Abstract
Free full text

γ-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin
Abstract
DNA double-strand breaks (DSBs) are extremely dangerous lesions with severe consequences for cell survival and the maintenance of genomic stability. In higher eukaryotic cells, DSBs in chromatin promptly initiate the phosphorylation of the histone H2A variant, H2AX, at Serine 139 to generate γ-H2AX. This phosphorylation event requires the activation of the phosphatidylinositol-3-OH-kinase-like family of protein kinases, DNA-PKcs, ATM, and ATR, and serves as a landing pad for the accumulation and retention of the central components of the signaling cascade initiated by DNA damage. Regions in chromatin with γ-H2AX are conveniently detected by immunofluorescence microscopy and serve as beacons of DSBs. This has allowed the development of an assay that has proved particularly useful in the molecular analysis of the processing of DSBs. Here, we first review the role of γ-H2AX in DNA damage response in the context of chromatin and discuss subsequently the use of this modification as a surrogate marker for mechanistic studies of DSB induction and processing. We conclude with a critical analysis of the strengths and weaknesses of the approach and present some interesting applications of the resulting methodology.
INTRODUCTION
DNA organization and histones
The accommodation of ~2 m of DNA in the ~10 µm nucleus of a human cell is made possible through its organization into chromatin. The basic unit of chromatin, the nucleosome, consists of 147 base pairs of DNA wrapped in nearly two (1.7) left-handed superhelical turns around a ~100 kDa octamer of histone proteins, each separated from the next one by a linker section of variable length (20–80 bp) (Figure 1A). As a result, the 6.4 × 109 bp of a human diploid cell are organized into over 30 million nucleosomes. Four small (100–135 aa), highly conserved histone proteins, H2A, H2B, H3 and H4, each sharing the histone-fold motif and present in two copies (Figure 1B), form the histone octamer and compact the DNA through the mediated wrapping by approximately 3-fold (1). For nucleosome assembly, DNA is first wrapped around the H3–H4 tetramer before the addition of two H2A–H2B dimers completes the core (2).
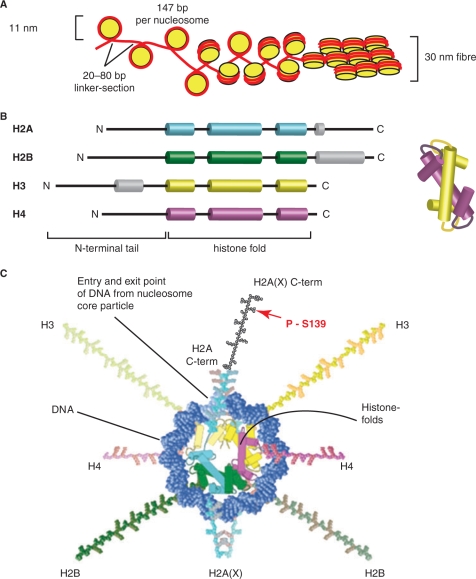
H2AX in the context of chromatin. (A) Organization of DNA in chromatin. One hundred and forty-seven base pairs of DNA (red) are wrapped around a nucleosome (yellow) consisting of eight histone proteins (two H2A/H2B dimers and two H3/H4 dimers), thus forming the 11 nm nucleosome. The histones dimerize via the histone fold motif and four histone dimers form the nucleosome core. Nucleosomes are separated by linker DNA sections of 20–80 bp in length. The DNA wraps in 1.7 turns around the nucleosome forming 142 hydrogen bonds at the DNA histone interface. The histone tails protrude from the nucleosome core and can be modified, for instance by acetylation, phosphorylation or ubiquitinylation. Further condensation of chromatin, as in the 30 nm fiber, allows a 100-fold compaction of DNA (schematic representation; the actual organization of the nucleosomes in the 30 nm fiber is still under investigation). (B) All histone proteins share the highly conserved histone fold motif (displayed in color) containing the three alpha helices involved in nucleosome core organization. Alpha helical domains outside the histone fold domain are shown in gray. The structure on the right illustrates how two histone fold domains interact for dimer formation. (C) A model of the nucleosome core particle showing DNA interactions with core histones (redrawn in a modified form from Ref. (148)).The DNA entry and exit points are localized at the H2A/H2B dimer. The H2AX C-terminus, which is 14 amino acids longer than that of H2A, is drawn here (there are no structural data available and the schematic drawing is only for demonstration purposes) in black with a red arrow marking the phosphorylation site within the SQEY motif.
Higher levels of chromatin condensation and nuclear organization (3) are mediated by nonhistone proteins, as well as by interactions between the N-terminal regions of core-histones in adjacent nucleosomes, and lead to the formation of the 30 nm fiber (Figure 1A), which achieves a 100-fold compaction of the DNA (4). The further condensation of the 30 nm fiber as well as higher levels of chromatin condensation, which culminate with the 10 000-fold compaction of the stretched DNA fiber in the ~700 nm metaphase chromosomes, are less well understood (5), but are facilitated by the linker histone H1 and condensins (6).
The organization of DNA into chromatin is not only important for resolving problems of spatial accommodation and organization, but it is also essential for the functional utilization of the DNA and the proper coordination of its metabolic activities (7,8). By organizing DNA, histones and nonhistone proteins generate a structural barrier to thousands of DNA-binding factors and DNA enzymes, whose uncontrolled access would compromise any meaningful activity and function of the DNA molecule. Therefore, it comes as no surprise that organization of DNA into chromatin is instrumental for the multitude of gene expression patterns observed in the different cell lineages of multicellular organisms.
The highly dynamic (0.25 s average residence time of histone core octamer on DNA) stability of chromatin (9) is ensured by 142 hydrogen bonds at the interface between DNA and the histone core. In addition, numerous hydrophobic interactions and salt linkages allow the wrapping around the nucleosome core of nearly any DNA sequence (1). To ensure accessibility and chromatin structure facilitating the different functions of the DNA, interactions with core histones are regulated by numerous covalent modifications, many of which are localized at the amino terminal histone tails protruding from the nucleosome core (Figure 1B and C), as well as by specialized variant core histones (10). These modifications reduce the affinity of histone tails for adjacent nucleosomes, thereby affecting chromatin structure. However, the most profound effect of histone modifications is their ability to attract specific proteins to a stretch of chromatin that has been appropriately modified (10,11). These recruited proteins define and initiate biological functions in a manner intimately coordinated with local chromatin structure.
DNA DSB repair in the context of chromatin
Modification of chromatin structure has been extensively studied in the context of transcriptional regulation. However, there is now ample evidence that modification of chromatin structure also plays a central role in the regulation of DNA repair (12). Broadly speaking, DNA lesions can be classified in two categories on the basis of their effect on chromatin integrity. The first category, which includes base and nucleotide damages as well as single interruptions of the sugar phosphate backbone, does not overly risk chromatin integrity or function, and error-free repair can be accommodated with limited, local modification of the chromatin structure using the complementary DNA strand as a template. The second category, however, which is mainly comprised of DNA double-strand breaks (DSBs), but may also include some types of DNA-protein crosslinks, can bring chromatin to a state severely undermining its integrity and function. This type of DNA lesion may be partly recognized by the resulting destabilization of chromatin with the resulting signaling and repair coordinated by associated modifications in chromatin structure. In comparison with other types of DNA lesions, DSBs generate the additional complication that error-free restoration is possible only through copying of lost sequence information from a different DNA molecule (or a different part of the same molecule), as the complementary strand is also damaged. In view of the specific requirements for error-free DSB repair, as well as the immediate risks a DSB generates to chromatin stability, it is not surprising that the DSB is among the most severe DNA lesions. Unrepaired or misrepaired DSBs induced by physical agents such as ionizing radiation, chemical agents such as topoisomerase inhibitors, oxidative stress, aberrant DNA replication, aberrant V(D)J or class switch recombination, etc., can cause genomic instability and cancer if the cell escapes death altogether (13,14).
Modification of chromatin structure will be important for all pathways utilized by the cell to repair DSBs. Particularly, homologous recombination repair (HRR), the only error-free pathway, will require extensive chromatin modification to facilitate its essential steps: initial processing of DNA ends, search for homology, invasion into the intact homologous double helix, formation of a Holiday junction, DNA synthesis with the associated branch migration and final resolution of the Holiday junction (Figure 2A) (15–17). Also, error-prone pathways utilized in the repair of DSBs have obvious requirements for chromatin modification. Thus, the search of homology required during single strand annealing (SSA) (Figure 2D) (17), as well as the end processing step, will be facilitated by chromatin modifications. Finally, the homology-independent removal of DSBs by nonhomologous end joining (NHEJ) (18) may require chromatin modification for efficient recognition and ligation (Figure 2B), although this modification will be more limited than that required for the other repair pathways and may depend upon the actual structure of chromatin in the vicinity of the break.
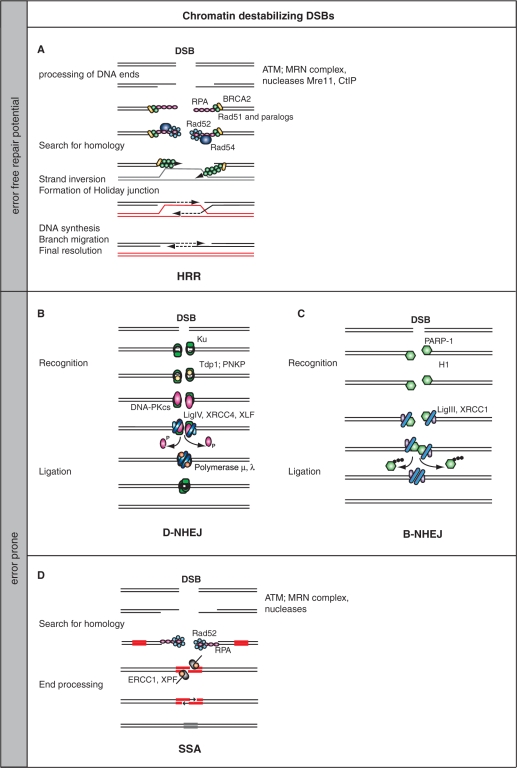
DSB repair pathways. (A) Homologous recombination repair (HRR). After the initial sensing of the DSB by MRN and the activation of ATM, H2AX is phosphorylated, which in turn elicits a sequence of signaling events thought to ultimately cause the activation of nucleases such as Mre11 and CtIP to process the DNA ends and generate ssDNA with 3′ overhangs. ssDNA is bound by RPA, which is subsequently exchanged by Rad51 and Rad51 paralogs. This exchange is facilitated by Rad52, Rad54 and BRCA2. The Rad51-decorated DNA fiber initiates strand invasion into an intact homologous DNA molecule that leads to the formation of a Holiday junction. The DNA sequence around the DSB is copied by DNA synthesis associated with branch migration, and the process is completed by resolution of the Holiday junction. HRR is a templated repair process and is therefore error free (15–17). (B) DNA-PK-dependent nonhomologous end joining (D-NHEJ). DNA ends are recognized by Ku, which recruits, after processing by Tdp1 or PNKP, DNA-PKcs. Upon end-binding, DNA-PKcs is activated and phosphorylates itself and possibly also other proteins (like H2AX on an adjacent nucleosome). Phosphorylated DNA-PKcs is thought to be released from the DNA end, which allows the DNA ligase IV/XRCC4/XLF complex to mediate end-ligation possibly with the help of a DNA polymerase that catalyzes gap filling (19,149,150). (C) Back up pathway of nonhomologous end joining (B-NHEJ). There is evidence that cells of higher eukaryotes with defects in D-NHEJ rejoin the majority of DSBs using an alternative repair pathway that is not utilizing any of the HRR-associated activities (19). This pathway is therefore termed backup NHEJ (B-NHEJ). Although details of this pathway remain to be elucidated, there is evidence that it utilizes the PARP-1/DNA Ligase III/XRCC1 repair module known to be involved in the repair of SSB and base damages (151–155), and that its function is facilitated by the linker histone H1 (156). (D) Single strand annealing (SSA). This repair pathway shares features of HRR and NHEJ, and is best described in yeast (17). After the initial sensing of the DSBs and processing of the ends by an exonuclease, possibly the MRN complex, the generated ssDNA tails are loaded with RPA. Ends are resected until homologous regions are exposed on the two DNA strands and pairing of these regions is facilitated by Rad52. After appropriate gap filling and removal of the overhangs by the ERCC1/XPF nuclease, a ligation step restores DNA integrity. The repair pathways described in B, C and D are associated with loss (and sometimes gain) of DNA material and are by nature error prone.
Despite their fundamental conceptual differences, the unifying characteristic of all pathways of DSB repair is that they restore the structural integrity of the DNA, which ensures the preservation of chromatin organization. This must have been a central consideration in the evolution of this constellation of DSB repair pathways, because two of them, NHEJ and SSA (Figure 2), fail to ensure sequence preservation, which is the fundamental characteristic of other repair pathways. The fact that error-prone repair pathways, particularly NHEJ, are predominantly used for the removal of the majority of DSBs in higher eukaryotes (19), allows the speculation that structural DNA integrity and the associated preservation of chromatin organization has taken priority over preservation of local DNA sequence in multicellular organisms.
Eukaryotic cells react to DNA damage with the so-called DNA damage response (DDR), a sophisticated molecular circuitry developed to detect, signal and repair DNA damage (20–22). Integral parts of the DDR are signaling cascades (checkpoints) that regulate key aspects of the cellular metabolism by interacting with the cell cycle engine. Chromatin modification is directly implicated in the development of these signaling cascades (23).
DNA damage recognition and processing in the context of chromatin will require chromatin modification and will elicit events aiming at the coordination of checkpoint signaling with DNA repair or apoptosis. The ultimate goal is the preservation of genomic integrity through the coupling of repair to other essential cellular metabolic activities such as gene expression, DNA replication, cell cycle progression and life or death decisions (20–22). It comes as no surprise, therefore, that efforts to describe and understand the mechanistic significance of DNA damage-associated histone modifications have been particularly intensified recently (23).
The histone variant H2AX is at the center of cellular responses to DSBs
Although several DNA damage-associated histone modifications have been described, here we focus on the most conspicuous one that has been at the center of research activities during the last several years: the modification of the H2A variant, H2AX. H2AX is one of the most conserved H2A-variants (Table 1 and Figure 3), and is present in chromatin at levels that vary between 2 and 25% of the H2A pool, depending on the cell line and tissue examined. H2AX moved to the center of cellular responses to DNA damage after the discovery that it becomes locally phosphorylated, to generate γ-H2AX, in the vicinity of DSBs (24–26). The combination of phosphospecific antibodies that recognize the phosphorylated S-139 residue of γ-H2AX with immunofluorescence microscopy documented the local phosphorylation through the formation of distinct foci in the vicinity of DSBs and allowed monitoring of their induction and repair (Figure 5C). Thus, a wide spectrum of applications could be developed ranging from mechanistic studies in biology to useful applications in oncology and radiation protection (see below).

Conserved H2AX variants. H2AX is highly conserved through evolution. Shown here is an amino acid sequence comparison between human, mouse, Xenopus and Drosophila. Identical amino acids are shown in dark boxes, and light boxes display blocks of similar amino acids. Light grey letters indicate nonsimilar amino acids, grey letters conserved and black letters weakly similar amino acids. The SQEY motif is underlined, and Serine 139, the residue that becomes phosphorylated upon induction of DSBs, is displayed in red. H2AX protein sequences were obtained from Swiss-Prot/TrEMBL and aligned using the VectorNTI software.
Table 1.
H2A Variants and Properties (147)
| Major variants––single band on SDS gel (no difference in function detected) | ||
| H2A1 | 10 genes | Six genes identical in sequence; four vary in up to four positions |
| Peptides are not resolvable in Triton X 100 gel electrophoresis | ||
| H2A2 | 1 gene (H2AO) | Leucin residue 51 is replaced by methionine |
| Altered electrophoretic mobility | ||
| Minor variants––peptide sequence differs considerably from bulk H2A | ||
| H2AX | 1 gene | Highly conserved |
| Varies primarily in the C-terminus | ||
| H2AZ | 1 gene | Highly conserved |
| Varies in many positions throughout the C-terminus | ||
| macroH2A1 | 1 gene | Recent in evolution |
| X-Chromosome silencing | ||
| macroH2A2 | 1 gene | Recent in evolution |
| X-Chromosome silencing | ||
| H2A-Bbd | 1 gene | Distantly related to H2A |
| Largely excluded from inactive X-Chromosome | ||
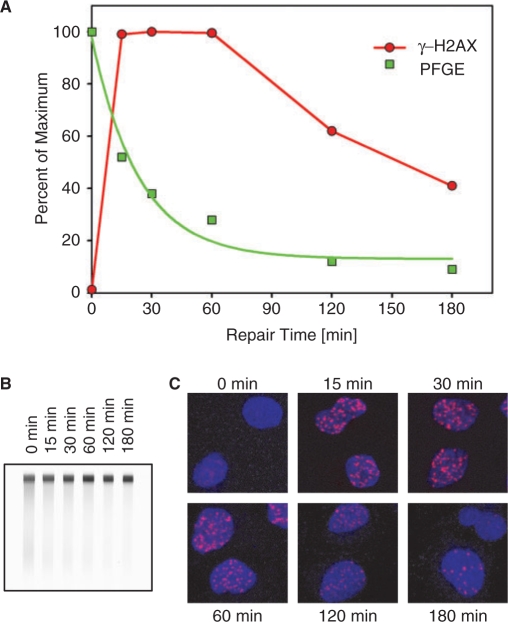
Comparison of DSB repair kinetics as measured by pulsed-field gel electrophoresis (PFGE) with the development of γ-H2AX foci. (A) Plateau-phase A549 cells were exposed to 20 Gy or 1 Gy X-rays and analyzed by PFGE (157) or γ-H2AX immunofluorescence (158), respectively, at the indicated time points. PFGE results (squares) have been normalized to the signal measured at 0 h, while the γ-H2AX results (circles) have been normalized to the maximum number of foci scored. The number of foci per cell was quantified using the Leica Q-Win software with the help of a special routine developed for foci counting. Foci were counted on 3D picture stacks generated on a Leica SP5 confocal microscope. γ-H2AX results were normalized to the maximum number of foci scored per cell—typically reached between 30 min and 1 h after IR. (B) Typical gel used to generate the PFGE results shown in A. DNA is stained with ethidium bromide. (C) Examples of γ-H2AX immunofluorescence at different times after exposure to IR (1 Gy). Cells were fixed with 2% paraformaldehyde, permeabilized with 0.2% Triton X-100 and stained with a phosphospecific primary anti-γ-H2AX antibody and an Alexa 568-labeled secondary antibody. Red shows γ-H2AX foci, blue nuclei stained with DAPI.
An important recognition from the above studies with far-reaching mechanistic consequences is that DSB sensing and processing are associated with a characteristic local modification and/or specific relocalization of the DDR proteins to distinct subnuclear structures observable by immunofluorescence microscopy that are commonly referred to as ‘foci’. When such foci are induced by ionizing radiation, they are referred to as IR-induced nuclear foci (IRIF) (27,28), and their analysis has provided important information on the molecular processes underlying the DDR. H2AX phosphorylation and γ-H2AX foci formation are now generally accepted as consistent and quantitative markers of DSBs, applicable even under conditions where only a few DSBs are present (29). In the following sections, we will review the mechanistic significance of H2AX phosphorylation in the cellular responses to DSBs and will analyze correlations between γ-H2AX foci formation and the processing of DSBs.
The significance of S-139 phosphorylation of H2AX
As outlined in Figure 3, the phosphorylation of H2AX occurs in the SQ motif that occupies a position of four residues from the carboxy terminus of the protein. SQ is followed by an acidic residue and the carboxy-terminus is hydrophobic. These characteristics are strictly evolutionarily conserved and are utilized for highly specific protein–protein interactions during DDR (see below). Notable also is that phosphorylation of S-139 in H2AX is one of the few modifications that occurs at the carboxy terminus of a histone, instead of the more frequently modified amino terminus (Figure 1B and C). It may be relevant that by virtue of the localization and orientation of H2A in the nucleosome, the S-139 phosphorylation will modify the nucleosome at a position right at the entry/exit points of the DNA (Figure 1C).
Phosphorylated H2AX is detected after only a few minutes in cells exposed to IR, and phosphorylation reaches a maximum ~30 min later. There is convincing evidence that the DNA lesion initiating this response is the DSB (both accidental and programmed) (26), and as a result the number of γ-H2AX foci scored approximates the number of DSBs induced (29). This tenant finds support in experiments where DSBs are induced by a 365 nm UVA laser in cells that have incorporated BrdU and are treated with Hoechst 33258 (24,30,31), as well as in experiments where DSBs are induced enzymatically (32).
One particularly relevant aspect of H2AX phosphorylation is that it is not limited to the immediate vicinity, but spreads instead to a large chromatin region surrounding the DSB. It has been estimated that in mammals 0.03% of H2AX is phosphorylated per DSB. From this value and the ~10% representation among H2A variants of H2AX in chromatin, it can be estimated that the modification spreads to a 2 Mbp region of chromatin and comprises ~2000 γ-H2AX molecules (25). However, immunofluorescence analyses indicate that regions fifteen times larger (up to 30 Mbp) can be modified, implying that not every contiguous H2AX molecule is phosphorylated (24,33). It is not clear how H2AX phosphorylation is spatially confined, but 4 Pi microscopy suggests that H2AX is not distributed randomly throughout bulk chromatin but exists in distinct clusters that define the boundaries of γ-H2AX spreading (34). Through extensive but still spatially restricted modification of H2AX, the presence of a dangerous DNA lesion that destabilizes chromatin is ‘translated’ and ‘communicated’ to the chromatin domain(s) at risk. The nearly 30 Mbp broad modification of chromatin through H2AX phosphorylation represents a major amplification when compared to the few damaged base pairs that constitute the initial DSB. Such a signal ‘amplifying’ chromatin modification is indicative of the gravity attached to DSBs by the cell, and may be considered as part of the preparatory platform the cell generates for mounting the associated signaling and repair processes (30).
The H2AX phosphorylation motif, SQ, is a common recognition site for the phosphatidylinositol-3-OH-kinase-like family of protein kinases (PIKKs), and the broad PI-3 kinase inhibitor wortmannin is effective in preventing formation of the corresponding foci (35). In principle, all three major PIKK members, ATM, ATR and DNA-PKcs, have the potential of phosphorylating H2AX, and there is evidence that each of them actually carries out this phosphorylation when others are genetically compromised (36–39). This flexibility among the kinases raises the question of actual contribution under physiological conditions. Among these PIKK kinases, ATM seems best suited for H2AX phosphorylation by virtue of its ability to become activated by immediate, local chromatin modifications associated with DNA breakage (40). Since chromatin modification encompasses entire chromatin domains, ATM will be able to phosphorylate several H2AX molecules within this domain. DNA-PKcs on the other hand, which become activated through its interaction with Ku, after Ku has directly bound to the DNA ends, is likely to have a reduced phosphorylation range requiring longer times for H2AX phosphorylation at longer distances (38,39). Indeed, ATM seems to be the main kinase associated with H2AX phosphorylation under normal physiological conditions (30,41–43), although it appears to require NBS1 for optimal activity (44). Furthermore, formation of γ-H2AX triggered by uncapped telomeres, as well as meiotic recombination-associated DSBs are largely dependent on ATM (45–47). Several aspects and details regarding ATM and DNA-PK activation, as well as their interplay in the phosphorylation of H2AX, remain unknown and are a fruitful area of future investigations (48).
In contrast to directly induced random breaks, DSBs associated with replication stress or UV damage are likely to be detected by ATR (49–51), which upon activation also phosphorylates H2AX. It should, however, be pointed out that ATR is activated through interaction with ATRIP, which recognizes single-stranded regions in the DNA. Such single-stranded regions can arise at stalled replication forks and also following repair of bulky DNA lesions (52). As such H2AX phosphorylation mediated by ATR does not necessarily reflect the presence of a DSB in the genome. This is important to keep in mind when γ-H2AX is scored as an indicator of DSBs, particularly in S-phase cells (see below for more discussion on this issue), and may partly explain the high number of γ-H2AX normally seen in S-phase cells in the absence of DNA-damage-inducing treatments.
The importance of PIKKs for H2AX phosphorylation is also supported by work in yeast. Thus, while S. cerevisiae does not have an H2AX variant, both major H2A isoforms, H2A1 and H2A2, have SQ motifs and are phosphorylated after the induction of DSBs in a Mec1- and Tel1-dependent (the homologs of ATR and ATM, respectively) manner (53). Since yeast mainly utilizes HRR for the removal of DSBs from the genome, it is intriguing that the SQ motif has been sequestered from the major isoforms to a histone variant, H2AX, after NHEJ became the predominant pathway for DSB repair in higher eukaryotes.
Dephosphorylation of γ-H2AX
If phosphorylation of H2AX signals chromatin destabilization through a DSB, it should be reverted to H2A after repair restores chromatin integrity and structure. Such resetting of chromatin could in principle be done either by replacing γ-H2AX in the nucleosome with H2AX, or by dephosphorylating γ-H2AX directly at the nucleosome. In mammalian cells, phosphatase 2A (PP2A) appears to be involved in the dephosphorylation of γ-H2AX (54). It is not clear whether the dephosphorylation takes place in situ, or whether it requires removal of γ-H2AX from chromatin. In this regard, the partial colocalization after DNA damage of γ-H2AX with PP2A is compatible with an in situ dephosphorylation. In yeast, on the other hand, the γ-H2AX homolog is first removed from chromatin and is subsequently dephosphorylated by the histone H2A phosphatase complex (HTP-C), the active subunit of which, Pph3, is 60% identical to PP2A (55), although it is not a direct homolog. As a result of this mode of action, foci loss is observed even in phosphatase-deficient yeast strains. Additional work shows that PP2Cγ likewise mediates γ-H2AX dephosphorylation and may act at the same time as a histone chaperone to deposit dephosphorylated H2A–H2B or H2AX–H2B dimers to incomplete nucleosomes (56). The exchange of H2AX with H2A has recently been shown to be mediated by FACT (for ‘FAcilitates Chromatin Transcription’), a heterodimer of Spt16 and SSRP1, and to be regulated by phosphorylation of H2AX and ADP-ribosylation of Spt16 (57). Notably, for γ-H2AX generated through phosphorylation by ATR during DNA replication, recent results implicate a PP4-phosphatase complex containing PP4C (the homolog of the yeast Pph3), PP4R2 and PP4R3b, in its dephosphorylation, which can occur within nucleosomes (58). More work is needed to better understand aspects of chromatin resetting after completion of DNA repair.
γ-H2AX is a specific and efficient coordinator of DDR signaling
Why does the cell initiate a spatially restricted modification of chromatin in the form of H2AX phosphorylation in response to DSBs? Which aspects of the DDR are facilitated through this modification? Is phosphorylation itself mediating local, nonspecific chromatin conformation changes, or is it instrumental in orchestrating the specific molecular interactions required for DNA damage signal generation and transmission? In this section, we address these questions with emphasis on signaling. In the following section, we then cover the possible role of H2AX in DSB repair.
One could reason that even the simple marking of chromatin with γ-H2AX at sites where DSBs have been induced could be sufficient for the initiation of the response required for their effective processing. Recent results also point to intriguing, highly specific molecular interactions that place γ-H2AX at the early stages of the signaling response.
As noted above, the most profound effect of histone tail modifications is their ability to attract specific proteins (10,11). Precisely, this function may be one of the most relevant contributions of γ-H2AX to DDR. Although γ-H2AX was shown to attract a number of proteins including NuA4, Ino80 and Swr1 of budding yeast (59–61), and the Tip60 chromatin remodeling complex of Drosophila (62), NBS1 (63), 53BP1 (64) and MDC1 (65), the specificity of several of these interactions remains uncertain. Specific recognition of γ-H2AX would require the presence of domains recognizing the carboxy terminus of γ-H2AX in a phosphor-specific manner in the candidate proteins. Two protein domains that are frequently found in proteins involved in DDR have been found to specifically recognize phosphorylated amino acid residues. The forkhead-associated (FHA) domain recognizes phosphorylated threonine residues in a specific sequence context (66). In addition, it has been observed that two consecutive BRCT domains (BRCA1 C-terminal domain) can create a structural element with phosphor–peptide binding capacity (67–70).
Several lines of evidence have recently converged to demonstrate that the BRCT repeats of MDC1 build the predominant recognition module of γ-H2AX in higher eukaryotes, binding with a relatively low Kd of 2.2 × 10−6 M (65,71–73). Crystallography data nicely reveal how this tandem BRCT domain is precisely tailored to recognize the γ-H2AX motif and demonstrate why the proximity of the phosphoserine to the C-terminus of H2AX remains invariable. Overall, these results explain the extremely low tolerance for mutations, as well as the high degree of evolutionary conservation observed in this region of the protein and provide a mechanism for regulating γ-H2AX dephosphorylation (72).
With the interaction between MDC1 and γ-H2AX, the site of the DSB is prepared for signaling and repair (Figure 4). This is because there is evidence that MDC1 also directly interacts in a highly dynamic manner (71) with NBS1 (74), which in the form of MRN complex is required for the activation of ATM (44,75). This interaction is mediated through phosphorylation of MDC1 by casein kinase 2 (CK2) that promotes phosphorylation-dependent interactions with NBS1 through its closely apposed FHA and twin BRCA domains (76). In this way, a positive feed-back loop is generated that extends H2AX phosphorylation to the Mbp regions described above, but how is the initial H2AX phosphorylation event induced? Based on the extremely high affinity of Ku for DNA ends, a likely scenario is phosphorylation through DNA-PK (Figure 4). However, if ATM can also be directly activated by DSBs, it could also function as the initial H2AX kinase—if mechanisms are in place to facilitate accessibility of the kinase to the DSB. In agreement with the model of a positive feedback loop, loss of MDC1 expression, or reduction of its cellular levels by siRNA treatment, reduces H2AX phosphorylation in response to IR probably as a result of a defect in recruiting ATM (65,72,77).
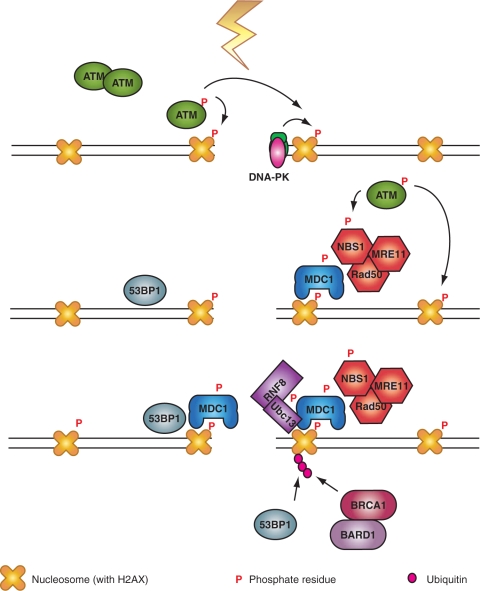
γ-H2AX is a specific and efficient coordinator of DDR signaling. Following the initial phosphorylation of H2AX by ATM, or DNA-PK, a nucleation reaction is initiated starting with the recruitment of MDC1 and continuing with that of the MRN complex to further activate ATM. This generates a feedback loop that leads to further phosphorylation of H2AX and the chromatin modifications required for the recruitment of 53BP1. The activation cascade culminates with the recruitment of RNF8 to phosphorylated MDC1 and the polyubiquitinylation of H2AX to recruit BRCA1/BARD1 (see text for details).
Can extensive phosphorylation of H2AX be mediated by other kinases of the PIKK family, such as DNA-PK? Although DNA-PK efficiently phosphorylates H2AX in the absence of ATM, it has been reported that it fails to do so when ATM is inhibited with the help of a specific inhibitor (42). This suggests a dominant negative effect of inhibited ATM on DNA-PK and is reminiscent to the dominant negative inhibition observed with inhibited DNA-PK in in vitro and possibly also in in vivo repair reactions (78). Notably, in NBS1-deficient cells, DNA-PK contributes significantly to H2AX phosphorylation even after inhibiting ATM (44), suggesting that the dominant negative action of ATM requires the MRN complex. These results and the presumed recruitment of ATM by NBS1 to the sites of DNA damage raise the interesting question of possible crosstalk between ATM/NBS1, on the one hand, and DNA-PKcs/Ku, on the other hand (Figure 4). Since free ends are likely to be initially recruited preferentially by Ku, some mechanism must exist to either block the interaction between DNA ends and Ku, or to facilitate their transition from Ku to the MRN complex. It is also notable that ATM and DNA-PKcs can be recruited to sites of DSBs by direct binding to MDC1 (77,79) providing thus an additional level of interaction and regulation. The transition of DNA ends from Ku to MRN may also be crucial for the regulation of repair pathway selection in cells of higher eukaryotes and requires further investigation.
The above outline provides an intriguing mechanism as to how an initial modification in a core histone is utilized to recruit DDR proteins to the sites of DSBs and to generate a platform for signaling and repair. In addition to MDC1, 53BP1 also has the ability to detect changes in chromatin upon the induction of DSBs. This protein also forms foci in cells exposed to IR with kinetics similar to those of γ-H2AX (80) and appears to detect DNA damage-induced changes in chromatin conformation. Recruitment of 53BP1 to sites of DSBs depends on a region of the protein that contains two consecutive Tudor domains that can bind directly to methylated histone H3 (64,81,82). Because this methylation is constitutive under physiological conditions, only structural modifications will be required to reveal it for a 53BP1 molecule to anchor on it (Figure 4).
While 53BP1 can be targeted to damaged chromatin by the above mechanism, its efficient accumulation and sustained retention within DSB-containing chromatin requires γ-H2AX and MDC1 (64,65,72,77,83). On the basis of these results and the fact that accumulation of MDC1 (and NBS1) at sites of DSBs proceeds faster than that of 53BP1 (71,84), it is possible that γ-H2AX-binding by MDC1 triggers changes in chromatin structure that lead to the exposure of the interaction-epitope for 53BP1. Recent genetic data support this view of sequential activation (85).
Importantly, although NBS1 and 53BP1 are recruited to sites of DSBs in the absence of MDC1, they fail to accumulate and prematurely dissociate from damaged chromatin (71,84). Also, laser-aided generation of DSBs in defined subnuclear volumes indicate that the initial distribution of NBS1, BRCA1 and 53BP1 to sites of DSBs does not require H2AX (86). This indicates that initiation and propagation stages may be mechanistically distinct processes. Work in yeast and Drosophila further indicates that the above events of protein accumulation and modification in response to DSBs may be further enhanced or facilitated by γ-H2AX-mediated recruitment of chromatin remodeling complexes (59–62,87,88).
The above outline mainly focuses on H2AX phosphorylation as a modification of chromatin involved in the coordination of DDR events. However, recent results point to yet another modification that contributes essentially to IRIF formation and the coordination of signaling/repair events: ubiquitinylation (89). While conjugation of ubiquitin chains via K48 generates a degradation signal, K63-linked polyubiquitin chains seem to be involved in DDR signaling (90,91). Recent publications identify the E3 ubiquitin ligase RNF8 as a key enzyme for this modification at the sites of DSBs (92–95) and place it in the chain of events initiated with the phosphorylation of H2AX (Figure 4). Thus, the recruitment of MDC1 to γ-H2AX and the associated consequential activation of ATM are thought to phosphorylate TQXF motifs in MDC1 that act as recruitment sites of the FHA domain of RNF8. This interaction anchors the E3 ligase to the site of the DSB, which then transfers with the help of the E2 conjugating enzyme Ubc13, ubiquitin residues to H2A and to H2AX (92,94,96). This modification is thought to reinforce, through as of yet uncharacterized mechanisms, the recruitment to chromatin of 53BP1, as well as the association of BRCA1–BARD1 complex on IRIF through the proteins Rap80 and Abraxas.
All the above-described events in aggregate place γ-H2AX at the center of a signaling cascade initiated by a DSB, and it may, therefore, come as a surprise that it is largely dispensable for checkpoint responses after exposure to relatively high radiation doses (30,53,83,97) (Figure 4). It has been, therefore, suggested that γ-H2AX is involved in the amplification step required for optimal checkpoint response at relatively low levels of DNA damage (30,98,99). It is evident that a network of interactions is initiated around γ-H2AX, which orchestrates the retention of many DDR proteins at sites of DSBs. While one could speculate that IRIF help to concentrate repair proteins to the sites of DNA damage, it is not clear how this accumulation will support DNA-PK-dependent NHEJ, the main pathway of DSB repair in higher eukaryotes, as the main players of the pathway are not detectable in such IRIF. It is precisely at this point that there is need for further information and additional work. Amplification of the checkpoint signal may be important for a small number of DSBs that require longer repair times and may help reduce checkpoint evasion in the presence of DNA damage (100,101).
Is the contribution of γ-H2AX to DSB repair direct?
The physiological role of H2AX phosphorylation in DNA repair is still a matter of intensive investigation. The first report that suggested a role for H2AX in DNA repair was a genetic study in yeast (53). This study showed that elimination of the C-terminal H2A residue led to an impairment in NHEJ. No clear effect was observed in HRR, which actually appeared increased in the absence of H2A. Further studies in this organism showed that H2A Ser-129 (the equivalent residue of S-139 in humans) is necessary for efficient repair of DSBs during DNA replication (97).
The analysis of H2AX-deficient embryonic stem (ES) cells in mice showed that although H2AX is not essential for NHEJ or HRR, it does somehow modulate the efficiency of these repair pathways (86,102–105). As a result, mouse cells lacking H2AX are radiosensitive and display deficits in DNA damage repair (106). Additionally, H2AX knock out mice show male-specific infertility and reduced levels of secondary immunoglobulin isotypes suggesting defects in class switch recombination (CSR) (83). Indeed, efficient resolution of DSBs induced during CSR in lymphocytes requires H2AX (102,104), and its absence is associated with chromosome abnormalities involving the immunoglobulin locus (107,108). More recent results in DT40 suggest an involvement (probably indirect) of γ-H2AX in HRR and a collaborative function with the Rad51 paralog, XRCC3 (109). Particularly intriguing is also the observation that H2AX is involved in the repair of a subset of DSBs, the repair of which requires, due to some as of yet unidentified reasons, the ATM kinase and the Artemis nuclease (110).
But how is γ-H2AX facilitating DSB repair? The results presented in the previous section suggest that this may be mediated by the contribution of γ-H2AX to signaling and the associated efficient activation of the checkpoint response. Alternatively, it has been suggested that DSB repair may be assisted directly by facilitating the synapsis of DNA ends (104,111). Chromatin reorganization mediated by γ-H2AX could prevent the separation of broken ends and thus facilitate rejoining. Notably, H2AX phosphorylation has been identified over the condensed XY chromosome in male meiotic prophase I (112). This pattern of phosphorylation is independent of meiotic recombination-associated DSBs (112,113), and it is also independent of ATM and DNA-PK (113). The observation that DSBs induce rapid local decreases in the density of chromatin (31), and that nucleosomes in the vicinity of the DSB are repositioned (114), points to the importance of appropriate changes in chromatin structure, which will facilitate the synapsis of broken DNA ends in preparation for rejoining.
Despite the above possible scenarios, the fact that direct effects of H2AX deficiency on DNA repair are subtle suggests that γ-H2AX supports repair of selected DSBs and/or that it specifically assists specific repair pathways (110). If the presumed function of concentrating DNA repair factors and tethering DNA ends together is of no consequence for the repair of the majority of DSBs, one can speculate a role in the repair of DSB clusters similar to those generated during CSR. More work is certainly required to clarify these important aspects of DDR.
Associations between γ-H2AX foci and DSBs: facts and caveats
The DSB-dependent formation of γ-H2AX not only opened the way to the intriguing mechanistic studies on DDR described above, but it also provided powerful means for indirectly visualizing DSBs in eukaryotic cells. The rational framework for the application of γ-H2AX foci formation to the quantification of DSBs was generated by a series of studies showing that lesions other than DSBs have no detectable foci formation potential, and by correlative studies suggesting that the numbers of DSBs estimated by scoring γ-H2AX foci is in agreement with extrapolations from other methods (29,110,115) (see below). Further support was also provided by experiments in which DSBs were specifically and rather exclusively induced in cellular DNA via the disintegration of 125I, incorporated into the DNA in the form of IdU (116). Under these conditions, a nearly one-to-one correlation was found between the calculated number of 125I disintegrations per cell, which closely approximates the number of DSBs, and the number of γ-H2AX foci scored (26). As a result of these studies, and together with the relative simplicity and sensitivity of the method, scoring of γ-H2AX foci became the most popular approach that is presently being used for measuring induction and repair of DSBs in cells exposed to various genotoxic agents under widely different experimental conditions and has allowed mathematical formulations of the repair kinetics (117).
When contrasted with other methods used in the past to measure DSBs, scoring of γ-H2AX foci comes with some distinctive advantages, but it is also associated with shortcomings that should be carefully considered in highly quantitative or purely mechanistic studies. Traditionally, the quantification of DSBs in cells is based on the associated size reduction of the DNA molecules, and is achieved by physical methods encompassing neutral sucrose density gradient centrifugation, neutral filter elution, as well as gel electrophoresis approaches including single-cell gel electrophoresis and pulsed-field gel electrophoresis (PFGE). Physical methods of DSB quantification typically have sensitivities that require the use of doses above 5 Gy for a reliable assessment of the rejoining kinetics. Since doses in this range largely compromise the reproductive integrity of the majority of human or rodent cells, it is considered a great advantage that scoring of γ-H2AX foci allows the measurement of DSBs at doses well below 5 Gy and, therefore, in a physiologically and therapeutically relevant range (29). In addition, physical methods of DSB detection require DNA free of histones and other DNA associated proteins which is usually achieved by lysis at high temperatures. It has been suggested that lysis at high temperatures transforms to DSBs heat labile lesions, which can confound the assessment of the rejoining kinetics (118). Scoring of γ-H2AX foci eliminates this potential source of error, which can also be reduced by running lysis in the physical methods of detection at low temperatures (118).
Despite these advantages of γ-H2AX as a marker of DSBs, the method has limitations mainly because it does not follow the actual fate of the physical DSB, but rather registers cellular metabolic activities initiated to facilitate and optimize DSB repair. This is implicit in the mechanism of γ-H2AX production described above and is also clearly reflected in the kinetics of appearance of γ-H2AX foci after exposure to IR. Figure 5 shows, in a typical experiment carried out in our laboratory using for plateau-phase A549 cells exposed to 1 Gy of X-rays, that full development of γ-H2AX foci requires 30 min and that there are no real signs of reduction before 1 h. PFGE, on the other hand, carried out with the same batch of cells, shows an immediate reduction in the DSB burden, proceeding with half times of ~20 min despite the higher radiation dose used (20 Gy). As a result of this rapid kinetics, DSBs cannot be detected by PFGE 1 h after irradiation when the levels of γ-H2AX foci are still at the maximum.
Since the higher dose used in PFGE is more likely to have slowed down, rather than to have sped up DSB rejoining, the above results indicate obvious disparities in the measurements of DSB repair kinetics with the two methods, particularly at a time resolution of the order of 1 h, and although the delayed kinetics of γ-H2AX foci development can be rationally explained by the time required to initiate and to sustain the precisely coordinated biochemical events leading to the development of a mature focus (see previous sections), it is evident that this fact compromises nevertheless the timely resolution of the repair kinetics obtained by scoring γ-H2AX foci. Furthermore, Figure 5 also shows that the kinetics of γ-H2AX decay, although prompt after 1 h, never approaches the initial speed of the DSB repair measured by PFGE (t50 ~100 versus 20 min—or 5-fold slower). Thus, in addition to the ~1 h uncertainty regarding the actual fate of the physical DSBs, there is also uncertainty with the kinetics of their removal.
The above limitations, which with variations have been observed in several cell systems (33,119) and have been reported to show a DSB-dose dependence (120), have no grave or immediate consequences for several applications that do not require fine resolution in the repair kinetics, or when emphasis is placed on the level of residual DSBs. However, as the DDR mechanism becomes better defined and an increasing amount of detail is added to its constituent steps, increased resolution in the kinetics will become important. In such cases, the above-discussed limitations should be carefully considered in the interpretation of the results obtained, and the means to overcome them should be developed.
The disparity between the actual removal of DSBs, as measured by physical methods of DSB detection, and the removal of γ-H2AX foci may increase when chemical or genetic manipulations that affect the phosphorylation cascade of H2AX are employed as tools, and may further depend on the severity of the DSB (121). Thus, use of PIKK or phosphatase inhibitors, or genetic manipulation of their activity, may alter foci formation and decay in a way that further uncouples it from the physical removal of the DSBs. This is experimentally illustrated in Figure 6, where we exposed A549 cells to 50 nM Calyculin A, a nonspecific inhibitor of PP2A, a phosphatase involved in the dephosphorylation of γ-H2AX (54). Although under the experimental conditions employed treatment with Calyculin A leads to a complete stop in γ-H2AX foci decay (122), it only has a small effect on the physical removal of DSBs as measured by PFGE. Experiments with elutriated HeLa, G1 cells show similar trends, suggesting that the effect is not cell line specific, although others have arrived to different conclusions (123). It would be inaccurate to conclude on the basis of γ-H2AX foci data shown in Figure 6 that DSB rejoining is completely inhibited by Calyculin A. Notably, delayed and stage-specific phosphorylation of H2AX was also observed in irradiated mouse embryos (124).

Effect of the phosphatase inhibitor Calyculin A on DSB repair kinetics and γ-H2AX foci development and decay. (A) Plateau-phase A549 cells were incubated with 0 or 50 nM Calyculin A 15 min prior to exposure to 20 or 1 Gy X-rays for PFGE or γ-H2AX immunofluorescence, respectively, and allowed to repair at 37°C for the indicated periods of time (other details of experimental design as in Figure 5). Results are shown normalized as described in Figure 5. (B, C) Typical PFGE gels used to generate the results shown in A for cells treated with 0 or 50 nM Calyculin A. DNA is stained with ethidium bromide. (D, E) γ-H2AX immunofluorescence at different times after irradiation and incubation with 0 or 50 nM Calyculin A. Other details as in Figure 5.
Discrepancies between γ-H2AX foci decay and DSB removal have also been reported in some cell systems in the absence of treatment. Thus, in one study, 30% of the initial γ-H2AX signal was present 8 h after IR, although no DSBs could be detected at that time (125). Therefore, when interpretating γ-H2AX results, the possibility should be considered that physical DSBs are removed from areas of chromatin that remain marked with γ-H2AX. It can be hypothesized that γ-H2AX continues marking the sites of some DSBs after resealing by NHEJ to facilitate additional processing by HRR (126,127).
Interesting results pointing to a divergence between physical repair of a DSB and development and decay of H2A foci have been recently reported in yeast. In this organism, the kinetics of γ-H2A loss (equivalent to γ-H2AX in higher eukaryotes) correlated with the appearance of early gene conversion intermediates rather than with the ultimate repair of the DSB (55). This indicates that the signal triggering γ-H2A loss might not be the completion of DSB repair but rather the completion of certain repair steps before the final sealing of the DSB. In this case, loss of foci will not immediately signify removal of the discontinuity in the DNA. As a result, HRR-defective strains of yeast have normal γ-H2A response despite their DSB repair defect. These observations, although opposite from those made in higher eukaryotes, further emphasize that high resolution analysis of DSB repair may be compromised when based exclusively on γ-H2AX analysis.
An additional confounding factor in the analysis of induction and repair of DSBs via γ-H2AX foci quantification comes from the observation that H2AX phosphorylation is diminished in areas of heterochromatin (128,129). We have also noted similar trends that are particularly striking in LIG4−/−-deficient MEFs exposed to high doses of IR. Figure 7 shows a representative example of a cell exposed to 16 Gy and analyzed 3 h later. Because of the DSB repair defect in these cells, γ-H2AX foci formation is still at a maximum at this time. It is evident that very few γ-H2AX foci are detected in the darkly stained areas of heterochromatin—despite the fact that the increased amount of DNA in these areas will lead to increased presence of DSBs after IR. Differential formation, or detection, of γ-H2AX in regions of chromatin with different organization will bias DSB analysis based on γ-H2AX foci formation. Weak H2AX phosphorylation in heterochromatin is also found in yeast, and in mouse fibroblasts, an increase of γ-H2AX foci size is observed after chromatin becomes more accessible (130). Notably, recent results indicate eviction of heterochromatin protein 1β (HP1 β) bound to lysine-9-methylated histone H3 after DNA damage through phosphorylation on Thr51 possibly by CK2 (131). This modification promotes H2AX phosphorylation and suggests a mechanism for γ-H2AX generation in areas of heterochromatin. Notably, a recent report postulates that ATM signaling temporarily perturbs heterochromatin via KAP-1 to facilitate DSB repair in these rather inaccessible regions of chromatin (132). However, H2AX phosphorylation is significantly slower in mitotic as compared to G1 CHO cells (119), in agreement with reduced γ-H2AX formation under conditions of condensed chromatin.
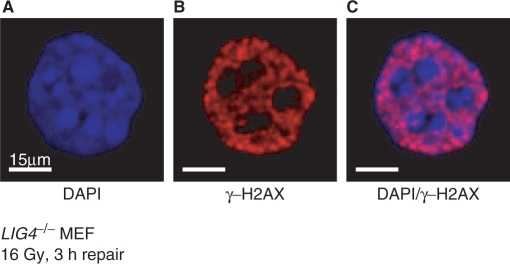
γ-H2AX foci are preferentially formed in regions of euchromatin. Elutriated G2 cells of DSB repair-deficient LIG4−/− MEFs were exposed to 16 Gy X-rays and analyzed for γ-H2AX immunofluorescence 3 h later. The picture on the left shows a DAPI-stained nucleus. Bright areas correspond to nuclear regions with increased DNA presence, thought to reflect densely packaged heterochromatin. The picture at the center shows γ-H2AX immunofluorescence obtained as described in Figure 5. The picture on the right shows an overlay of the two images with DNA displayed in blue and γ-H2AX foci in red. Note the nearly complete absence of γ-H2AX foci from heterochromatic areas, as well as from areas in the nucleus with reduced amounts of DNA.
Whether or not unrejoined DSBs always underlie visible γ-H2AX foci, it is clear that foci presence signifies a detectable, DSB-related modification of chromatin. If areas of heterochromatin have diminished levels of γ-H2AX, one can speculate that γ-H2AX is not required for DSB repair in condensed chromatin; in fact, γ-H2AX may facilitate DSB repair in euchromatin by conferring heterochromatin-like organization (discussed above), which may also explain the observed inhibition of transcription at the foci sites (133). Such a mechanism of γ-H2AX action will favor NHEJ but will act inhibitory on HRR. Further insight is required to address this important aspect of γ-H2AX function.
The afore-outlined potential confounding factors will need to be carefully considered when γ-H2AX is used to analyze DSB repair within short time intervals—for example, in experiments designed to investigate responses in specific phases of the cell cycle. Under these circumstances, it will be important to employ alternative methods to support any conclusions drawn on the basis of γ-H2AX foci formation. An interesting twist to the role of γ-H2AX foci is the recent observation that the immobilization of signaling molecules such as NBS1, MRE11, MDC1 or ATM on chromatin can generate DDR as measured by the generation of γ-H2AX in the absence of DNA damage (134). Similar results were also obtained in yeast when Mec1–Ddc2 and the PCNA-like 9-1-1 complex were immobilized (135). These observations point to hierarchical structures in DDR, which are likely to have important mechanistic ramifications.
APPLICATIONS OF γ-H2AX DETECTION
Analysis of γ-H2AX foci has found numerous applications. One of them is analysis and prediction of cell radiosensitivity to killing. A correlation was reported between the half-times of loss of γ-H2AX as measured by flow cytometry and clonogenic survival in cell lines of differing radiosensitivity to killing (136,137). It was also shown for 18 human tumor cell lines that a number of <3 γ-H2AX foci 24 h after irradiation was predictive for clonogenic survival (138). Similar results were obtained for cells from xenograft tumors of irradiated mice (139). On the other hand, γ-H2AX was unable to predict the efficacy of antioxidant radioprotective compounds (140). Thus, γ-H2AX has the potential of developing to a useful predictor of cellular radiosensitivity to killing and may find application in the clinic during treatment of human tumors with ionizing radiation, in the evaluation of interindividual variations in radiosensitivity (141,142), in the analysis of the endogenous DSB load (143) and even in the prediction of dose in nuclear accidents or terrorist attacks involving radioactive materials. In line with this expectation, γ-H2AX fluorescence intensity is taken as a sensitive test for the diagnosis of AT syndrome (144,145), and γ-H2AX foci formation has been used as a measure for whole body dose after radiotherapy (146).
FUNDING
Deutsche Forschungsgemeinschaft (DFG), Bundes Ministerium fuer Bildung und Forschung (BMBF), European Union (EU). Funding for open access charge: DFG.
ACKNOWLEDGEMENTS
Special thanks go to Dr Dennis Leeper and Nancy Mott for comments on the manuscript.
REFERENCES
Articles from Nucleic Acids Research are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/nar/gkn550
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/nar/article-pdf/36/17/5678/7182069/gkn550.pdf
Free to read at nar.oxfordjournals.org
http://nar.oxfordjournals.org/cgi/content/abstract/36/17/5678
Free to read at nar.oxfordjournals.org
http://nar.oxfordjournals.org/cgi/reprint/36/17/5678.pdf
Free to read at nar.oxfordjournals.org
http://nar.oxfordjournals.org/cgi/content/full/36/17/5678
Citations & impact
Impact metrics
Article citations
Chitosan biomineralized with ions-doped nano-hydroxyapatite tunes osteoblasts metabolism and DNA damage.
J Biol Eng, 18(1):60, 25 Oct 2024
Cited by: 0 articles | PMID: 39456111 | PMCID: PMC11515322
Matrine alkaloids modulating DNA damage repair in chemoresistant non-small cell lung cancer cells.
BMC Cancer, 24(1):1283, 16 Oct 2024
Cited by: 0 articles | PMID: 39415176 | PMCID: PMC11481340
In Silico Analysis Uncovers FOXA1 as a Potential Biomarker for Predicting Neoadjuvant Chemotherapy Response in Fine-Needle Aspiration Biopsies.
J Cancer, 15(18):6052-6072, 30 Sep 2024
Cited by: 0 articles | PMID: 39440050 | PMCID: PMC11493000
Super-silencer perturbation by EZH2 and REST inhibition leads to large loss of chromatin interactions and reduction in cancer growth.
Nat Struct Mol Biol, 20 Sep 2024
Cited by: 1 article | PMID: 39304765
GABA(A) Receptor Activation Drives GABARAP-Nix Mediated Autophagy to Radiation-Sensitize Primary and Brain-Metastatic Lung Adenocarcinoma Tumors.
Cancers (Basel), 16(18):3167, 15 Sep 2024
Cited by: 0 articles | PMID: 39335139 | PMCID: PMC11430345
Go to all (698) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Distribution and dynamics of chromatin modification induced by a defined DNA double-strand break.
Curr Biol, 14(19):1703-1711, 01 Oct 2004
Cited by: 334 articles | PMID: 15458641 | PMCID: PMC4493763
Mechanism of elimination of phosphorylated histone H2AX from chromatin after repair of DNA double-strand breaks.
Mutat Res, 685(1-2):54-60, 12 Aug 2009
Cited by: 36 articles | PMID: 19682466
Review
Quantification of gammaH2AX foci in response to ionising radiation.
J Vis Exp, (38):1957, 06 Apr 2010
Cited by: 13 articles | PMID: 20372103 | PMCID: PMC3164074
Gamma-H2AX - a novel biomarker for DNA double-strand breaks.
In Vivo, 22(3):305-309, 01 May 2008
Cited by: 695 articles | PMID: 18610740
Review





