembled the bld2–1 single-mutant strain. The BLD2 gene is likely to act upstream of the UNI genes. This result is consistent with the interpretation that the Uni gene products play a role in basal body function or assembly.
The uni3–1 strain has a higher frequency of both aflagellate and biflagellate cells than was observed in uni1–1 strains (Huang et al., 1982 and Table Table1).1). To address the mechanism involved in generating this pattern of flagellar number, we undertook a pedigree analysis of the flagellar phenotypes of mitotic progeny. We wanted to know whether the phenotypes occurred randomly. In other words, does the phenotype of a parental cell have an effect on the phenotypes of the progeny, or does the phenotype of the parent have no influence on the phenotypes of the progeny? As shown in Figure Figure2A,2A, the phenotypes of the progeny of a particular cell are not random; the phenotype of a cell in one generation is highly predictive of the phenotypes of the two daughter cells produced during mitosis. To confirm this result, we followed single cells through three successive cell cycles (Figure (Figure2B).2B). In summary, we found that aflagellate cells produced one uniflagellate and one aflagellate daughter (45 of 46). Uniflagellate (29 of 30) and biflagellate cells (23 of 25) produced one biflagellate and one aflagellate daughter cell. The phenotypes in a population of uni3–1 cells are likely to be a reflection of the ages of the cells in the population. In the remainder of the work described, we concentrated our analysis on the molecular characterization of the uni3–1 mutation because the allele was tagged.
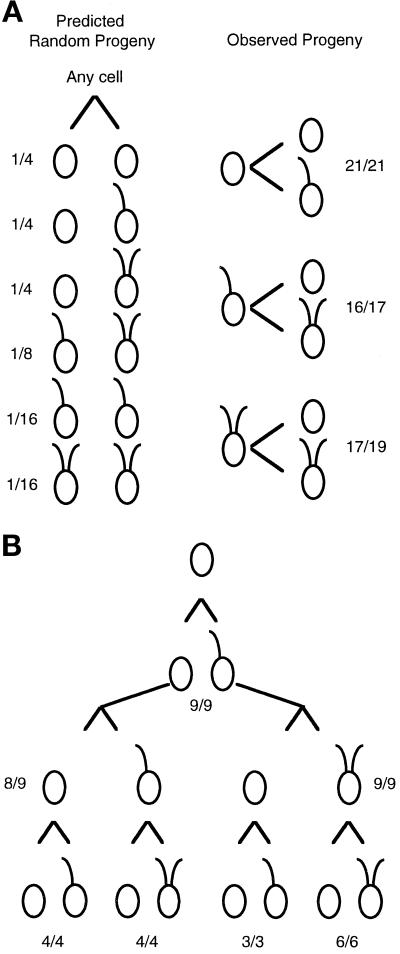
Pedigree analysis of the mitotic progeny from uni3–1 cells. Individual cells were placed in 40 μl rich medium in microtiter wells. Upon mitotic cell division, each daughter cell was transferred into a new well, and the swimming phenotype was monitored under a dissecting microscope with 100× magnification. (A) In the left panel, the predicted fraction of pairs of daughter cells if the phenotype is random and independent of lineage. The fractions were calculated as simple probabilities for independent events. In the right panel are the observed numbers. The fractions indicate the number of cells with the diagrammed phenotype over the number of cells successfully transferred and observed. In the three pairs that did not follow the pattern diagrammed, both cells had no flagella when observed. (B) Twelve aflagellate cells were placed individually in microtiter wells, and progeny for three successive cell divisions were monitored. The fractions indicate the number of cells that showed the diagrammed pattern over the number successfully transferred and monitored. As in part A, cells that did not show the diagrammed pattern produced two aflagellate cells.
Molecular Characterization of the UNI3 Locus
In Chlamydomonas, transforming DNA inserts nonhomologously into the nuclear genome and provides a “tag” that can be used for isolating DNA flanking the insertion site (Tam and Lefebvre, 1993; Pazour et al., 1995; Smith and Lefebvre, 1996). Many insertional events in Chlamydomonas are associated with the partial loss of transforming plasmid DNA as well as genome rearrangements. These genomic rearrangements include deletions as well as more complicated events (Smith and Lefebvre, 1996). The uni3–1 mutation was generated after transformation with the plasmid pγΔ-1, which carries the ARG7 gene in a pBR329-based vector flanked by two short segments of the γ-tubulin gene (Figure (Figure3A).3A). These segments of the γ-tubulin gene provided insulation of the ARG7 gene against deletion of transforming DNA and would provide single-copy probes for Southern analysis, if sequences needed for plasmid rescue were lost. In the uni3–1 strain, a single insertion of the transforming DNA was observed using pBR329 DNA as the probe (diagrammed in Figure Figure3B).3B). Southern blot analysis of the insertion was performed using pBR329 and γ-tubulin sequences as probes. The 2.5 kb at the right end of the plasmid as diagrammed contain the ampicillin resistance gene and the origin of replication; these sequences were deleted in the uni3–1 strain. This precluded the possibility of using plasmid rescue for cloning the DNA adjacent to the site of insertion. The left end was intact and was used for constructing a size-selected library (see MATERIALS AND METHODS).
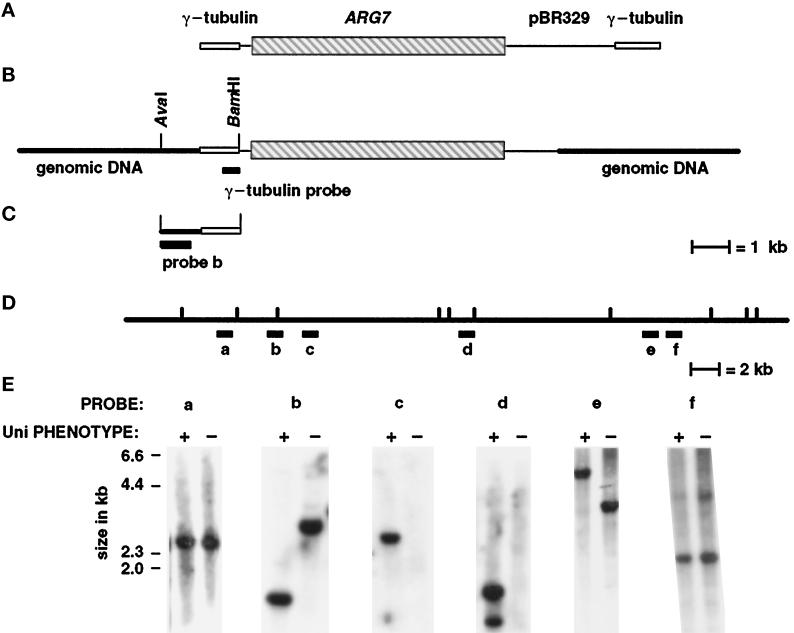
Diagram of the pΔγ-1 vector, the genomic insertion site, the size-selected genomic DNA clone with flanking sequences, and demonstration that uni3–1 is a deletion allele. (A) The vector pΔγ-1 carries the ARG7 gene (Debuchy et al., 1989), which is diagrammed as the striped box, and pBR329 sequences, which are diagrammed as black lines. At the ends, when the plasmid is linearized with EcoRI, are 1049-bp and 750-bp segments of the Chlamydomonas γ-tubulin gene, which are diagrammed as open boxes. (B) The orientation of the plasmid at the insertion site in uni3–1 cells. Most of the pBR329 and all of the γ-tubulin sequences were deleted from the right side as diagrammed. The 1,049 bp of γ-tubulin sequences from the left side of the pΔγ-1 plasmid remained in the transformant. (C) The cloned genomic DNA from the size-selected library made from AvaI–BamHI digested DNA from uni3–1 cells. Probe b was used to screen the phage libraries and contains single-copy DNA from the site of the insertion. (D) Restriction map of uni3–1 and wild-type DNA with the enzyme NheI. The location of probes a–f on the wild-type map are shown. Probe b in parts C and D are the same probe. (E) Southern blots of genomic DNA from uni3–1 and wild-type cells hybridized with probes a–f. The left lane in each panel contains wild-type DNA (+) and the right lane contains uni3–1 DNA (−).
A 2.3-kb fragment with approximately 1.3 kb of flanking genomic DNA was cloned from the size-selected library made from BamHI–AvaI-digested uni3–1 DNA using a short γ-tubulin sequence as the probe (Figure (Figure3C).3C). A 870-bp AvaI–SacI fragment derived from the flanking genomic sequence recognized a restriction fragment length polymorphism (RFLP) between the uni3–1 strain and the parental strain. This RFLP was linked to the uni3–1 phenotype in 16 meiotic progeny from a cross of uni3–1 and a wild-type strain. These data support the idea that genomic DNA flanking the insertion site was cloned. This 870-bp genomic DNA was used as a probe to begin a chromosome walk in two independent genomic DNA phage libraries (see MATERIALS AND METHODS).
Four overlapping λ clones that span 54 kb of wild-type DNA were obtained from the walk that began with the 870-bp probe (labeled probe b in Figure Figure3,3, C and D). The structure of the uni3–1 mutation was determined by hybridizing genomic DNA from wild-type and uni3–1 cells with single-copy probes from various points along the walk (Figure (Figure3E).3E). The uni3–1 hybridization patterns detected by probes a and f were indistinguishable from the wild-type patterns. For probe b, as noted above, and for probe e, RFLPs were observed. Each RFLP presumably occurred because a restriction enzyme site was missing or altered in the mutant DNA compared with the wild-type DNA (probes b and e, Figure Figure3E).3E). For probes c and d, no signal was detected in the uni3–1 mutant DNA. Based on these data as well as Southern blots from several other probes between b and c that are not shown, we estimated that a 27-kb deletion is present in the uni3–1 strain compared with the DNA from wild-type or parental strains.
Rescue of the uni3–1 Strains by Transformation
To identify the UNI3 gene, we cotransformed a uni3–1 nit1–1 NIT2 strain with pMN56, a plasmid carrying the NIT1 gene (Nelson and Lefebvre, 1995), and one of the four overlapping phage (λCU-1, λCU-2, λCU-3, and λCU-4) found in the walk (Figure (Figure4A).4A). We selected for the presence of the NIT1 gene on medium containing sodium nitrate as the sole nitrogen source; 64, 148, 211, and 31 Nit1+ transformants were generated in these experiments with λCU-1, λCU-2, λCU-3, and λCU-4, respectively. Because the frequency of cotransformation is often low (Ferris and Goodenough, 1994), we expected only a subset of transformants to show rescue of the Uni− phenotype. These rescued cells would be biflagellate and able to swim; 4.7% of the cotransformants with λCU-2 and λCU-3 DNAs restored wild-type function, while no rescued strains were observed with λCU-1 or λCU-4 DNAs. Light microscopic analysis of the rescued strains revealed wild-type or nearly wild-type distributions of biflagellate, uniflagellate, and aflagellate cells and wild-type frequencies of cells with cleavage defects (n = 500). The presence of transforming DNA was confirmed by Southern blots of genomic DNA from 17 rescued transformants. Genomic DNA from transformants with the nonrescuing clones, λCU-1 or λCU-4, was also examined by Southern blots. The λ DNA was incorporated at a similar frequency, but without rescue of the mutant phenotype. Phage λCU-2 and λCU-3 overlapped each other by 9.5 kb, and we conclude that this overlapping region is sufficient for rescuing the Uni− phenotype. Furthermore, this result demonstrates that the uni3–1 allele is a null mutation as the region needed for rescue is deleted in the uni3–1 allele.
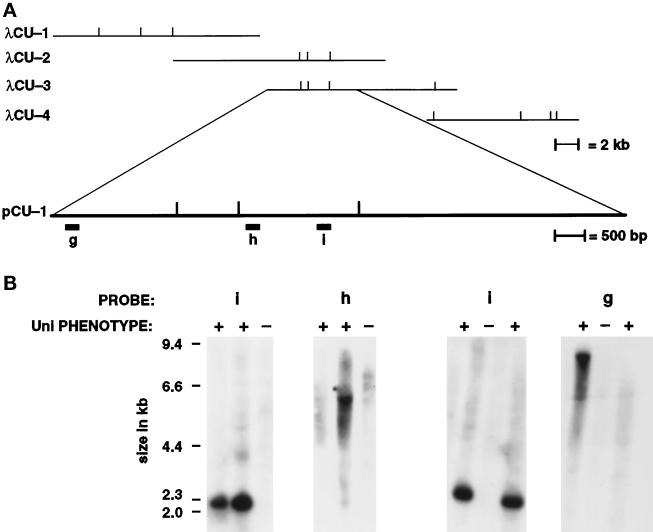
The λ phage and plasmids used for transformation rescue and in vivo deletion analysis. (A) Phage λCU-1, λCU-2, λCU-3, and λCU-4 were obtained in the chromosome walk and were used for assaying rescue after transformation. The vertical bars indicate the recognition sites for the enzyme NheI. The subclone pCU-1 showed rescue of the Uni− phenotype. (B) Southern blots of phenotypically rescued uni3–1 and control strains. Probes g, h, and i were hybridized against DNA from the rescued strains (+) and control uni3–1 strains (−). Probes g and h are found in the same NheI fragment as probe d in Figure Figure3D.3D. Probe i was present in all strains shown as well as 13 other rescued strains. Probes g and h were present in some, but not all, of the rescued strains. The loss of portions of the transforming DNA in rescued strains was used to further delineate the DNA needed for the restoration of the wild-type phenotype to ~5.5 kb.
We took advantage of in vivo deletions of transforming DNA to further define the sequences needed for rescue. Using probes g, h, and i, we found that only 5.5 kb of DNA were needed to rescue the mutant phenotypes (Figure (Figure4B).4B). Probes g and h were present in some, but not all, rescued transformants as shown in Figure Figure4B.4B. Signal from probe i was present in all 17 strains with a rescued phenotype. Plasmid pCU-2, which has a 5.68-kb insertion, also rescued the mutant phenotype.
The UNI3 Gene Encodes a Novel Tubulin
The 5681 bp of genomic DNA needed for rescue was sequenced, and a single-copy cDNA was isolated from a library made from nitrogen-starved cells after screening multiple cDNA libraries (see MATERIALS AND METHODS). The cDNA sequence appeared to be incomplete and was extended using RT-PCR; 133 bp of the suspected coding region and 90 bp of the noncoding 5′ region were found by RT-PCR. The predicted protein has 534 amino acids with a predicted molecular weight of 55,806 and a pI of 6.6. Comparison of the 5.7 kb of genomic DNA needed for rescue and 1.97 kb of cDNA/RT-PCR sequences suggested that 10 exons and 9 introns are present (Figure (Figure5).5). Each exon–intron boundary is marked by the consensus for Chlamydomonas introns (LeDizet and Piperno, 1995; Figure Figure5).5). Many genes in Chlamydomonas show extreme codon bias (reviewed in LeDizet and Piperno, 1995). We observed a similar bias toward codons that have a G or C in the third position (88.2%).
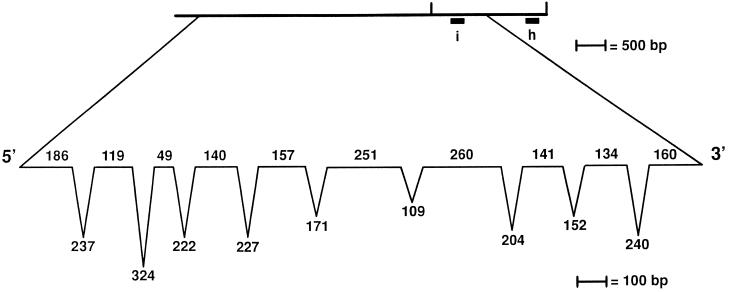
Exon-intron distribution in the UNI3 gene. The length of the exons (on the line) and introns (below the line) are indicated and were determined based on comparisons of cDNA and RT-PCR products with the genomic sequence. At the exon–intron boundaries, we find the sequence G GT[GA] where the boundary is indicated by a vertical line (
GT[GA] where the boundary is indicated by a vertical line ( ) and at intron-exon boundaries, we find the sequence CAG
) and at intron-exon boundaries, we find the sequence CAG [GC]. Both of these sequences agree with the consensus for Chlamydomonas intron boundaries (LeDizet and Piperno, 1995).
[GC]. Both of these sequences agree with the consensus for Chlamydomonas intron boundaries (LeDizet and Piperno, 1995).
Comparison of DNA and protein from various databases and the predicted amino acid sequence of Uni3p using several different sequence analysis algorithms suggested that this rescuing fragment encoded a tubulin (Table (Table2).2). Using the database search programs BLAST or FASTA, the first several hundred matches included only complete or partial fragments of tubulin. Using the motif search programs PROSITE or BLOCKS, the predicted amino acid sequence met the criteria for a tubulin. Using the profile method Pfam-AHMM, the predicted sequence had a high probability of being a tubulin (Table (Table2).2).
Table 2
Search algorithms used on Uni3p sequence
| Algorithm | Result | Reference |
|---|---|---|
| BLAST | First 398 sequences retrieved were tubulins. Best match: sum (P) = 6.2 × 10−41 | Altschul et al. (1990) |
| FASTA | First 190 sequences retrieved were tubulins. Best match: Z score = 1.2 × 10−21 | Pearson and Lipman (1988) |
| BLOCKS | Match for 4/4 tubulin BLOCKS. Score = 1446 (P) < 1.3 × 10−9 | Henikoff and Henikoff (1994) |
| PROSITE | Match only to tubulin site [SAG]-G-G-T-G-[SA] | Bairoch (1993) |
| Pfam-AHMM | (P) = 2−140 of alignment to 198 full-length tubulin sequences by chance | Sonnhammer et al. (1997) |
The predicted amino acid sequence of the UNI3 gene does not correspond to the two identical α-tubulin proteins (James et al., 1993), the two identical β-tubulin proteins (Youngblood et al., 1984), or the γ-tubulin from Chlamydomonas (Vassilev et al., 1995 and Figure Figure6).6). The predicted Uni3 protein (Uni3p) is 23%, 26%, and 27% identical to Chlamydomonas α-, β-, and γ-tubulin, respectively. The motif [SAG]GGTG[AG], which is the PROSITE motif for tubulins, is present in the predicted Uni3p as are other conserved regions indicated by shaded boxes in Figure Figure6.6. The sequence analysis and the lack of identity with the known tubulins of Chlamydomonas suggests that the Uni3 protein may represent a new member of the tubulin superfamily.
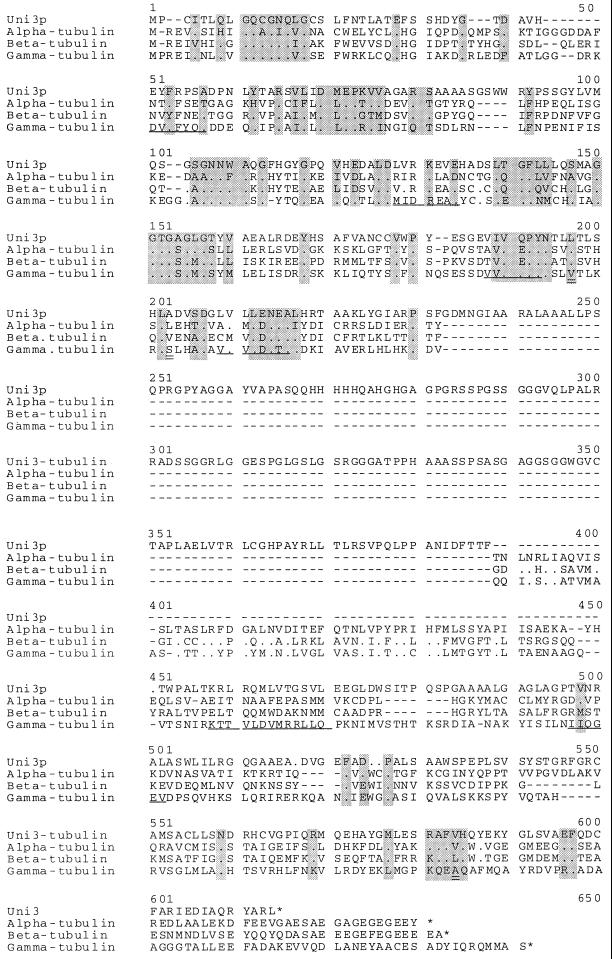
Comparison of the predicted Uni3p with α-, β-, and γ-tubulin from Chlamydomonas. The sequences were aligned using the Pfam method (Sonnhammer et al., 1997). The γ-tubulin sequence was generated in our laboratory (see MATERIALS AND METHODS). At the amino acid level, this γ-tubulin sequence (GenBank Accession number AF013109) differs at three amino acids positions from the GenBank entry U31545 of γ-tubulin (C. D. Silflow, University of Minnesota, St. Paul). Using the numbering in U31545, the changes are V190L, S196T, and A411R. These amino acids are indicted by double underlines in this Figure at positions 197, 203, and 584. Gaps introduced by the alignment program to optimize the alignment are indicated by (−). Identity of amino acids is indicated by (.) and a stop codon is indicated by (*). There is greater similarity/identity in the amino terminus than in the carboxy terminus. The GenBank accession number for Uni3 tubulin is AF013108.
We have also compared Uni3p by domains with other tubulins (Burns, 1995). We find that the first 217 amino acids of the N-terminal region, which are encoded by the first five exons, were more similar to the γ-tubulins than they were to other tubulins. However, the Uni3p sequence is lacking some of the consensus sequences considered diagnostic of a γ-tubulin. These conserved γ-tubulin motifs are DVFFYQ at position 51, M/IIDREAE/D at position 128, VVVQPYN at position 188, VVVLDN at position 209, KTTVLDVMRLL at position 458, and IIQGEA at position 497 (Figure (Figure6);6); they are not present in Uni3p. The last 145 amino acids, which are encoded by the last three exons, were more similar to the β-tubulins than to the other tubulins. The amino acids from 391 to 450 (as numbered in Figure Figure6)6) in the Chlamydomonas α-, β-, and γ-tubulins are 30% identical, but there is no corresponding region in Uni3p. Comparison of UNI3 exons 6 and 7 failed to find any significant sequence identity in SwissProt or GenBank. However, these exons showed the same codon bias and dinucleotide frequencies as observed in other exons.
To further investigate the relationship of the Uni3 protein to the three known groups of the tubulin superfamily, we constructed phylogenetic trees using tubulins from a wide range of eukaryotic organisms (Figure (Figure7).7). For the tree shown, the alignment was made using Pileup (Devereux et al., 1984), and the phylogeny was constructed using the Fitch-Margoliash method (1967). We observed clustering of the α-, β-, and γ-tubulins, as expected. However, the Uni3p sequence was equally distant from the other three groups of the tubulin superfamily.
Included in the phylogenetic tree analysis were sequences of TUB4 from Saccharomyces cerevisiae (Sobel and Synder, 1995) and a gamma-like tubulin from Caenorhabditis elegans (Sulston et al., 1992). These two genes were found in genome-wide sequencing efforts. Different groups have suggested that these genes encode either γ-tubulins (Sobel and Synder, 1995) or new members of the tubulin superfamily (Burns, 1995). In all of the trees that we constructed with Uni3p, the yeast and worm sequences fell within the cluster of γ-tubulin sequences, but had longer branch lengths than other γ-tubulin sequences (Figure (Figure7).7). This result suggested that these tubulins are bona fide γ-tubulins, but are more divergent. This interpretation agrees with the analysis of Keeling and Doolittle (1996).
We have also constructed trees using UGPMA, neighbor-joining, and parsimony methods with alignments from CLUSTAL-W (Thompson et al., 1994) and Pfam (Sonnhammmer et al., 1997). All trees generated were qualitatively the same; the Uni3p branch was always separated from the other three groups although the absolute length of the branches differed with other methods (our unpublished observations). We also constructed trees with the amino acids encoded by exons 6 and 7 of Uni3 removed and a similar number of amino acids from the other tubulins removed to determine whether the trees were strongly influenced by this nontubulin-like region. The trees were qualitatively unaffected by the absence of these sequences. Thus, it appears that the UNI3 gene encodes a new member of the tubulin superfamily.
DISCUSSION
Chlamydomonas cells are normally biflagellate, but in the presence of mutations in any of three genes (UNI1, UNI2, or UNI3), an increased percentage of cells assemble zero or one flagellum. The uniflagellate cells share another property. In uniflagellate cells, the single flagellum is found on the basal body opposite the eyespot, which is the older of the two basal bodies in wild-type cells (Holmes and Dutcher, 1992). These mutations allow us to distinguish between the assembly properties of the two basal bodies, which in wild-type cells appear coordinated. This flagellar assembly phenotype in combination with the disrupted morphology of the basal bodies, as observed by electron microscopy, suggest that the Uni+ gene products may be required for basal body assembly.
By electron microscopy, basal bodies from uni3–1 cells have a unique phenotype. Whereas in wild-type Chlamydomonas cells, the proximal portion of the basal bodies consist of a blade of three microtubules (Ringo, 1967; Johnson and Porter, 1968; Goodenough and Weiss, 1978), in uni3–1 cells there was an absence of triplet microtubules in the basal bodies. The C tubule, in particular, appears to be missing. Triplet microtubules are considered a hallmark of centrioles and basal bodies (reviewed by Stubblefield and Brinkley, 1967). However, very few images of triplet microtubules have been published (Fulton, 1971). Centrioles have been recently observed in Drosophila melanogaster that have primarily singlet and some doublet microtubules during the syncytial divisions (McDonald and Morphew, 1993; Callaini et al., 1997), in pole cell centrosomes (McDonald, personal communication), and in isolated centrosomes from embryos (Moritz et al., 1995), but triplet microtubules have been observed during oogenesis (Mahowald and Strassheim, 1970) and spermatogenesis. Clearly, not all cells require centrioles with triplet microtubules. However, it may be that triplet microtubules are required in basal bodies to nucleate flagella. It will be interesting to determine whether triplet microtubules in centrioles are needed in other cell types in which centrioles may have a specialized function.
The ultrastructural phenotype of uni3–1 basal bodies suggests several possible locations for Uni3p. We have recently obtained a polyclonal serum against the Uni3p and find that it localized to the basal bodies by indirect immuofluorescence (Dutcher and Preble, manuscript in preparation). Thus, Uni3p could act as a seed for the C tubule, as a protofilament in the seam of the C and B tubules, or as a major component of the C tubule. Immunoelectron microscopy will be needed to address the localization.
The uni3–1 cells have another phenotype that suggests they have a defect in basal body function. We had observed previously that the correct placement of the cleavage furrow and mitotic spindle was lost in bld2–1 cells, which fail to assemble basal bodies (Ehler et al., 1995). The uni3–1 cells, but not the uni1–1 or uni2–1 cells, have misplaced cleavage furrows with respect to the mitotic spindles. This phenotype suggests that different aspects of basal body function may be altered in the various uni− mutants. Recently, a tagged allele at the UNI2 locus was obtained. The gene was cloned, but it has no similarity to other genes in the database (Wu, Tam, Lefebvre, and Silflow, personal communication).
Uni3p Defines a New Member of the Tubulin Superfamily
The UNI3 gene encodes a protein that represents a new class of tubulins, which clearly differs from the well known α-, β-, and γ-tubulins (Figures (Figures66 and and7).7). The Uni3 tubulin differs from other tubulins in several ways. First, a cell with a deletion of the UNI3 gene is viable; this gene is not essential. Null alleles in single-copy α-, β-, or γ-tubulin genes in a variety of organisms have lethal phenotypes (reviewed by Cabral et al., 1984; Huffaker et al., 1987). Southern blots of genomic DNA with genomic or cDNA UNI3 probes did not reveal any additional hybridization signals, even under conditions of reduced stringency (Preble and Dutcher, work in progress). This result suggests that there are not additional genes with similar sequence that may provide redundant function. A second difference is the presence of two additional exons in the UNI3 gene that encode a region not present in other known tubulins. The function of this region in the protein is unknown. These two exons contain a histidine-rich region and many glycine and proline residues.
The UNI3 gene does not appear to be unique to Chlamydomonas as we have found a sequence in the mouse EST database that is likely to be a homolog. Clone 371244 (Accession no. W53427) from 13.5–14.5 d mouse embryos is 62%/51% similar/identical to Uni3p over 129 amino acids. This clone is also 43% identical to human γ-tubulin, but lacks several motifs conserved in γ-tubulins. We are currently sequencing the entire EST to determine the extent of similarity.
Burns (1995) proposed that new tubulin genes found in the sequencing projects of C. elegans and S. cerevisiae are each new members of the tubulin superfamily and named them δ and ε, respectively. As illustrated in Figure Figure7,7, these sequences are found in the branch with other γ-tubulin sequences, but are more divergent. The phylogenetic analysis of Keeling and Logsdon (1996) suggested that these genes represent divergent members of the γ-tubulin family rather than founding members of new subfamilies. Furthermore, functional analysis suggests that the TUB4 gene in S. cerevisiae is essential and encodes γ-tubulins (Spang et al., 1996). Tub4p localizes to the spindle pole body, which is the MTOC equivalent in yeast (Sobel and Synder, 1995; Marschall et al., 1996; Spang et al., 1996). The yeast genome is completely sequenced, and no tubulin genes in addition to the known α- and β-tubulin genes were found (J.M. Cherry, C. Adler, C. Ball, S. Dwight, S. Chervitz, Y. Jia, G. Juvik, S. Weng, and D. Botstein [1996], Saccharomyces genome database, http://genome-www.stanford.edu/Saccharomyces/). In C. elegans, no other γ-tubulin gene has been identified by the sequencing project, which is about 80% completed. The C. elegans γ-like tubulin gene is found in the emb-30 operon, but definitive evidence for an embryonic lethal mutation in the γ-like tubulin gene is not yet available (Tabish, Khan, and Siddiqui, personal communication). The essential nature of the γ-tubulin gene in A. nidulans (Oakley et al., 1990), Schizosaccharomyces pombe (Stearns et al., 1991; Horio et al., 1991), or D. melanogaster (Sunkel et al., 1995), as well as yeast, is in contrast to the nonessential nature of the UNI3 gene. This difference underscores the conclusion that Uni3 tubulin is unique compared with other known tubulins.
Recently another gene with a tubulin motif has been described. The Misato gene in D. melanogaster encodes a protein with both tubulin-like and myosin-like motifs (Miklos et al., 1997). A null mutation in Misato results in flies with irregular chromosome segregation. Misato is no more similar to Uni3p than it is to other tubulins.
Centriole/Basal Body Maturation Requires Multiple Cell Cycles
Differences between old and new centrioles and basal bodies have been documented in a variety of organisms. Centriole/basal body maturation may be involved in promoting different structures and functions for the old (parental) and new (daughter) centrioles. In pig epithelial cells, an electron-dense halo surrounds the parental centriole during metaphase, and this centriole is perpendicular to the spindle axis. In interphase, the parental centriole has appendages, but the daughter centriole does not. This maturation requires more than one cell cycle to achieve (Vorobjev and Chentsov, 1982). In PtK2 cells, morphological differences between the two centrioles have also been observed (Reider and Borisy, 1982; Paintrand et al., 1992). In many biflagellate algal cells, the two flagella differ from one another in size, structure, and function (Melkonian et al., 1987), although, to date, no morphological differences in basal bodies have been noted in green algae. The progression of flagellar morphology in these algae is postulated to require the maturation of the basal bodies that template the flagella. For example, in Pleurochrysis carterae, the two flagella are functionally and structurally dissimilar. This heterogeneity requires three generations to achieve. A short, nonhairy flagellum is associated with the daughter basal body, a longer, nonhairy flagellum is associated with the parental basal body, and a longer, hairy flagellum is associated with the grandparental basal body (Beech et al., 1988). The pattern of staining of the monoclonal antibody against the protein cenexin also suggests that this centriolar epitope may require more than one generation to be present on the parental centriole (Lange and Gull, 1995).
Our pedigree analysis of flagellar assembly in the uni3–1 mutant strain is consistent with the requirement for three generations for maturation events. In the absence of the Uni3 gene product, the predominant class is aflagellate cells. We propose that these aflagellate cells contain a new basal body and a basal body that has just become a parent. This pair of basal bodies, in the absence of the Uni3 gene product, cannot assemble flagella. When a parental basal body completes another cell cycle, the pair of basal bodies assembles a single flagellum on the older basal body. When the basal body has participated in at least three cell cycles, the cell assembles two flagella.
One explanation for the pattern of flagellar assembly that we observe in the pedigree studies is that another gene product promotes assembly in the basal body pairs that have a two-generation- or three-generation-old basal body. However an alternate explanation for the pedigree observations is that the Uni3p is required for the normal segregation of basal bodies. In wild-type cells, a parental and a daughter basal body always go to each pole. If two parental basal bodies go to one pole and the two new daughter basal bodies go to the other pole, this could generate the biflagellate and aflagellate class. The uniflagellate class could arise from segregation of a parental and daughter basal body from the other parental and daughter, as we observed in uni1–1 cells (Holmes and Dutcher, 1989). This would require that two alternative segregation patterns occurred with reproducible frequencies.
Is there a reason for a cell to distinguish between grandparent, parent, and daughter centrioles? If these centrioles play roles in establishing asymmetries in the cell, then perhaps centrosomes with different levels of maturity of their centrioles could provide positional information for the cell that could be used in orienting spindles, cleavage furrows or other cellular components. For example, the PIE-1 protein is initially localized to both centrosomes, but then disappears from the centrosome that will be in the cell destined to be a somatic cell (Mello et al., 1996). It is possible that that differences in the age of the centrosomes could contribute.
ACKNOWLEDGMENTS
We are grateful for the genorosity and kindness of Dr. Ursula Goodenough who volunteered to do electron microscopy on uni3–1 cells. This manuscript would have never seen the light of day without her help. We thank Rogene Schnell, Andy Wang, and Jeff Woessner for cDNA libraries, Jean-David Rochaix and Saul Purton for the pARG7.8 plasmid, David Johnson and Anthony Palombella for genomic phage libraries, and Andrea Preble for Southern blots with cDNA probes. We thank Gary Stormo for advice on sequence and phylogenetic analysis and Shmuel Pietrokovski, Steve Henikoff, and Sean Eddy for their help. We thank Scott Schuyler and Tom Giddings for the electron microscopic analysis of longitudinal sections. We thank Sylvia Fromherz, Anthony Palombella, Andrea Preble, and Gary Stormo for their discussion and critical reading of this manuscript. This work was supported by a grant from the National Institutes of Health (GM-32843). S.K.D. was supported in part by a University of Colorado Faculty Fellowship from the Committee on Creative Work and Research. The Perkin Elmer-Cetus thermal cycler was a gift from the Colorado Chapter of the American Cancer Society.
REFERENCES
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. [Abstract] [Google Scholar]
- Bairoach A. The PROSITE dictionary of sites and patterns in proteins, its current status. Nucleic Acids Res. 1993;21:3097–3103. [Europe PMC free article] [Abstract] [Google Scholar]
- Beech PL, Wetherbee R, Pickett-Heaps JD. Transformation of the flagella and associated flagellar components during cell division in the Coccolithophorid Pleurochysis carterie. Protoplasma. 1988;145:37–46. [Google Scholar]
- Burns RG. Identification of two new members of the tubulin family. Cell Motil Cytoskeleton. 1995;31:255–258. [Abstract] [Google Scholar]
- Cabral F, Schibler M, Kuriyama R, Abraham I, Whitfield C. Genetic analysis of microtubule function in CHO cells. In: Borisy GG, Cleveland DW, Murphy DB, editors. Molecular Biology of the Cytoskeleton. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1984. pp. 305–317. [Google Scholar]
- Callaini G, Whitfield WG, Riparbelli MG. Centriole and centrosome dynamics during the embryonic cell cycles that follow the formation of the cellular blastoderm in Drosophila. Exp Cell Res. 1997;234:183–190. [Abstract] [Google Scholar]
- Debuchy R, Purton S, Rochaix J-D. The argininosuccinate lyase gene of Chlamydomonas reinhardtii: an important tool for nuclear transformation and for correlating the genetic and molecular maps of the ARG7 locus. EMBO J. 1989;8:2803–2809. [Europe PMC free article] [Abstract] [Google Scholar]
- Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. [Europe PMC free article] [Abstract] [Google Scholar]
- Dutcher SK. In: Genetic properties of linkage group XIX in Chlamydomonas reinhardtii. In: Extrachromosomal Elements in Lower Eukaryotes. Wickner RB, Hinnebusch A, Lambowitz AM, Gunsalus IC, editors. A. Hollaender, New York: Plenum Press; 1986. pp. 303–325. [Abstract] [Google Scholar]
- Dutcher SK. Mating and tetrad analysis in Chlamydomonas reinhardtii. In: Dentler W, Witman G, editors. Cilia and Flagella. San Diego, CA: Academic Press; 1995a. pp. 531–540. [Abstract] [Google Scholar]
- Dutcher SK. In: Purification of basal bodies and basal body complexes from Chlamydomonas reinhardtii. In: Cilia and Flagella. Dentler W, Witman G, editors. San Diego, CA: Academic Press; 1995b. pp. 325–329. [Google Scholar]
- Ehler LL, Holmes JA, Dutcher SK. Loss of spatial control of the mitotic spindle apparatus in a Chlamydomonas reinhardtii mutant strain lacking basal bodies. Genetics. 1995;141:945–960. [Europe PMC free article] [Abstract] [Google Scholar]
- Félix M-A, Antony C, Wright M, Maro B. Centrosome assembly in vitro role of γ-tubulin recruitment in Xenopus sperm aster formation. J Cell Biol. 1994;124:19–31. [Europe PMC free article] [Abstract] [Google Scholar]
- Felsenstein J. Inferring phylogenies from protein sequences by parsimony, distance, and likelihood methods. Methods Enzymol. 1996;266:418–427. [Abstract] [Google Scholar]
- Fernández E, Schnell R, Ranum LP, Hussey SC, Silflow CD, Lefebvre PA. Isolation and characterization of the nitrate reductase gene of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1989;86:6449–6453. [Europe PMC free article] [Abstract] [Google Scholar]
- Ferris PJ, Goodenough UW. The mating-type locus of Chlamydomonas reinhardtii contains highly rearranged DNA sequences. Cell. 1994;76:1136–1145. [Abstract] [Google Scholar]
- Fulton C. Centrioles. In: Reinert J, Ursprung H, editors. Origin and Continuity of Cell Organelles. New York, NY: Springer-Verlag; 1971. pp. 170–221. [Google Scholar]
- Fitch WM, Margoliash E. Construction of phylogenetic trees. Science. 1967;155:279–284. [Abstract] [Google Scholar]
- Foster KW, Smyth RD. Light antennas in phototactic algae. Microbiol Rev. 1981;44:572–636. [Europe PMC free article] [Abstract] [Google Scholar]
- Goodenough UW, Weiss RL. Interrelationships between microtubules, a striated fiber, and the gametic mating structure of Chlamydomonas reinhardtii. J Cell Biol. 1978;76:430–438. [Europe PMC free article] [Abstract] [Google Scholar]
- Gumpel NJ, Rochaix J-D, Purton S. Studies on homologous recombination in the green alga Chlamydomonas reinhardtii. Curr Genet. 1994;26:438–442. [Abstract] [Google Scholar]
- Harris EH. The Chlamydomonas Sourcebook. San Diego, CA: Academic Press; 1989. [Google Scholar]
- Henikoff S, Henikoff GJ. Protein family classification based on searching a database of “blocks.” Genomics. 1994;19:97–107. [Abstract] [Google Scholar]
- Holmes JA, Dutcher SK. Cellular asymmetry in Chlamydomonas reinhardtii. J Cell Sci. 1989;94:273–283. [Abstract] [Google Scholar]
- Holmes JA, Dutcher SK. Genetic approaches to the study of cytoskeletal structure and function in Chlamydomonas. In: Menzel D, editor. The Cytoskeleton of the Algae. Boca Raton, FL: CRC Press; 1992. pp. 347–367. [Google Scholar]
- Holmes JA, Johnson DE, Dutcher SK. Linkage group XIX of Chlamydomonas reinhardtii has a linear map. Genetics. 1991;133:865–874. [Europe PMC free article] [Abstract] [Google Scholar]
- Horio T, Uzawa S, Jung MK, Oakley BR, Tanaka K, Yanagida M. The fission yeast gamma-tubulin is essential for mitosis and is localized at microtubule organizing centers. J Cell Sci. 1991;99:693–700. [Abstract] [Google Scholar]
- Horst CJ, Witman GB. ptx1, a nonphototactic mutant of Chlamydomonas, lacks control of flagellar dominance. J Cell Biol. 1993;120:733–741. [Europe PMC free article] [Abstract] [Google Scholar]
- Huffaker TC, Hoyt AM, Botstein D. Genetic analysis of the yeast cytoskeleton. Annu Rev Genet. 1987;21:259–284. [Abstract] [Google Scholar]
- Huang B, Ramanis Z, Dutcher SK, Luck DJL. Uniflagellar mutant of Chlamydomonas: evidence for the role of basal bodies in the transmission of positional information. Cell. 1982;29:745–753. [Abstract] [Google Scholar]
- James SW, Silflow CD, Lefebvre PA. A mutation in the α1-tubulin gene of Chlamydomonas reinhardtii confers resistance t anti-microtubule herbicides. J Cell Sci. 1993;106:209–218. [Abstract] [Google Scholar]
- Johnson DE, Dutcher SK. Molecular studies of linkage group XIX of Chlamydomonas reinhardtii: evidence against a basal body location. J Cell Biol. 1991;113:339–346. [Europe PMC free article] [Abstract] [Google Scholar]
- Johnson UG, Porter KR. Fine structure of cell division in Chlamydomonas reinhardtii. Basal bodies and microtubules. J Cell Biol. 1968;38:403–425. [Europe PMC free article] [Abstract] [Google Scholar]
- Keeling PJ, Doolittle WF. α-Tubulin from early-diverging eukaryotic lineages and the evolution of the tubulin family. Mol Biol Evol. 1996;3:1297–1305. [Abstract] [Google Scholar]
- Keeling PJ, Logsdon JM., Jr Highly divergent Caenorhabditis and Saccharomyces tubulins evolved recently from genes encoding γ-tubulin. Trends Cell Biol. 1996;6:375. [Abstract] [Google Scholar]
- Kindle KL. High frequency nuclear transformation of Chlamydomnas reinhardtii. Proc Natl Acad Sci USA. 1990;87:1228–1232. [Europe PMC free article] [Abstract] [Google Scholar]
- King SJ, Dutcher SK. Chlamydomonas reinhardtii phototactic mutant strains reveal a function of phosphoregulation of an inner dynein arm complex. J Cell Biol. 1997;136:177–191. [Europe PMC free article] [Abstract] [Google Scholar]
- Kochanski RS, Borisy GG. Mode of centriole duplication and distribution. J Cell Biol. 1990;110:1599–1605. [Europe PMC free article] [Abstract] [Google Scholar]
- Lange B, Gull K. A molecular marker for centriole maturation in the mammalian cell cycle. J Cell Biol. 1995;130:919–927. [Europe PMC free article] [Abstract] [Google Scholar]
- LeDizet M, Piperno G. ida4–1, ida4–2, and ida4–3 are intron splicing mutations affecting the locus pf28, a light chain of Chlamydomonas axonemal inner dynein arms. Mol Biol Cell. 1995;6:713–723. [Europe PMC free article] [Abstract] [Google Scholar]
- Lux FG, III, Dutcher SK. Genetic interactions at the FLA10 locus: suppressors and synthetic phenotypes that affect the cell cycle and flagellar function in Chlamydomonas reinhardtii. Genetics. 1991;128:549–561. [Europe PMC free article] [Abstract] [Google Scholar]
- Mahowald AP, Strassheim JM. Intercellular migration of centrioles in the germarium of Drosophila melanogaster. An electron microscopic study. J Cell Biol. 1970;45:306–320. [Europe PMC free article] [Abstract] [Google Scholar]
- Marschall L, Jeng RL, Mulholland J, Stearns T. Analysis of Tub4p, a yeast gamma-like protein: implications for microtubule-organizing center function. J Cell Biol. 1996;134:443–454. [Europe PMC free article] [Abstract] [Google Scholar]
- McDonald K, Morphew MK. Improved preservation of ultrastructure in difficult-to-fix organisms by high pressure freezing and freeze substitution: I. Drosophila melanogaster and Strongylocentrus purpuratus embryos. Microsc Res Tech. 1993;24:465–473. [Abstract] [Google Scholar]
- Melkonian M, Reize IB, Preising HR. Maturation of a flagellum/basal body requires more than one cell cycle in algal flagellates: studies on Nephroselmis olivacea (Prasinphyceae) In: Wiessner W, Robinson DG, Starr RC, editors. Algal Development. Berlin: Springer-Verlag; 1987. pp. 102–113. [Google Scholar]
- Mello CC, Schubert C, Draper B, Zhang W, Lobel R, Priess JR. The PIE-1 protein and germline specification in C. elegans embryos. Nature. 1996;382:710–712. [Abstract] [Google Scholar]
- Miklos GL, Yamamoto M, Burns RG, Maleszka R. An essential cell division gene of Drosophila, absent from Saccharomyces, encodes an unusual protein with tubulin-like and myosin-like peptide motifs. Proc Natl Acad Sci USA. 1997;94:5189–5194. [Europe PMC free article] [Abstract] [Google Scholar]
- Moestrup Ø. Flagellar structure in algae: a review, with new observations particularly on the Chrysophyceae, Phaeophyceae (Fucophyceae), Euglenophyceae and Reckertia. Phycologia. 1982;21:427–528. [Google Scholar]
- Moritz M, Braunfeld MB, Fung Jennifer, C, Sedat JW, Alberts BM. Three-dimensional structural characterization of centrosomes from early Drosophila embryos. J Cell Biol. 1995;130:1149–1159. [Europe PMC free article] [Abstract] [Google Scholar]
- Myster S, Knott J, O’Toole E, Porter ME. The Chlamydomonas Dhc1 gene encodes a dynein heavy chain subunit involved in the assembly of an inner dynein arm. Mol Biol Cell. 1997;8:607–620. [Europe PMC free article] [Abstract] [Google Scholar]
- Nelson JA, Lefebvre PA. Targeted disruption of the NIT8 gene in Chlamydomonas reinhardtii. Mol Cell Biol. 1995;15:5762–5769. [Europe PMC free article] [Abstract] [Google Scholar]
- Oakley CE, Oakley BR. Identification of gamma-tubulin, a new member of the tubulin superfamily encoded by mipA of Aspergillus nidulans. Nature. 1989;338:662–664. [Abstract] [Google Scholar]
- Oakley BR, Oakley CE, Yoon Y, Jung MK. Gamma-tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergillus nidulans. Cell. 1990;61:1289–1301. [Abstract] [Google Scholar]
- Paintrand M, Moudour M, Delacroix H, Bornens M. Centrosome organization and centriole architecture: their sensitivity to divalent cations. J Struct Biol. 1992;108:107–128. [Abstract] [Google Scholar]
- Pazour GJ, Sineshchekov OA, Witman GB. Mutational analysis of the phototransduction pathway of Chlamydomonas reinhardtii. J Cell Biol. 1995;131:427–440. [Europe PMC free article] [Abstract] [Google Scholar]
- Pearson WR, Lipman DJ. Improved tools for biological sequence analysis. Proc Natl Acad Sci USA. 1988;85:2444–2448. [Europe PMC free article] [Abstract] [Google Scholar]
- Porter ME, Power J, Dutcher SK. Extragenic suppressors of paralyzed flagellar mutations in Chlamydomonas reinhardtii identify loci that alter the inner dynein arms. J Cell Biol. 1992;118:1163–1176. [Europe PMC free article] [Abstract] [Google Scholar]
- Ranum LP W, Thompson MD, Schloss JA, Lefebvre PA, Silflow CD. Mapping flagellar genes in Chlamydomonas using restriction fragment length polymorphisms. Genetics. 1988;120:109–122. [Europe PMC free article] [Abstract] [Google Scholar]
- Reider CL, Borisy GG. The centrosome cycle in PtK2 cells. Asymmetric distribution and structural changes in the pericentriolar material. Biol Cell. 1982;44:117–132. [Google Scholar]
- Ringo DL. Flagellar motion and fine structure of the flagellar apparatus in Chlamydomonas. J Cell Biol. 1967;13:383–391. [Europe PMC free article] [Abstract] [Google Scholar]
- Rüffer U, Nultsch W. Comparison of the beating of the cis- and trans-flagella of Chlamydomonas cells held on micropipettes: II. changes in flagellar beat patterns. Cell Motil. 1987;18:269–278. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Smith E, Lefebvre PA. PF16 encodes a protein with armadillo repeats and localizes to a single microtubule of the central apparatus in Chlamydomonas flagella. J Cell Biol. 1996;132:359–370. [Europe PMC free article] [Abstract] [Google Scholar]
- Sobel SG, Synder M. A highly divergent gamma-tubulin gene is essential for cell growth and proper microtubule organization in Saccharomyces cerevisiae. J Cell Biol. 1995;131:1775–1788. [Europe PMC free article] [Abstract] [Google Scholar]
- Sonnhammer ELL, Eddy SR, Durbin R. Pfam: a comprehensive database of protein families based on seed alignments. Proteins. 1997;28:405–420. [Abstract] [Google Scholar]
- Spang A, Geissler S, Grein K, Schiebel E. The γ-tubulin-like Tub4p of Saccharomyces cerevisiae is associated with the spindle pole body substructure that organizes microtubules and is required for mitotic spindle formation. J Cell Biol. 1996;134:429–441. [Europe PMC free article] [Abstract] [Google Scholar]
- Stearns T, Kirschner M. In vitro reconstitution of centrosome assembly and function: the central role of gamma-tubulin. Cell. 1994;76:623–637. [Abstract] [Google Scholar]
- Stearns T, Evans L, Kirschner M. Gamma-tubulin is a highly conserved component of the centrosome. Cell. 1991;65:825–836. [Abstract] [Google Scholar]
- Stubblefield E, Brinkley BR. Architecture and function of the mammalian centriole. In: Warren KB, editor. Formation and Fate of Cell Organelles. Vol. 6. New York, NY: Academic Press; 1967. pp. 175–218. [Google Scholar]
- Sulston J, et al. The C. elegans genome sequencing project: a beginning. Nature. 1992;356:37–41. [Abstract] [Google Scholar]
- Sunkel CE, Gomes R, Sampaio P, Perdigao J, Gonzalez C. Gamma-tubulin is required for the structure and function of the microtubule organizing centre in Drosophila melanogaster. EMBO J. 1995;14:28–36. [Europe PMC free article] [Abstract] [Google Scholar]
- Tam L-W, Lefebvre PA. Cloning of flagellar genes in Chlamydomonas reinhardtii by DNA insertional mutagenesis. Genetics. 1993;135:375–384. [Europe PMC free article] [Abstract] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL-W: improving sensitivity of progressive multiple sequence alignment through sequence weighting, positioning-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. [Europe PMC free article] [Abstract] [Google Scholar]
- Vassilev A, Kimble M, Silflow CF, LaVoie M, Kuriyama M. Identification of intrinsic dimer and overexpressed monomeric forms of gamma-tubulin in Sf9 cells infected with baculovirus containing the Chlamydomonas gamma-tubulin sequence. J Cell Sci. 1995;108:1083–1092. [Abstract] [Google Scholar]
- Vorobjev IA, Chentsov YS. Centrioles in the cell cycle. I. Epithelial cells. J Cell Biol. 1982;98:938–949. [Europe PMC free article] [Abstract] [Google Scholar]
- Wetherbee R, Platt SJ, Beech PJ, Pickett-Heaps JD. Flagellar transformation in the heterokont Epipyxis pulchra (Chrysophycaeae): direct observation using image enhanced light microscopy. Protoplasma. 1988;145:47–54. [Google Scholar]
- Youngblood J, Schloss JA, Silflow CD. The two β-tubulin genes of Chlamydomonas reinhardtii code for identical proteins. Mol Cell Biol. 1984;4:2686–2696. [Europe PMC free article] [Abstract] [Google Scholar]
- Zheng Y, Wong ML, Alberts B, Mitchinson T. Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature. 1995;378:578–583. [Abstract] [Google Scholar]
Articles from Molecular Biology of the Cell are provided here courtesy of American Society for Cell Biology
Full text links
Read article at publisher's site: https://doi.org/10.1091/mbc.9.6.1293
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc25351?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Towards understanding centriole elimination.
Open Biol, 13(11):230222, 15 Nov 2023
Cited by: 3 articles | PMID: 37963546 | PMCID: PMC10645514
Review Free full text in Europe PMC
Cleavage of delta and epsilon tubulin PCR products is observed upon PCR purification.
MicroPubl Biol, 2023, 10 Oct 2023
Cited by: 0 articles | PMID: 37881246 | PMCID: PMC10594130
The Tubulin Superfamily in Apicomplexan Parasites.
Microorganisms, 11(3):706, 09 Mar 2023
Cited by: 5 articles | PMID: 36985278 | PMCID: PMC10056924
Translational regulation of δ-tubulin through its 5'-untranslated region.
Mol Biol Rep, 50(4):3451-3458, 09 Feb 2023
Cited by: 0 articles | PMID: 36757552
Appearing and disappearing acts of cilia.
J Biosci, 48:8, 01 Jan 2023
Cited by: 2 articles | PMID: 36924208 | PMCID: PMC10005925
Review Free full text in Europe PMC
Go to all (129) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Nucleotide Sequences (4)
- (2 citations) ENA - U31545
- (1 citation) ENA - W53427
- (1 citation) ENA - AF013109
- (1 citation) ENA - AF013108
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Three-dimensional organization of basal bodies from wild-type and delta-tubulin deletion strains of Chlamydomonas reinhardtii.
Mol Biol Cell, 14(7):2999-3012, 04 Apr 2003
Cited by: 81 articles | PMID: 12857881 | PMCID: PMC165693
Mutations in alpha-tubulin promote basal body maturation and flagellar assembly in the absence of delta-tubulin.
J Cell Sci, 117(pt 2):303-314, 01 Jan 2004
Cited by: 14 articles | PMID: 14676280
Role of delta-tubulin and the C-tubule in assembly of Paramecium basal bodies.
BMC Cell Biol, 2:4, 07 Mar 2001
Cited by: 52 articles | PMID: 11255590 | PMCID: PMC29069
Long-lost relatives reappear: identification of new members of the tubulin superfamily.
Curr Opin Microbiol, 6(6):634-640, 01 Dec 2003
Cited by: 39 articles | PMID: 14662361
Review
Funding
Funders who supported this work.
NIGMS NIH HHS (2)
Grant ID: R01 GM032843
Grant ID: GM-32843




