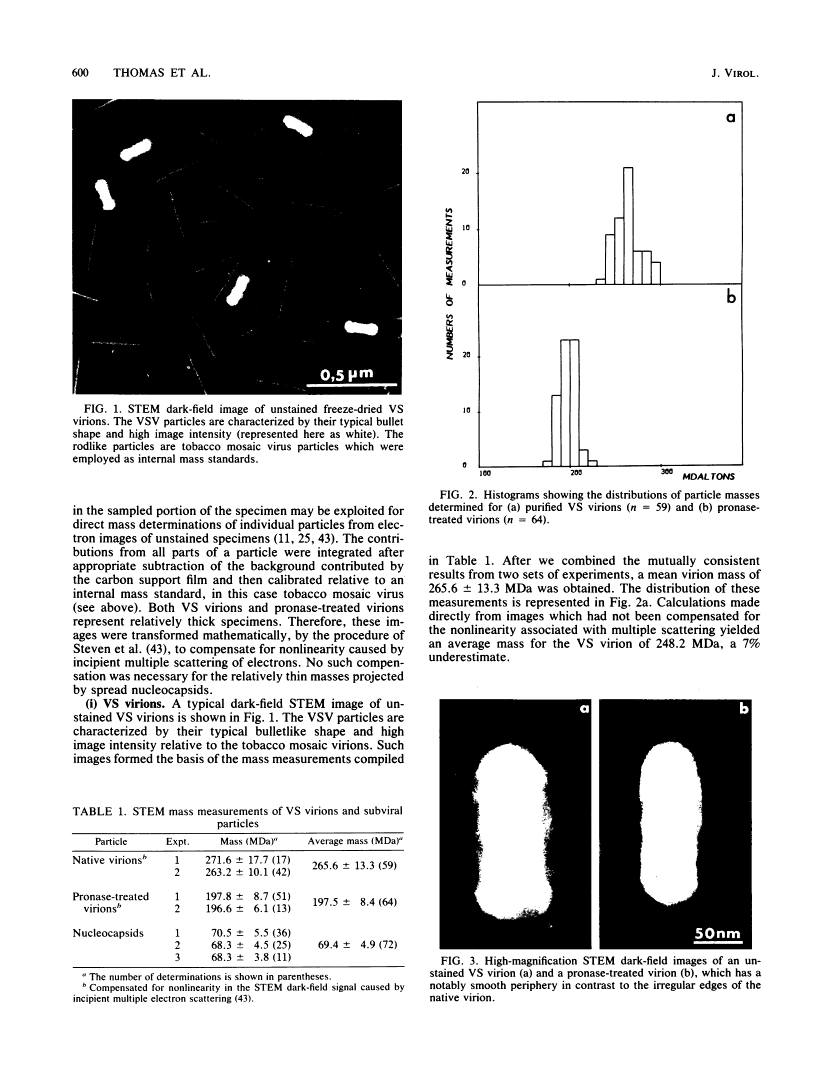
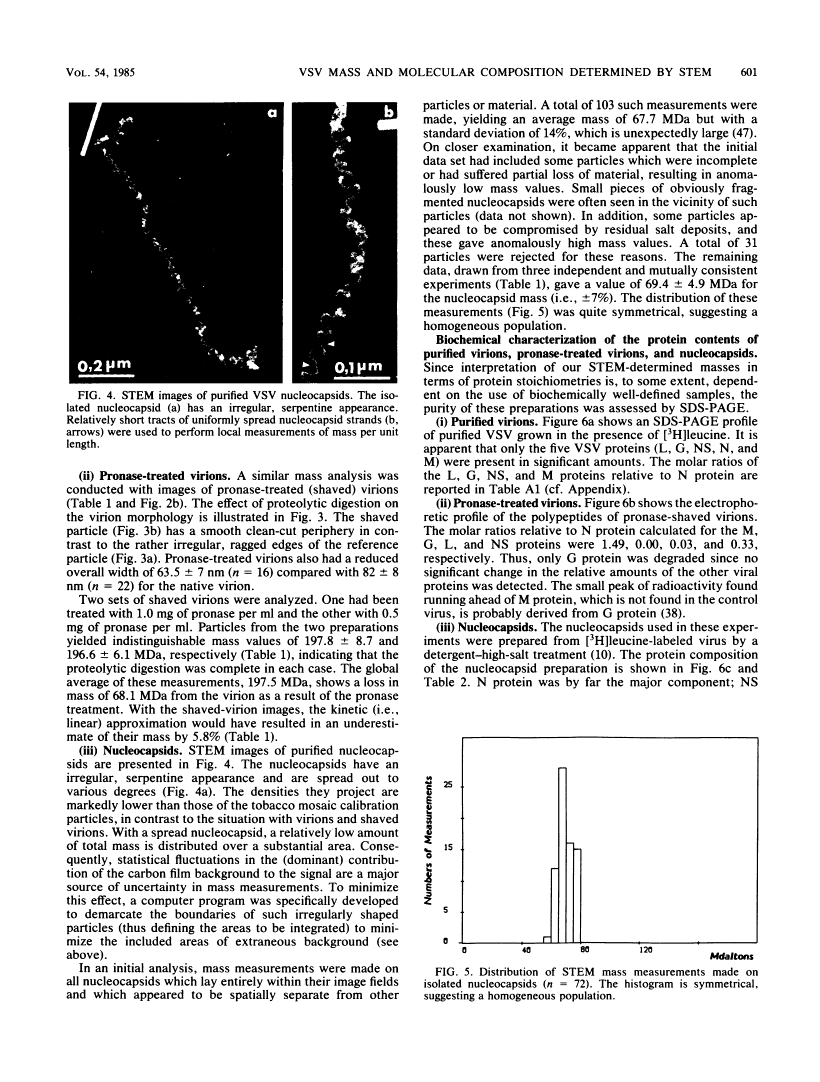
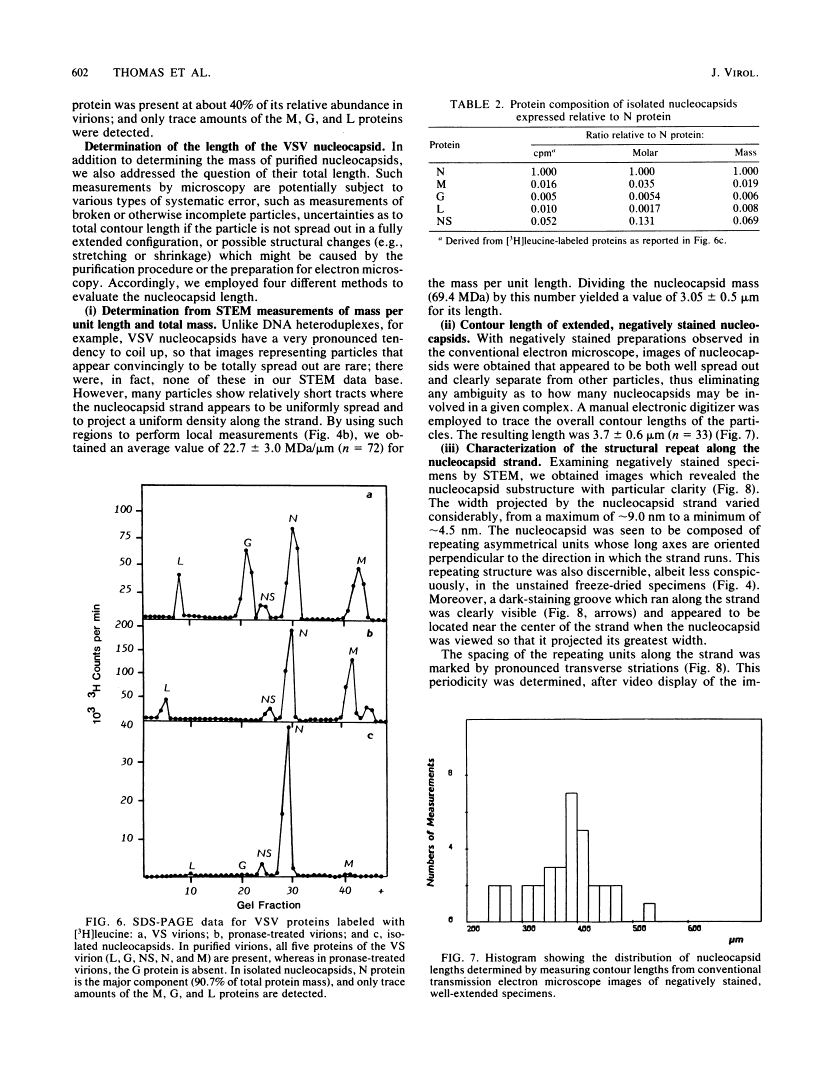
Abstract
Free full text

Mass and molecular composition of vesicular stomatitis virus: a scanning transmission electron microscopy analysis.
Abstract
Dark-field scanning transmission electron microscopy was used to perform mass analyses of purified vesicular stomatitis virions, pronase-treated virions, and nucleocapsids, leading to a complete self-consistent account of the molecular composition of vesicular stomatitis virus. The masses obtained were 265.6 +/- 13.3 megadaltons (MDa) for the native virion, 197.5 +/- 8.4 MDa for the pronase-treated virion, and 69.4 +/- 4.9 MDa for the nucleocapsid. The reduction in mass effected by pronase treatment, which corresponds to excision of the external domains (spikes) of G protein, leads to an average of 1,205 molecules of G protein per virion. The nucleocapsid mass, after compensation for the RNA (3.7 MDa) and residual amounts of other proteins, yielded a complement of 1,258 copies of N protein. Calibration of the amounts of M, NS, and L proteins relative to N protein by biochemical quantitation yielded values of 1,826, 466, and 50 molecules, respectively, per virion. Assuming that the remaining virion mass is contributed by lipids in the viral envelope, we obtained a value of 56.1 MDa for its lipid content. In addition, four different electron microscopy procedures were applied to determine the nucleocapsid length, which we conclude to be 3.5 to 3.7 micron. The nucleocapsid comprises a strand of repeating units which have a center-to-center spacing of 3.3 nm as measured along the middle of the strand. We show that these repeating units represent monomers of N protein, each of which is associated with 9 +/- 1 bases of single-stranded RNA. From scanning transmission electron microscopy images of negatively stained nucleocapsids, we inferred that N protein has a wedge-shaped, bilobed structure with dimensions of approximately 9.0 nm (length), approximately 5.0 nm (depth), and approximately 3.3 nm (width, at the midpoint of its long axis). In the coiled configuration of the in situ nucleocapsid, the long axis of N protein is directed radially, and its depth corresponds to the pitch of the nucleocapsid helix.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.5M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernhard W. A new staining procedure for electron microscopical cytology. J Ultrastruct Res. 1969 May;27(3):250–265. [Abstract] [Google Scholar]
- Bishop DH, Roy P. Dissociation of vesicular stomatitis virus and relation of the virion proteins to the viral transcriptase. J Virol. 1972 Aug;10(2):234–243. [Europe PMC free article] [Abstract] [Google Scholar]
- Blumberg BM, Giorgi C, Kolakofsky D. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell. 1983 Feb;32(2):559–567. [Abstract] [Google Scholar]
- Carroll AR, Wagner RR. Inhibition of transcription by immunoglobulins directed against the ribonucleoprotein of homotypic and heterotypic vesicular stomatitis viruses. J Virol. 1978 Feb;25(2):675–684. [Europe PMC free article] [Abstract] [Google Scholar]
- Cartwright B, Smale CJ, Brown F, Hull R. Model for vesicular stomatitis virus. J Virol. 1972 Aug;10(2):256–260. [Europe PMC free article] [Abstract] [Google Scholar]
- Cartwright B, Talbot P, Brown F. The proteins of biologically active sub-units of vesicular stomatitis virus. J Gen Virol. 1970 Jun;7(3):267–272. [Abstract] [Google Scholar]
- Cornell BA, Middlehurst J, Separovic F. The molecular packing and stability within highly curved phospholipid bilayers. Biochim Biophys Acta. 1980 May 23;598(2):405–410. [Abstract] [Google Scholar]
- Crewe AV. High-resolution scanning transmission electron microscopy. Science. 1983 Jul 22;221(4608):325–330. [Abstract] [Google Scholar]
- Emerson SU, Wagner RR. L protein requirement for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1973 Dec;12(6):1325–1335. [Europe PMC free article] [Abstract] [Google Scholar]
- Engel A. Molecular weight determination by scanning transmission electron microscopy. Ultramicroscopy. 1978;3(3):273–281. [Abstract] [Google Scholar]
- Gallione CJ, Greene JR, Iverson LE, Rose JK. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. [Europe PMC free article] [Abstract] [Google Scholar]
- Hainfeld JF, Wall JS, Desmond EJ. A small computer system for micrograph analysis. Ultramicroscopy. 1982;8(3):263–270. [Abstract] [Google Scholar]
- Hartford SL, Lesnaw JA, Flygare WH, MacLeod R, Reichmann ME. Physical properties of New Jersey serotype of vesicular stomatitis virus and its defective particles. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1202–1205. [Europe PMC free article] [Abstract] [Google Scholar]
- Hunt DM, Wagner RR. Inhibition by aurintricarboxylic acid and polyethylene sulfonate of RNA transcription of vesicular stomatitis virus. J Virol. 1975 Nov;16(5):1146–1153. [Europe PMC free article] [Abstract] [Google Scholar]
- Hunt RC, Brown JC. Surface glycoproteins of mouse L cells. Biochemistry. 1974 Jan 1;13(1):22–28. [Abstract] [Google Scholar]
- Lamvik MK. Muscle thick filament mass measured by electron scattering. J Mol Biol. 1978 Jun 15;122(1):55–68. [Abstract] [Google Scholar]
- Lenard J. Virus envelopes and plasma membranes. Annu Rev Biophys Bioeng. 1978;7:139–165. [Abstract] [Google Scholar]
- Lenard J, Compans RW. The membrane structure of lipid-containing viruses. Biochim Biophys Acta. 1974 Apr 8;344(1):51–94. [Europe PMC free article] [Abstract] [Google Scholar]
- Lodish HF, Porter M. Heterogeneity of vesicular stomatitis virus particles: implications for virion assembly. J Virol. 1980 Jan;33(1):52–58. [Europe PMC free article] [Abstract] [Google Scholar]
- McSharry JJ, Compans RW, Choppin PW. Proteins of vesicular stomatitis virus and of phenotypically mixed vesicular stomatitis virus-simian virus 5 virions. J Virol. 1971 Nov;8(5):722–729. [Europe PMC free article] [Abstract] [Google Scholar]
- McSharry JJ, Wagner RR. Lipid composition of purified vesicular stomatitis viruses. J Virol. 1971 Jan;7(1):59–70. [Europe PMC free article] [Abstract] [Google Scholar]
- Mosesson MW, Hainfeld J, Wall J, Haschemeyer RH. Identification and mass analysis of human fibrinogen molecules and their domains by scanning transmission electron microscopy. J Mol Biol. 1981 Dec 15;153(3):695–718. [Abstract] [Google Scholar]
- Mudd JA. Glycoprotein fragment associated with vesicular stomatitis virus after proteolytic digestion. Virology. 1974 Dec;62(2):573–577. [Abstract] [Google Scholar]
- Nagpal ML, Brown JC. Protein and glycoprotein components of phagosome membranes derived from mouse L cells. Int J Biochem. 1980;11(2):127–138. [Abstract] [Google Scholar]
- Nakai T, Howatson AF. The fine structure of vesicular stomatitis virus. Virology. 1968 Jun;35(2):268–281. [Abstract] [Google Scholar]
- Newcomb WW, Brown JC. Role of the vesicular stomatitis virus matrix protein in maintaining the viral nucleocapsid in the condensed form found in native virions. J Virol. 1981 Jul;39(1):295–299. [Europe PMC free article] [Abstract] [Google Scholar]
- Newcomb WW, Tobin GJ, McGowan JJ, Brown JC. In vitro reassembly of vesicular stomatitis virus skeletons. J Virol. 1982 Mar;41(3):1055–1062. [Europe PMC free article] [Abstract] [Google Scholar]
- Pinney DF, Emerson SU. In vitro synthesis of triphosphate-initiated N-gene mRNA oligonucleotides is regulated by the matrix protein of vesicular stomatitis virus. J Virol. 1982 Jun;42(3):897–904. [Europe PMC free article] [Abstract] [Google Scholar]
- Reading CL, Penhoet EE, Ballou CE. Carbohydrate structure of vesicular stomatitis virus glycoprotein. J Biol Chem. 1978 Aug 25;253(16):5600–5612. [Abstract] [Google Scholar]
- Repik P, Bishop DH. Determination of the molecular weight of animal RNA viral genomes by nuclease digestions. I. Vesicular stomatitis virus and its defective T particle. J Virol. 1973 Nov;12(5):969–983. [Europe PMC free article] [Abstract] [Google Scholar]
- Rose JK, Gallione CJ. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. [Europe PMC free article] [Abstract] [Google Scholar]
- Rothman JE, Engelman DM. Molecular mechanism for the interaction of phospholipid with cholesterol. Nat New Biol. 1972 May 10;237(71):42–44. [Abstract] [Google Scholar]
- Ruigrok RW, Andree PJ, Hooft van Huysduynen RA, Mellema JE. Characterization of three highly purified influenza virus strains by electron microscopy. J Gen Virol. 1984 Apr;65(Pt 4):799–802. [Abstract] [Google Scholar]
- Schloemer RH, Wagner RR. Association of vesicular stomatitis virus glycoprotein with virion membrane: characterization of the lipophilic tail fragment. J Virol. 1975 Aug;16(2):237–240. [Europe PMC free article] [Abstract] [Google Scholar]
- Schmidt MF, Schlesinger MJ. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 1979 Aug;17(4):813–819. [Abstract] [Google Scholar]
- Schubert M, Harmison GG, Meier E. Primary structure of the vesicular stomatitis virus polymerase (L) gene: evidence for a high frequency of mutations. J Virol. 1984 Aug;51(2):505–514. [Europe PMC free article] [Abstract] [Google Scholar]
- Simpson RW, Hauser RE. Structural components of vesicular stomatitis virus. Virology. 1966 Aug;29(4):654–667. [Abstract] [Google Scholar]
- Smith PR. An integrated set of computer programs for processing electron micrographs of biological structures. Ultramicroscopy. 1978;3(2):153–160. [Abstract] [Google Scholar]
- Steven AC, Hainfeld JF, Wall JS, Steer CJ. Mass distributions of coated vesicles isolated from liver and brain: analysis by scanning transmission electron microscopy. J Cell Biol. 1983 Dec;97(6):1714–1723. [Europe PMC free article] [Abstract] [Google Scholar]
- Steven AC, Wall J, Hainfeld J, Steinert PM. Structure of fibroblastic intermediate filaments: analysis of scanning transmission electron microscopy. Proc Natl Acad Sci U S A. 1982 May;79(10):3101–3105. [Europe PMC free article] [Abstract] [Google Scholar]
- Ware BR, Raj T, Flygare WH, Lesnaw JA, Reichmann ME. Molecular weights of vesicular stomatitis virus and its defective particles by laser light-scattering spectroscopy. J Virol. 1973 Jan;11(1):141–145. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jvi.54.2.598-607.1985
Read article for free, from open access legal sources, via Unpaywall:
https://jvi.asm.org/content/jvi/54/2/598.full.pdf
Free after 4 months at jvi.asm.org
http://jvi.asm.org/cgi/reprint/54/2/598
Free to read at jvi.asm.org
http://jvi.asm.org/cgi/content/abstract/54/2/598
Citations & impact
Impact metrics
Article citations
Recombinant origin and interspecies transmission of a HERV-K(HML-2)-related primate retrovirus with a novel RNA transport element.
Elife, 13:e80216, 22 Jul 2024
Cited by: 0 articles | PMID: 39037763 | PMCID: PMC11379458
Absolute quantification of viral proteins from pseudotyped VSV-GP using UPLC-MRM.
Microbiol Spectr, 12(8):e0365123, 25 Jun 2024
Cited by: 0 articles | PMID: 38916347 | PMCID: PMC11302727
Chandipura viral glycoprotein (CNV-G) promotes Gectosome generation and enables delivery of intracellular therapeutics.
Mol Ther, 32(7):2264-2285, 03 May 2024
Cited by: 0 articles | PMID: 38702887
Directional change during active diffusion of viral ribonucleoprotein particles through cytoplasm.
Biophys J, 123(17):2869-2876, 25 Apr 2024
Cited by: 1 article | PMID: 38664967
Mechanisms of active diffusion of vesicular stomatitis virus inclusion bodies and cellular early endosomes in the cytoplasm of mammalian cells.
PLoS One, 19(3):e0290672, 14 Mar 2024
Cited by: 0 articles | PMID: 38483897
Go to all (144) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Stereo images of vesicular stomatitis virus assembly.
J Virol, 57(3):922-932, 01 Mar 1986
Cited by: 45 articles | PMID: 3005636 | PMCID: PMC252823
Role of the vesicular stomatitis virus matrix protein in maintaining the viral nucleocapsid in the condensed form found in native virions.
J Virol, 39(1):295-299, 01 Jul 1981
Cited by: 62 articles | PMID: 6268817 | PMCID: PMC171289
Assembly of vesicular stomatitis virus nucleocapsids in vivo: a kinetic analysis.
J Virol, 32(1):304-313, 01 Oct 1979
Cited by: 20 articles | PMID: 232181 | PMCID: PMC353554
The nucleocapsid of vesicular stomatitis virus.
Sci China Life Sci, 55(4):291-300, 01 Apr 2012
Cited by: 5 articles | PMID: 22566085
Review
Funding
Funders who supported this work.
NCRR NIH HHS (1)
Grant ID: RR-01777
NIGMS NIH HHS (1)
Grant ID: GM-34036