Abstract
Free full text

CREB Modulates the Functional Output of Nucleus Accumbens Neurons: A CRITICAL ROLE OF N-METHYL-D-ASPARTATE GLUTAMATE RECEPTOR (NMDAR) RECEPTORS*
Abstract
Nucleusaccumbens(NAc)mediumspinyneuronscyclebetween two states, a functionally inactive downstate and a functionally active upstate. Here, we show that activation of the transcription factor cAMP-response element-binding protein (CREB), a common molecular response to several drugs of abuse, increases both duration of the upstate and action potential firing during the upstate. This effect of CREB is mediated by enhanced N-methyl-D-aspartate glutamate receptor (NMDAR) function: increased CREB activity increases both NMDAR-mediated synaptic currents and surface level of NMDARs, while inhibition of NMDARs abolishes the effect of CREB on upstate duration. Furthermore, mimicking the effect of CREB by pharmacological enhancement of NMDAR function in the NAc in vivo suppressed novelty- and cocaine-elicited locomotor activity. These findings suggest that by enhancing NMDAR-mediated synaptic transmission, CREB activation promotes the proportion of time NAc neurons spend in the upstate. This effect, along with the CREB enhancement of NAc membrane excitability (Dong, Y., Green, T., Saal, D., Marie, H., Neve, R., Nestler, E.J., and Malenka, R.C.(2006)Nat. Neurosci.9,475–477), may counteract drug-induced maladaptations in the NAc and thus ameliorate the addictive state.
The nucleus accumbens (NAc)3 plays a key role in translating rewarding, motivational, and emotional stimuli into behaviors (2). By inducing molecular and cellular adaptations, drugs of abuse modify NAc circuitry, and thereby contribute importantly to the pathophysiological emotional and motivational responses underlying addiction. In vivo NAc neurons oscillate between two functional states (3), the “downstate” and the “upstate.” During downstates, the NAc neurons rest at a hyperpolarized membrane potential (approximately −75 mV), which prevents neurons from firing action potentials. Accordingly, NAc neurons in the downstate largely remain quiescent. In contrast, during upstates, NAc neurons dwell at a more depolarized plateau potential (approximately −55 mV), which facilitates neuron ability to fire action potentials. Both in vitro and in vivo results suggest that the upstate is initiated and maintained by synchronous excitatory synaptic inputs to NAc neurons; the summated activation of postsynaptic glutamate receptors elevates the membrane potential of NAc neurons from the downstate to the upstate (4, 5).
As NAc neurons fire action potentials primarily during the upstate, it has been proposed that the upstate is the time when NAc neurons execute their behaviorally relevant functions (6). In awake behaving animals, the NAc neurons actively process emotional and motivational inputs. Thus, it is not surprising that NAc neurons in behaving rats spend most of their time dwelling at the upstate membrane potentials (7). In contrast, NAc neurons spend less time in the upstate in anesthetized animals (6, 8). In the context of drug addiction, it has been reported that chronic exposure to methamphetamine decreases the upstate action potential firing of NAc neurons (9, 10), whereas withdrawal from amphetamine increases the proportion of NAc neurons that exhibit oscillations between down- and upstates (8). These observations suggest that the behavioral consequences of exposure to drugs of abuse may, in part, involve modifications of down- and upstate transitions in NAc neurons in a manner that would influence their functional output.
Here we examine whether changes in the activity of the transcription factor cAMP-response element-binding protein (CREB) influence the down- and upstates of NAc neurons. CREB is activated in NAc neurons following exposure to several drugs of abuse (2) and this activation appears to counteract the pathological adaptations caused by these substances (1, 2, 11). To determine whether CREB activation affects the down- and upstates of NAc neurons, we used viral vectors to express constitutively active (ca) or dominant-negative (dn) forms of CREB in NAc slice cultures. We show that expression of caCREB increases both the duration of individual upstates and the action potential firing during upstates. Further results suggest that the CREB-induced enhancement of upstates is achieved via increased N-methyl-D-aspartate receptor (NMDAR)-mediated synaptic transmission. Along with previous work, our results suggest that CREB activation increases the functional output of NAc neurons via several mechanisms, which together help explain the behavioral role of CREB in the NAc in addiction.
EXPERIMENTAL PROCEDURES
Virus-mediated Gene Transfer
Recombinant sindbis pseudovirions expressing caCREB-IRES-GFP (Y134F mutation in CREB), dnCREB-IRES-GFP (S133A mutation in CREB) or GFP alone were generated as previously reported (1, 12). To exclude potential nonspecific effects of the sindbis viral vectors, a herpes virus (bicistronic HSV amplicon) (13) was also used in several experiments in an interleaved manner. There was no difference in the results obtained using these two types of viruses, and thus the data were combined.
NAc Slice Culture and Electrophysiology
Young (P19–21) Sprague-Dawley rats were deeply anesthetized with isoflurane and decapitated. Coronal NAc slices (200-μm thick) were obtained in ice-cold sterile low Ca2+ solution (containing in mM: 126 NaCl, 1.6 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 0.625 CaCl2, 18 NaHCO3, and 11 glucose), and then placed on Millicell Millipore culture plate inserts in wells containing Neurobasal-A Media with 4% B-27 and 1% Glutamax-I Supplements (Invitrogen). For viral infection, concentrated virus solution (1 μl) was carefully dropped onto the NAc shell 1 h after slice preparation (1).
Standard whole-cell recordings were performed 1 day following virus infection. Recordings were made from the medium spiny neurons in the NAc shell (ventral-medial area), which was identified by anatomical landmarks (14). To achieve whole-cell configurations, a seal of at least a gigaohm was formed between the electrode tip and the cell membrane and then the membrane underlying the electrode tip was ruptured to produce low-resistance electrical access to the cell interior. Recordings were made in either current or voltage clamp mode with a Multi-Clamp 700B amplifier (Molecular Device). The intracellular and extracellular solutions used depended on the individual experiment and can be found in our published papers (15–18). In general, Cs+-based internal solutions were used for all experiments except those that involved examining the action potential firing, for which a K+-based internal solution was used (in mM: 130 potassium methanesulfate, 10 KCl, 10 HEPES, 0.4 EGTA, 2.0 MgCl2, 2.5 MgATP, 0.25 Na3GTP, pH 7.2–7.4; 275–285 mOsm). The recording bath contained (in mM): 126 NaCl, 1.6 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, 18 NaHCO3, and 11 glucose, and equilibrated at 31–34 °C with 95% O2/5% CO2. Some cells were filled with Alexa568 (0.2 mg/ml, Molecular Probes, OR). To compare evoked action potentials (Fig. 3, D and E), the resting membrane potential was maintained at −80 mV for all experiments.
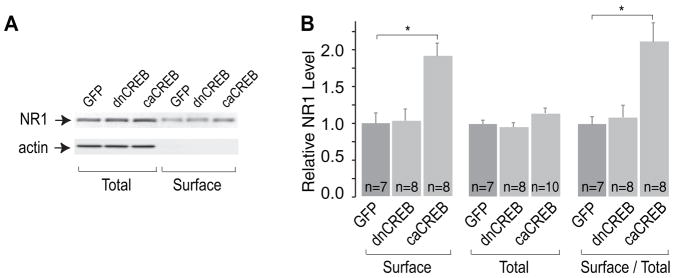
A, sample Western blots showing surface and total levels of NR1 subunits in NAc neurons expressing GFP alone, dnCREB, or caCREB. B, summary showing that both the surface level and the ratio of surface to total level of NR1 subunits were increased by caCREB in the NAc, with no significant effect observed on total NR1 levels. *, p < 0.05.
Definitions of Upstate and Upstate NAc Neuron
We operationally defined that during the 5-min whole-cell current-clamp recordings, any depolarizing membrane oscillations with amplitude >7 mV, duration >0.1 s, and >1 action potential firing represented the upstate (19). We also defined that any NAc neurons that exhibited such membrane oscillations greater than 10 times during the 5-min recording were upstate NAc neurons. Upstate neurons were also operationally called bimodal neurons in this article. In most upstate NAc neurons, the frequency (0.1–1.5 Hz) and amplitude (10–35 mV) of downstate-to-upstate transitions were relatively regular. Of all recorded NAc neurons, none fired action potentials from their hyperpolarized downstates.
Procedure for Obtaining AMPAR and NMDAR EPSCs
To examine excitatory postsynaptic currents (EPSCs), the extra-cellular solution routinely contained picrotoxin (0.1 mM) to block GABAAR-mediated currents, which would otherwise confound measures of glutamate receptor-mediated currents. Stimuli were applied through bipolar stainless steel electrodes, which were placed in the dorsolateral region of the recorded neurons (~100 μm). Stimulation parameters included: frequency, 0.1 Hz; intensity, 50–200 μA; duration, 0.1 msec. The membrane potential of recorded neurons was first held at −70 mV. Presynaptic stimulations elicited inward currents, which are primarily mediated by AMPARs (because at this holding potential the NMDARs are largely blocked by Mg2+). When the holding potential was switched to +40 mV, the same presynaptic simulation elicited dual component responses (mediated by both AMPARs and NMDARs) because at this voltage the Mg2+ blockade of NMDARs is released (the outward current trace as indicated in Fig. 2G). After obtaining 30 traces as baseline, the NMDAR-selective antagonist D-APV (50 μM) was applied and thus pure AMPAR-mediated EPSCs were obtained (Fig. 2G). Subtraction of the AMPAR EPSCs from the dual component EPSCs generated the NMDAR EPSCs (as labeled in Fig. 2G).
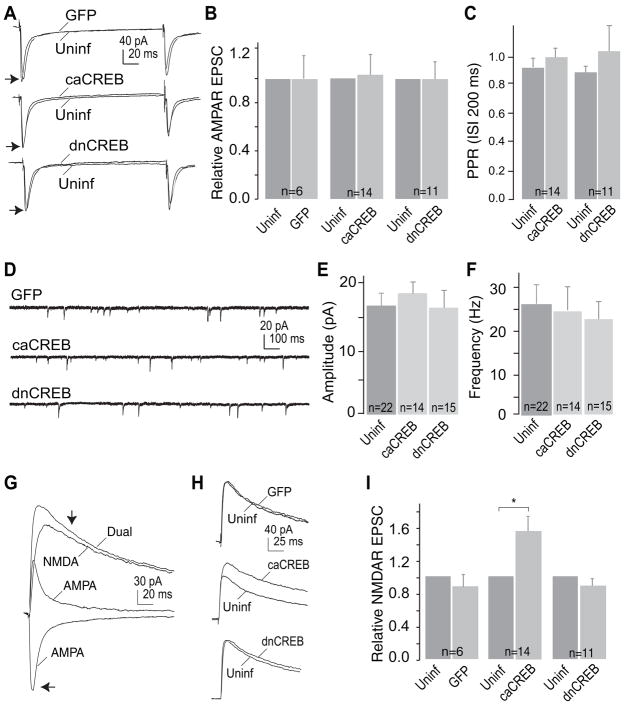
A, sample traces showing AMPAR EPSCs recorded (holding potential −70 mV) from uninfected control neurons and the adjacent manipulated neurons upon stimulation of the same set of presynaptic inputs. B, summary showing that the amplitude of AMPAR EPSCs was not affected by expression of GFP alone, caCREB-GFP, or dnCREB-GFP. C, summary showing that the paired-pulse ratio (PPR) of AMPAR EPSCs was not affected by expression of caCREB or dnCREB. D, sample mEPSCs from GFP-, caCREB-, or dnCREB-expressing NAc neurons. E and F, neither amplitude nor frequency of mEPSCs was affected by expression of caCREB or dnCREB. G, sample traces showing that dual components (mediated by AMPARs and NMDARs as indicated) were recorded from NAc neurons by holding the membrane potential at +40 mV. The dual current at 30 ms (indicated by the upper arrow) after onset was primarily mediated by NMDARs, and, thus, was operationally measured as the amplitude of NMDAR EPSCs. The amplitude of AMPAR EPSCs was measured at the peak (indicated by the lower arrow). H, sample traces showing the dual EPSCs from paired uninfected and manipulated cells. I, summary showing that expression of caCREB significantly increased the amplitude of NMDAR EPSCs in NAc neurons.
Western Blot Analysis of Surface NMDARs
The NAc shell was taken and incubated in 1 mg/ml NHS-SS-biotin (Pierce) to biotinylate surface proteins for 30 min at 4 °C. After removing ambient or nonspecifically bound NHS-SS-biotin, the tissue was homogenized and sonicated in lysis buffer containing proteinase and phosphatase inhibitors (in mM, 20 Tris, 50 NaCl, 1 EDTA, 1 EGTA, and 1% Triton X-100, 0.1% SDS, pH 7.4). The homogenates were centrifuged and the supernatants collected. The same amount of proteins was incubated with Neutravidin-linked beads (Pierce) to capture biotinylated surface protein at 4 °C for 2 h. The surface proteins were eluted for Western blotting. Antibodies included NR1 antibody (provided by Dr. Morrison, Mt. Sinai School of Medicine), and β-actin monoclonal antibody (Sigma). Band intensities were quantified by NIH image software.
Behavioral Testing
Rats were anesthetized with zyket (ketamine 87 mg/kg + xylazine 13 mg/kg) and placed into a stereotaxic apparatus. Bilateral 26 gauge stainless steel guide cannulae (Plastics One, Roanoke, VA) were implanted into the NAc shell (1.0 from bregma, ±1.0 mm from midline and −6.0 mm from skull). Rats recovered 1 week prior to experimentation. On the testing day, intra-NAc injections were done using a 33-gauge stainless steel needle connected to PE-20 tubing leading to a 1.0-μl Hamilton syringe. The needles were lowered 1 mm below the guide cannulae, and a volume of 0.5 μl was delivered with an infusion pump over a period of 90 s. The needles were allowed to remain in place for 30 s following the injection. A microinjection of saline was always given prior to the first day of test microinjections to adapt the animals to the procedure. At the end of the experiment, all cannulae placements were verified with neutral red staining of coronal brain sections.
Two main studies were conducted: For one set of studies, rats were microinjected with either saline or DCS (10 μg/0.5 μl/side) 20 min prior to being placed into the locomotor chamber for the first time (15.5″L × 8″W × 8″H). Horizontal activity was monitored automatically by photocell beam interruptions. These animals were subsequently tested for their response to cocaine (15 mg/kg, intraperitoneal) after microinjection of saline or DCS given in a counterbalanced order. Activity was monitored every 5 min for at least 20 min. A second set of studies was then conducted to test whether the interval between the microinjection of DCS and behavioral testing (20 min) might be too long to observe maximal behavioral effects. Therefore, we administered saline or DCS microinjections 5 min prior to testing. In this set of experiments, animals were tested in a different box (16″ L × 16″ W × 12″ H), in which rats could reside for several days, so that spontaneous locomotor activity could also be monitored. The experimental design was such that animals were first tested for novelty-induced locomotor activity after intra-NAc saline or DCS injection, allowed 2 days to live in the photocell cages, then tested for the effects of intra-NAc injection of saline or DCS on spontaneous locomotor activity and, 2 days later, tested for the effects of intra-NAc saline or DCS on cocaine-induced locomotor activity. We did not find any differences in DCS-induced suppression of activity, whether it was given 5 or 20 min prior to testing, or whether rats were tested in the large or smaller apparatus, so the data for novelty and cocaine-induced activity were combined and are represented as percent of intra-NAc saline controls.
Statistical Analysis
Over 50% of the data were collected and analyzed without knowledge of the molecular manipulations that the slices/animals had received. No differences were found from blinded and nonblinded experiments, and therefore results were combined. All values are expressed as mean ± S.E. Statistical significance was assessed using a Student t test for electrophysiological and biochemical experiments and a two-way ANOVA with a repeated measure over time for behavioral assays. The p values were presented as <0.05, <0.01. If >0.05, results were indicated as not significant.
RESULTS
Two Functional States of NAc Neurons in One-day-old Slice Cultures
In one-day-old slice cultures, NAc neurons maintained their characteristic spiny morphology (Fig. 1, A1–3) and electrophysiological properties (1). Whole-cell current-clamp recordings revealed that ~45% of NAc neurons (n = 48/104) periodically oscillated between two distinct membrane potentials commonly referred to as the upstate and downstate (4). Analysis of the distribution of membrane potentials during upstates and downstates indicated that the average downstate potential was −75 mV (±6 mV, n = 24), while the average upstate potential was −62 mV (±5 mV, Fig. 1C). These membrane potentials are similar to those seen during upstates and downstates in vivo (5–7).
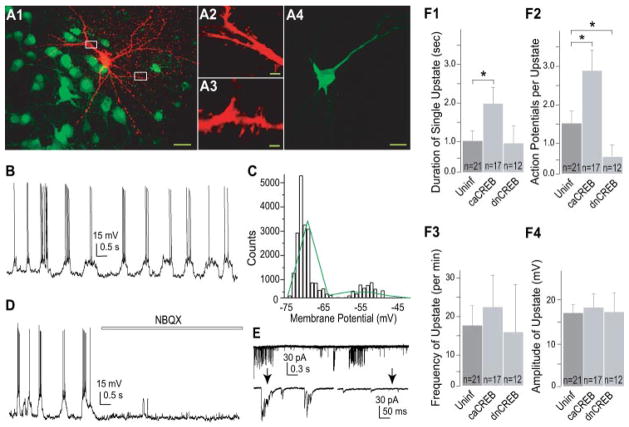
A, an example neuron filled with Alexa 568 exhibiting typical spiny morphology shown at low (A1) and high (A2,3) magnifications. Neurons expressing caCREB were detected by co-expression of GFP. Calibration bar: 10 μm in A1, 1 μm in A2,3, and 15 μm in A4. B, sample bimodal membrane property of a NAc neuron. C, membrane potential of the bimodal neuron in B was fit by two-Gaussian distribution (green line). D, upstates of NAc neurons were abolished by NBQX. E, whole-cell voltage-clamp recordings showing the occurrence of EPSC bursts in a NAc neuron. Below traces are two segments from the upper trace (indicated by arrows) that were expanded on a slower time scale. F, CREB regulated the upstate duration (F1) and the upstate action potential firing (F2), but not the upstate frequency (F3) or amplitude (F4) in NAc neurons. *, p < 0.05.
Both in vitro and in vivo observations suggest that the upstate potential is generated by bursting/synchronous glutamatergic synaptic inputs (4–6). We therefore first examined whether the upstate potential is mediated by glutamatergic synaptic inputs in the slice culture preparation. The two types of glutamate receptors that mediate most fast excitatory synaptic transmission are α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid type receptors (AMPARs) and NMDARs. Under physiological conditions, activation of NMDARs requires the prior activation of AMPARs to depolarize the membrane potential. Thus, blocking AMPARs can functionally block activation of both AMPARs and NMDARs. In slice cultures, the upstate of NAc neurons was abolished by application of the AMPAR antagonist NBQX (3 μM, Fig. 1D, n = 5), indicating that the upstate of NAc neurons is mediated by activation of AMPARs and/or NMDARs. We next examined whether the excitatory synaptic activity in the upstate-downstate cycling NAc neurons has a bursting pattern, because only bursting synaptic activity can be temporally summated to generate a stable depolarization plateau of the membrane potential (i.e. the upstate membrane potential plateau). To test this, we used whole-cell voltage-clamp techniques to record spontaneous EPSCs (sEPSCs) in NAc neurons. We found that sEPSCs for the upstate neurons indeed occurred in a bursting pattern (Fig. 1E, n = 5). These observations suggest that the upstate of NAc neurons in slice culture is mediated by bursting sEPSCs.
Activation of CREB Prolongs the Upstate of NAc Neurons
Exposure to drugs of abuse has been shown to alter the bimodal states (i.e. up- and downstate) of NAc neurons in vivo (8–10). A common molecular change in NAc neurons following exposure to drugs of abuse is the activation of the transcription factor CREB (2, 20). To examine whether activation of CREB influences the bimodal property of NAc neurons, we manipulated CREB activity within individual neurons using virus-mediated gene transfer to express caCREB or dnCREB tagged with GFP. As reported previously (1, 12), both caCREB and dnCREB readily entered the nucleus of infected neurons (Fig. 1, A4). Expression of either CREB constructs or GFP alone did not affect the percentage of neurons exhibiting bimodal states (43–50% of neurons, n > 40 for each manipulation). However, the duration of individual upstates was significantly longer in NAc neurons expressing caCREB-GFP than in either dnCREB-expressing or uninfected neurons (Fig. 1, F1, in seconds: uninfected, 1.0 ± 0.3, n = 21; caCREB, 1.9 ± 0.4, p < 0.05, n = 17; dnCREB, 1.0 ± 0.4, n = 12). In addition, the number of action potentials observed during individual upstates was significantly increased by expression of caCREB-GFP and decreased by expression of dnCREB-GFP (Fig. 1, F2, uninfected, 1.5 ± 0.3, n = 21; caCREB, 2.8 ± 0.7, p < 0.05, n = 17; dnCREB, 0.6 ± 0.5, p < 0.05, n = 12). In contrast, neither the frequency (number of upstates per min: uninfected, 17.3 ± 6.2, n = 21; caCREB, 22.5 ± 8.6, n = 17; dnCREB, 15.2 ± 12.7, n = 12, Fig. 1, F3) nor the amplitude (in mV: uninfected, 16.8 ± 1.9, n = 21; caCREB, 18.5 ± 4.1, n = 17; dnCREB, 17.1 ± 4.8, n = 12, Fig. 1, F4) of individual upstates was significantly altered by expression of caCREB or dnCREB.
Activation of CREB Selectively Enhances NMDAR-mediated Synaptic Current
Because AMPAR- and NMDAR-mediated synaptic currents are the driving force for the upstates of NAc neurons (4–6), we next examined whether the CREB effect on upstates results from changes in AMPAR or NMDAR EPSCs. We performed pairwise comparisons of both AMPAR- and NMDAR-mediated EPSCs between infected and adjacent uninfected NAc neurons using identical afferent stimulation (12). Briefly, we first made a whole-cell voltage-clamp recording from one cell (either infected or uninfected) and held the membrane potential at −60 mV. Because of the voltage-dependent Mg2+ blockade of NMDARs, these receptors were minimally activated at this holding potential. The recorded EPSCs were therefore primarily mediated by AMPARs (AMPAR EPSCs). Then, without changing the parameters for presynaptic stimulation, the membrane potential was switched to +40 mV. Because this depolarized potential removes the voltage-dependent Mg2+ blockade, NMDARs could be activated and together with AMPARs mediated dual-component EPSCs (AMPAR EPSC + NMDAR EPSC). Because of the fast inactivation of AMPAR EPSCs, the dual-component EPSC at 30 ms after onset was mainly mediated by NMDARs (Fig. 2G), and thus was operationally measured as the amplitude of NMDAR EPSCs. Therefore, both AMPAR and NMDAR EPSCs could be measured from the first cell by changing the holding potentials. Immediately after finishing recording from the first neuron, without changing the presynaptic stimulation, we made the same measurements from an adjacent cell of opposite phenotype (i.e. uninfected or infected; the sequence in which recordings were made was alternated during the course of experiments). By keeping the stimulation parameters constant, roughly the same set of presynaptic fibers was stimulated to activate similar numbers of synapses on adjacent pairs of neurons. When normalized to the peak amplitude of EPSCs in adjacent uninfected neurons, the AMPAR EPSCs were not significantly altered by expression of either GFP (1.01 ± 0.18, n = 6), caCREB (1.05 ± 0.15, n = 14), or dnCREB (0.99 ± 0.14, n = 11) (Fig. 2, A and B).
The paired-pulse ratio (PPR, 2nd:1st) is often used as a sensitive measurement to detect changes in presynaptic release. The PPR (interpulse interval = 200 ms) of AMPAR EPSCs was not affected by expression of caCREB (uninfected, 0.93 ± 0.07; caCREB, 0.98 ± 0.04, n = 14) or of dnCREB (uninfected, 0.88 ± 0.04; dnCREB, 1.01 ± 1.22, n = 11, Fig. 2C), suggesting no significant presynaptic alterations.
Another sensitive electrophysiological assay to detect changes in presynaptic release and postsynaptic responsiveness is the measurement of miniature (m) EPSCs. In general, a change in the frequency of mEPSCs reflects a change in presynaptic release, whereas a change in the amplitude reflects a change in postsynaptic responsiveness. In the presence of tetrodotoxin (TTX), which blocks spontaneous action potentials in presynaptic terminals, we measured mEPSCs in uninfected and infected NAc neurons and observed that neither the amplitude (in pA: uninfected, 16.5 ± 2.2, n = 22; caCREB, 17.2 ± 1.9, n = 14; dnCREB, 16.3 ± 3.1, n = 15), nor the frequency (in Hz: uninfected, 24.9 ± 4.1, n = 22; caCREB, 24.1 ± 5.2, n = 14; dnCREB, 22.3 ± 3.8, n = 15), of AMPAR-mediated mEPSCs was significantly altered by manipulations of CREB activity (Fig. 2 D–F). These findings suggest that neither the presynaptic release of glutamate nor postsynaptic AMPAR function were altered by expression of caCREB or dnCREB.
In contrast to AMPAR EPSCs, NMDAR EPSCs were substantially altered by changes in CREB activity. NAc neurons expressing caCREB had significantly larger NMDAR EPSCs than control neurons, whereas expression of either GFP alone or dnCREB had no significant effects (peak amplitude relative to adjacent uninfected neurons: GFP, 0.89 ± 0.15, n = 6; caCREB, 1.57 ± 0.19, n = 14, p < 0.01; dnCREB, 0.88 ± 0.11, n = 15, Fig. 2, G–I). Because CREB activation selectively up-regulates NMDAR EPSCs without affecting AMPAR EPSCs, the ratio of AMPAR:NMDAR should be lower in caCREB-expressing neurons than in uninfected control neurons. This prediction was also confirmed in the pairwise recordings (uninfected, 0.82 ± 0.12; caCREB, 0.54 ± 0.16; n = 14, p < 0.05, paired Student’s t test).
Two of the most likely mechanisms through which caCREB could increase NMDAR EPSCs are an increase in the function of pre-existing synaptic NMDARs or an addition of new NMDARs on the cell surface. To address the latter possibility, we examined whether expression of caCREB-GFP in NAc neurons affected the surface or total level of the NMDAR subunit NR1. Because functional NMDARs require NR1, surface expression of NR1 reflects the total number of functional NMDARs on the plasma membrane (21, 22). In this set of experiments, we first dissected the NAc tissue and briefly (10 min) incubated it with viral vectors before placing it on the tissue culture film. Twenty-four hours later, >90% of the NAc neurons were infected (indicated by their GFP signals, data not shown). We then performed Western blot analysis of biotinylated surface NR1 subunits in NAc neurons (see “Experimental Procedures”), which revealed a significant increase induced by expression of caCREB but not dnCREB nor GFP alone (relative intensity of NR1 protein bands: GFP, 1.00 ± 0.15, n = 7; dnCREB, 1.05 ± 0.163, n = 8; caCREB, 1.89 ± 0.21, n = 8, p < 0.01, Fig. 3). The total level of NR1 subunits was not significantly altered by any of these manipulations (relative density: GFP, 1.00 ± 0.04, n = 7; dnCREB, 0.95 ± 0.08, n = 8; caCREB, 1.16 ± 0.09, n = 10). Accordingly, the ratio of surface NR1 to total NR1 was increased by caCREB expression (normalized to the ratio of GFP group: dnCREB, 1.10 ± 0.20, n = 7; caCREB, 2.09 ± 0.30, n = 8, p < 0.01, Fig. 3). The increase in surface but not total NR1 suggests that caCREB likely influences NMDAR trafficking to the surface or their stability on the plasma membrane (21, 23).
NMDARs Mediate CREB-induced Enhancement of Upstate of NAc Neurons
Although the AMPAR- and NMDAR-mediated synaptic currents together mediate the upstate of NAc neurons, their contributions are different. Computational studies suggest that AMPAR-mediated synaptic inputs preferentially mediate the initiation of the upstate, whereas NMDAR-mediated synaptic inputs preferentially mediate the elongation of the upstate (24). Given our observations that caCREB selectively prolongs the duration of upstates (Fig. 1, H1) and that caCREB up-regulates surface NMDARs (Figs. 2 and and3),3), we hypothesized that the enhancement of NMDAR EPSCs by caCREB is sufficient to lead to prolonged upstate duration. To test this hypothesis, we first examined whether NMDARs indeed contribute to the duration of upstates of NAc neurons in the slice culture preparation. As shown in Fig. 4, A and B, in uninfected NAc neurons, acute application of the NMDAR antagonist D-APV (50 μM) significantly shortened the duration of upstates (relative change, 29.4 ± 8.1%, n = 7, p < 0.05), indicating that NMDARs influence the duration of upstates of NAc neurons in slice cultures.
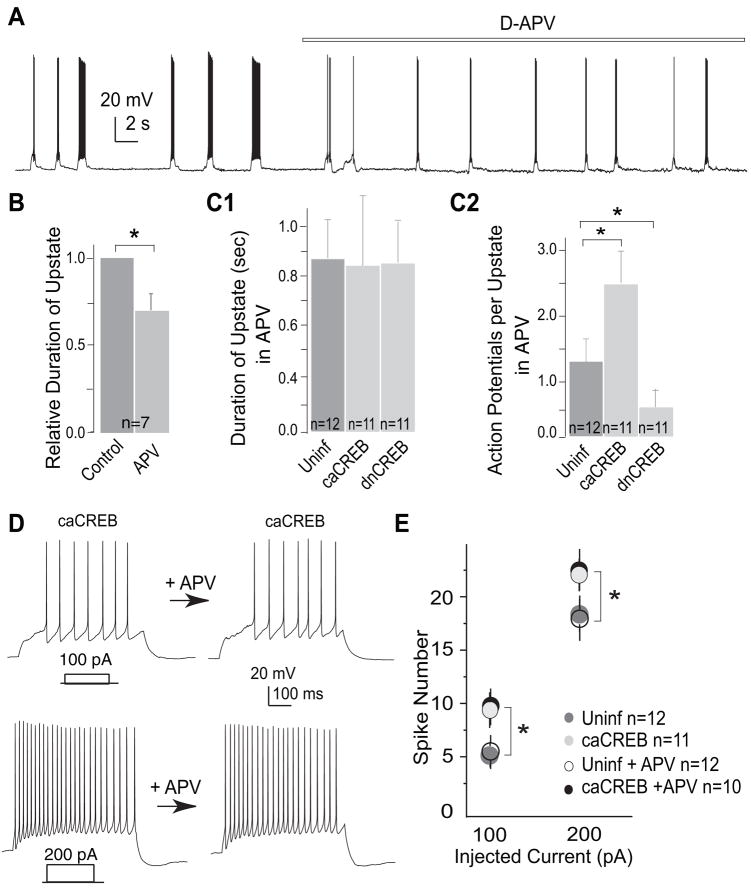
A, sample traces from an uninfected NAc neuron showing the bimodal activity before and during application of D-APV. B, summary showing that inhibition of NMDARs reduced the upstate duration of uninfected NAc neurons. C1–2, summaries showing that inhibition of NMDARs abolished the effect of CREB on the upstate duration (C1), but not the upstate action potential firing (C2), in NAc neurons. D, sample voltage traces showing action potential firing elicited by 500 ms of depolarizing current injections (100 and 200 pA) before and during application of D-APV. E, summary showing that activation of CREB up-regulated the evoked action potential firing of NAc neurons, and that inhibition of NMDARs did not influence this CREB effect. *, p < 0.05.
We next examined whether up-regulation of NMDARs is the primary mechanism by which caCREB prolongs the upstates of NAc neurons. In caCREB-expressing neurons, acute inhibition of NMDARs by D-APV abolished the increase in the duration of the upstate (in seconds: uninfected, 0.87 ± 0.13, n = 12; caCREB, 0.84 ± 0.29, n = 11; dnCREB, 0.85 ± 0.18, n = 11, Fig. 4, C1). This result suggests that the effect of increased CREB activity on the upstate duration is mediated by NMDARs.
In contrast to the significant effect on upstate duration, application of D-APV did not affect either the caCREB-induced up-regulation or the dnCREB-induced suppression of action potential firing during the upstate (number of action potentials per upstate in APV: uninfected, 1.26 ± 0.42, n = 2; caCREB, 2.28 ± 0.58, n = 11, p < 0.05; dnCREB, 0.52 ± 0.41, n = 11, p < 0.05, Fig. 4, C2). This result suggests that the effect of CREB on action potential firing is independent of activation of NMDARs. Indeed, previous work has shown that caCREB expression increases the excitability of NAc neurons by regulating a set of voltage-gated ion channels (1). To further test the role or lack thereof of NMDARs in CREB-mediated regulation of action potential firing, we examined evoked action potential firing by depolarizing current pulses in the presence of D-APV. As shown in Fig. 4, D and E, the caCREB-induced enhancement of action potential firing in NAc neurons was not affected by application of D-APV (number of action potentials elicited by 100 pA: uninfected, 4.9 ± 0.9, uninfected-APV, 5.1 ± 0.7, n = 12; caCREB, 9.8 ± 1.1, caCREB-APV, 10.0 ± 1.6, n = 11; by 200 pA: uninfected, 18.2 ± 2.1, uninfected-APV, 17.2 ± 2.4, n = 12; caCREB, 20.8 ± 1.4, n = 11, caCREB-APV, 21. 4 ± 1.1, n = 10, p < 0.05, Fig. 4, D and E).
Taken together, these observations suggest that CREB activation increases both the upstate duration and action potential firing in NAc neurons but does so via distinct molecular mechanisms within NAc neurons: activation of CREB increases upstate duration by enhancing NMDAR-mediated synaptic transmission and increases membrane excitability by modulating voltage-gated ion channels (1).
Mimicking CREB-induced Up-regulation of NAc NMDARs Suppresses Locomotor Activity in Rats
We next addressed whether the up-regulation of NMDAR-mediated synaptic transmission in the NAc contributes to the behavioral consequences of increased CREB activity in this brain structure. One widely used approach to stimulate NMDAR-mediated functions is to inject NMDA into the NAc. However, injection of NMDA activates NMDARs in the absence of endogenously released glutamate, often resulting in nonspecific effects. Instead, we used DCS, which is a co-agonist of NMDARs; it binds to the glycine site of NR1 subunits and potentiates the function of NMDARs only when they are also bound to endogenous glutamate (25). This approach minimizes the potential nonspecific effects that can accompany application of NMDA itself.
To validate this approach, we examined the effect of DCS on NMDAR EPSCs of NAc neurons in acute slices. Whole-cell voltage-clamp recordings were made at −30 mV in the presence of AMPAR and GABAAR antagonists. After obtaining stable NMDAR EPSCs, we switched to DCS (10 μM)-containing bath solution and observed a significant increase in the peak amplitude of NMDAR EPSCs (Fig. 5, A and B). Furthermore, the potentiated currents were blocked by the NMDAR-selective antagonist D-APV (50 –100 μM) (Fig. 5A), indicating that the DCS-enhanced currents were mediated by NMDARs. Having verified the effectiveness of DCS, we injected it, using a behaviorally relevant dose (10 μg/0.5 μl/side) (26), into the NAc shell of rats through chronic guide canulae (Fig. 5C) and examined locomotor activity under three different conditions: spontaneous locomotor activity, novelty-induced locomotor activity, and cocaine-induced locomotor activity. Spontaneous locomotor activity reflects the basal motor function of animals, whereas novelty- and drug-induced locomotor enhancement have been related to the sensitivity of animals to the reinforcing effects of addictive drugs (27). Bilateral intra-NAc injections of DCS alone did not affect spontaneous locomotor activity (Fig. 5D). However, rats that received these same injections prior to placement in the activity monitors exhibited significantly reduced novelty-induced locomotor activity compared with animals that received intra-NAc injections of saline (p < 0.05, F1, 22 = 5.741, ANOVA, n = 13 and 11 for saline and DCS group, respectively; Fig. 5E). Furthermore, cocaine-induced locomotor activity was also significantly lower following intra-NAc injections of DCS compared with saline-injected controls (p < 0.05, F1, 22 = 6.467, ANOVA, n = 10 and 14 for saline and DCS group, respectively; Fig. 5F). These results suggest that the effect of CREB activation on NMDARs, and thus the upstates of NAc neurons, may act to decrease the sensitivity of animals to drug-related stimulation.
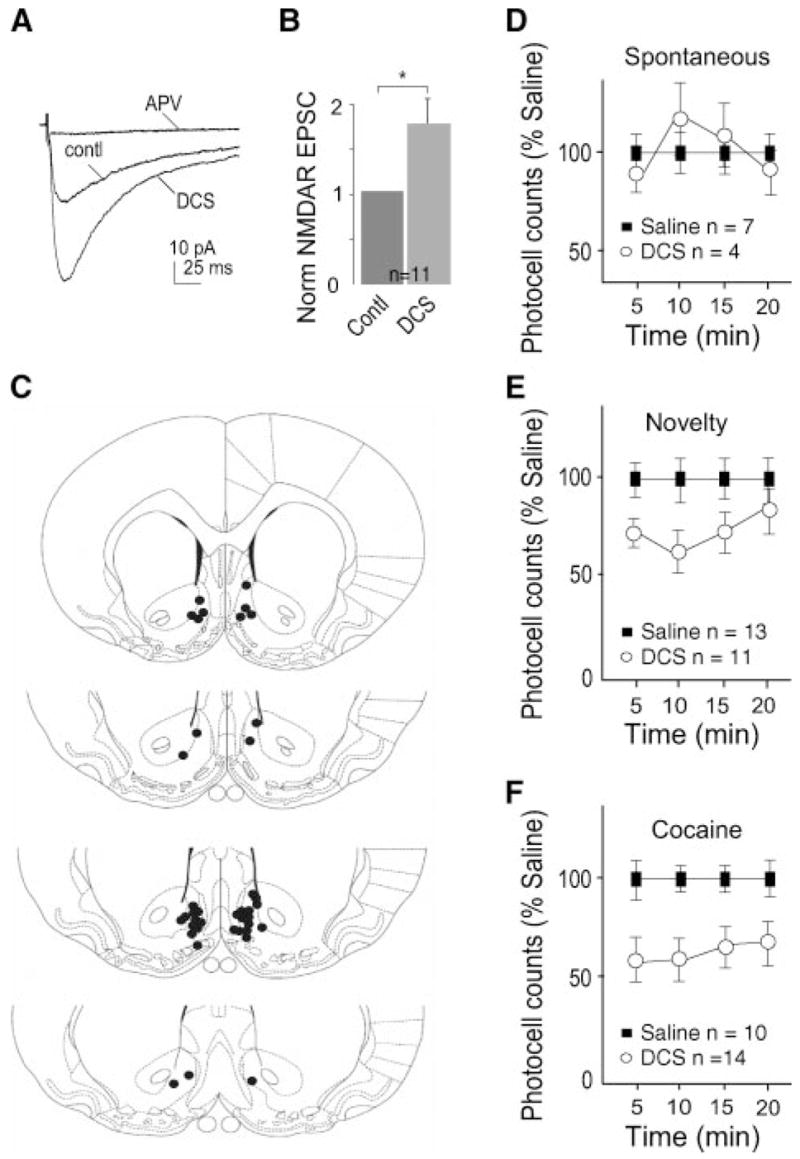
A, sample traces showing that application of DCS enhanced NMDAR EPSCs in a NAc neuron. B, summary showing that the DCS effect on NMDAR EPSCs was consistently observed in all 11 recorded NAc neurons. C, diagrams showing the injection sites of DCS. The filled dots represent the positions of the tips of cannula that deliver DCS. D, summary showing that rats given intra-NAc injection of DCS did not exhibit significant alteration in their spontaneous locomotor activity. E, summary showing that rats given intra-NAc injection of DCS exhibited significantly reduced novelty-induced horizontal locomotor activity, p < 0.01. F, summary showing that rats given intra-NAc injection of DCS exhibited significantly reduced cocaine-induced locomotor behaviors. (p < 0.01).
Activation of CREB in the NAc is a common molecular adaptation following exposure to drugs of abuse. An evolving body of evidence shows that activation of CREB in NAc suppresses addictive behaviors (1, 11, 28), but the underlying mechanism remains largely unclear. The CREB-NMDAR-upstate pathway identified in this study may provide a mechanism that links the cellular effect of CREB to its overall inhibitory role in regulating drug responsiveness.
DISCUSSION
NAc neurons, like medium spiny neurons in the dorsal striatum (4, 5), oscillate between a functionally active upstate and quiescent downstate (6). The upstate, initiated and maintained by synchronous glutamatergic synaptic inputs, is the time window for NAc neurons to execute their functions. Here, we demonstrate that increasing CREB activity in individual NAc neurons prolongs the upstate duration as well as increases the amount of action potential firing during the upstate. Furthermore, we present evidence that the CREB-mediated prolongation of upstate duration is due to the selective enhancement of NMDAR EPSCs. Finally, this change in NMDAR-mediated synaptic transmission has behavioral consequences, since pharmacologically enhancing NMDAR function in the NAc in vivo reduces novelty- and cocaine-elicited locomotor responses.
CREB Modulates the Functional Output of NAc Neurons through NMDAR-dependent and NMDAR-independent Mechanisms
Although both AMPARs and NMDARs are important for driving the membrane potential toward the upstate, NMDARs appear to be particularly important in maintaining the upstate potential (24, 29), perhaps because of the slow decay time course of NMDAR EPSCs (30). Therefore, the results that activation of CREB prolongs upstates (Fig. 1H) and increases synaptic NMDARs (Fig. 2I) in NAc neurons suggest that the effect of CREB on the upstate is mediated by CREB-enhanced NMDAR function. This notion is further supported by additional evidence showing that inhibiting NMDARs completely abolishes the effect of CREB on upstates of NAc neurons (Fig. 4, C1).
The ability of neurons to fire action potentials in response to membrane depolarization is usually referred to as their intrinsic membrane excitability. In bimodal NAc neurons, the upstate membrane potential brings the neuron closer to or beyond the threshold of action potentials, whereas the intrinsic membrane excitability sets the threshold for action potential firing and determines the frequency of action potential firing past threshold. Therefore, the final functional output of NAc neurons, which by definition is the degree of action potential firing, is determined by the action potential firing during upstates. Thus, a predicted direct consequence of increased upstate duration by CREB activation is that NAc neurons will, on average, fire more action potentials even if their intrinsic membrane excitability is not altered. This prediction was confirmed by our result showing that the upstate action potential firing was indeed up-regulated in caCREB-expressing neurons (Fig. 1, H2). Moreover, when the duration of upstates was normalized by inhibiting NMDARs, caCREB-expressing neurons still fired more action potentials during each upstate (Fig. 4, C2). These results indicate that, in addition to utilizing an NMDAR-dependent mechanism to increase the time window (upstate duration) for action potential firing, CREB also mediates an NMDAR-independent mechanism to upregulate intrinsic membrane excitability (1). These two independent effects of CREB ensure that, upon CREB activation, the functional output of NAc neurons will be increased.
Timing and Complexity of CREB Activation in Cocaine-treated Animals
How do our results influence our understanding of the in vivo role of CREB in the NAc? To study the cellular electrophysiological consequences of CREB activation in NAc neurons, we employed viral vectors to express caCREB, an approach that ensures a persistently (12–20 h) high level of CREB activation. This relatively long duration of CREB activation was essential for the current study because briefer activation may result in smaller, undetectable cellular changes. However, the activation and inactivation of CREB in vivo are more temporally dynamic. Thus, the timing of CREB activity must be considered when applying our data to complex in vivo conditions. Specifically, it has been shown that CREB in NAc neurons is rapidly activated (~10 min) following cocaine exposure but quickly (<40 min) returns to baseline (20). Such brief activation of CREB may not be capable of triggering all of the cellular alterations observed when CREB is persistently activated, as is seen with chronic cocaine exposure (2). Moreover, the same cellular targets of CREB may also be modulated in the opposite direction by other drug-activated molecular mechanisms (31). Therefore, under chronic cocaine conditions, the net changes in these cellular targets reflect the combined modulatory effects of CREB as well as many other drug-activated mechanisms.
One example that illustrates this point is the complex modulation of the intrinsic membrane excitability of NAc neurons in response to cocaine treatment. We previously demonstrated that activation of CREB up-regulated the intrinsic membrane excitability of NAc neurons (1), predicting that, if cocaine-induced CREB activation is the predominant modulatory factor, the intrinsic membrane excitability of NAc neurons would be increased in cocaine-treated animals. However, the opposite change, decreased membrane excitability of NAc neurons, was observed (1). Apparently, other as yet unknown cocaine-induced mechanisms dominate the effects of cocaine on this parameter of NAc function. Thus, it is likely that activation of CREB is only one of several molecular processes triggered by cocaine exposure, and that the dynamic summation of all of these adaptations determines the net cellular alterations observed. Manipulating CREB or CREB-dependent signaling may therefore be a clinical strategy to counteract drug-induced maladaptations in the NAc.
Cellular Mechanisms and Implication of CREB Modulation of NMDARs
Expression of caCREB in NAc neurons caused an increase in the surface level of the essential NMDAR subunit NR1 as well as an increase in NMDAR EPSCs. Given that surface NR1 subunits appear to exist only in functional NMDARs (21), our findings suggest that the caCREB-induced enhancement of NMDAR EPSCs results from an increased number of functional synaptic NMDARs. In contrast, caCREB had no effect on the total level of NR1 in this brain region. Several mechanisms could underlie the selective increase in the surface level of NR1, including modifications in the trafficking machinery responsible for delivering NMDARs to synapses (21), or changes in synaptic structure such as generation of new synaptic connections that only express NMDARs (but not AMPARs) (12). Although the gene for NR1 possesses a putative CRE binding site (30), it appears that transcription and translation of NR1 subunits are not affected by activation of CREB in NAc neurons. Indeed, the lack of caCREB effect on total levels of NR1 is consistent with a recent microarray study in which no change in NR1 mRNA levels was detected in NAc neurons upon CREB activation (31).
Although both biochemical and electrophysiological assays show an increase in surface NMDARs upon activation of CREB, the effects are quantitatively different (~50% increase in the electrophysiological assay and ~90% in the biochemical assay, Figs. 2I and and3B).3B). A likely reason for this discrepancy is differences in the exact population of NMDARs that are detected. In Western blot analysis, the total number of surface NMDARs is measured, regardless of their functional state or cellular location. In contrast, the electrophysiologically measured NMDAR EPSC represents the function and number of synaptic NMDARs. Therefore, although the level of NMDARs measured by Western blot is increased by 90%, the function and subcellular location of these NMDARs is not known. Furthermore, technical considerations may also contribute to the quantitative differences in the biochemical and electrophysiological results such as: 1) the non-linear property of Western blot analysis, 2) imperfect separation of intracellular versus surface NMDARs by the surface biotinylation approach, or 3) potential nonspecific binding of antibodies. Importantly, despite the profound differences in how the two assays were performed, the biochemical and electrophysiological results were qualitatively consistent, both showing an enhancing effect of CREB on NMDARs.
In contrast to the robust effect of caCREB, expression of dnCREB did not affect NMDAR EPSCs, surface levels of NMDARs, or the duration of upstates in NAc neurons (Figs. 1–3). These results are consistent with a previous study in hippocampal neurons in which synaptic NMDARs were particularly sensitive to caCREB but not dnCREB (12). The lack of an effect of dnCREB is likely not due to the low basal activity of CREB because substantial basal activity of CREB has been observed in NAc neurons (20). In addition, the lack of an effect is likely not due to the insufficient inhibition of endogenous CREB by expressed dnCREB. This form of dnCREB, once expressed, can form dimers with endogenous CREB, CREM (cAMP-element modulator), and ATF-1 (activating transcription factor-1); the homo- or heterodimers still bind to CREs, but they are transcriptionally inactive and occlude the binding of wild-type CREB dimers both in vitro and in vivo (32). Furthermore, expression of this form of dnCREB in NAc neurons produces a clear effect on membrane excitability that is opposite to that of CREB activation (1). It is therefore more likely that surface expression of NMDARs and its reverse process, internalization of surface NMDARs, are mediated by distinct mechanisms with different sensitivities to CREB. Indeed, the trafficking mechanisms mediating surface expression and internalization of NMDARs are clearly very different (21, 33, 34).
It has been shown that CREB activation in the hippocampus lowers the threshold for eliciting NMDAR-dependent long-term potentiation (LTP) of AMPAR EPSCs and increases its magnitude (12, 35). Thus, an expected cellular consequence of CREB-mediated up-regulation of NMDARs in NAc neurons is facilitation of LTP of AMPAR EPSCs. Given that in vivo administration of cocaine elicits long-term depression (LTD, a process opposite to LTP) of AMPAR EPSCs in NAc neurons (36), which appears to be required for the expression of drug-induced behavioral sensitization (37), a CREB-mediated enhancement of LTP in the NAc would possibly counteract the drug-induced LTD as well as behavioral sensitization. Furthermore, in vivo administration of cocaine decreases the intrinsic membrane excitability of NAc neurons, a cellular change facilitating locomotor sensitization (1), whereas activation of CREB increases the intrinsic membrane excitability of NAc neurons (1). These observations taken together further support the notion stated earlier that, at the cellular level, CREB activation in NAc neurons acts to counteract the drug-induced adaptations that contribute to drug sensitization. Thus, we hypothesize that exposure to drugs of abuse simultaneously triggers both drug-facilitating and drug-counteracting mechanisms, with activation of CREB representing a latter such mechanism. This hypothesis is supported by previous (1, 11) as well as the current behavioral results (Fig. 5).
Behavioral Implications of CREB Modulation of NMDARs in NAc Neurons
Activation of CREB is an important drug-induced molecular adaptation that produces a variety of behavioral consequences (2). In the context of our studies that focused on the effects of CREB on locomotor activity it has been found that increasing NAc CREB activity decreases cocaine-induced locomotor responses (38), whereas suppressing NAc CREB increases drug-induced locomotor responses (39). To examine the role of the CREB-induced enhancement of NMDAR EPSCs in mediating these effects of CREB on locomotor activity, we injected DCS into the rat NAc in vivo after first confirming that it enhances NMDAR EPSCs. This manipulation, like CREB itself, decreased cocaine-induced locomotor activity as well as the increase in locomotor behavior elicited by novelty. In contrast, experimentally decreasing NAc neuronal excitability has been shown to enhance locomotor activity (1). Therefore, the effect of CREB both on NAc NMDARs and on intrinsic membrane excitability appears to contribute to CREB’s inhibitory effects on behavioral responses to cocaine. This work thereby provides further insight into the molecular and cellular basis of CREB action in the NAc, information, which can be exploited to develop improved treatments for cocaine addiction.
Acknowledgments
We thank Drs. Bryan Slinker, Tung Fong, and Thomas Green for their intellectual and technical assistance.
Footnotes
*This work was supported in part by the Program of Alcohol and Drug Abuse Research Program at Washington State University (to Y. D. and B. A. S.), NARSAD (to Y. H. H. and M. M. H.), and the National Institutes of Health (to R. L. N., R. S. Z., B. A. S., E. J. N., R. C. M., and Y. D.).
3The abbreviations used are: NAc, nucleus accumbens; CREB, cAMP-response element-binding protein; EPSC, excitatory postsynaptic current; EPSP, excitatory postsynaptic potential; NMDAR, N-methyl-D-aspartate glutamate receptor; AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid glutamate receptor; ANOVA, analysis of variance; DCS, D-cycloserine; GFP, green fluorescent protein.
References
Full text links
Read article at publisher's site: https://doi.org/10.1074/jbc.m706578200
Read article for free, from open access legal sources, via Unpaywall:
http://www.jbc.org/content/283/5/2751.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
CRF regulates pain sensation by enhancement of corticoaccumbal excitatory synaptic transmission.
Mol Psychiatry, 29(7):2170-2184, 07 Mar 2024
Cited by: 0 articles | PMID: 38454083
Regulation of Phosphorylated State of NMDA Receptor by STEP61 Phosphatase after Mild-Traumatic Brain Injury: Role of Oxidative Stress.
Antioxidants (Basel), 10(10):1575, 05 Oct 2021
Cited by: 11 articles | PMID: 34679709 | PMCID: PMC8533270
Potential evidence of peripheral learning and memory in the arms of dwarf cuttlefish, Sepia bandensis.
J Comp Physiol A Neuroethol Sens Neural Behav Physiol, 207(4):575-594, 14 Jun 2021
Cited by: 0 articles | PMID: 34121131
Key transcription factors mediating cocaine-induced plasticity in the nucleus accumbens.
Mol Psychiatry, 27(1):687-709, 02 Jun 2021
Cited by: 31 articles | PMID: 34079067 | PMCID: PMC8636523
Review Free full text in Europe PMC
The potential for one drug, administered at the earliest preclinical stage, to prevent the subsequent decline of cognition that eventuates in dementia.
Alzheimers Dement (N Y), 6(1):e12084, 01 Oct 2020
Cited by: 4 articles | PMID: 33024811 | PMCID: PMC7528321
Go to all (54) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A silent synapse-based mechanism for cocaine-induced locomotor sensitization.
J Neurosci, 31(22):8163-8174, 01 Jun 2011
Cited by: 130 articles | PMID: 21632938 | PMCID: PMC3286116
Morphine-induced inhibition of Ca2+ -dependent d-serine release from astrocytes suppresses excitability of GABAergic neurons in the nucleus accumbens.
Addict Biol, 22(5):1289-1303, 29 May 2016
Cited by: 11 articles | PMID: 27239019
NMDA receptor within nucleus accumbens shell regulates propofol self-administration through D1R/ERK/CREB signalling pathway.
Addict Biol, 29(5):e13401, 01 May 2024
Cited by: 1 article | PMID: 38782631 | PMCID: PMC11116088
Reflections on: "A general role for adaptations in G-Proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function".
Brain Res, 1645:71-74, 29 Dec 2015
Cited by: 23 articles | PMID: 26740398 | PMCID: PMC4927417
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIDA NIH HHS (3)
Grant ID: R01 DA023206-01A2
Grant ID: R01 DA023206
Grant ID: R37 DA023206




