Abstract
Free full text

Fas-mediated elimination of antigen-presenting cells and autoreactive T cells contribute to prevention of autoimmunity
Abstract
Summary
Fas (Apo-1, CD95) receptor has been suggested to control T cell expansion by triggering T cell-autonomous apoptosis. This paradigm is based on the extensive lymphoproliferation and systemic autoimmunity in mice and humans lacking Fas or its ligand. However, with systemic loss of Fas, it is unclear whether T cell-extrinsic mechanisms contribute to autoimmunity. We found that tissue-specific deletion of Fas in mouse antigen presenting cells (APC) was sufficient to cause systemic autoimmunity, implying that normally APC are destroyed during immune responses via a Fas-mediated mechanism. Fas expression by APC was increased by exposure to microbial stimuli. Analysis of mice with Fas loss restricted to T cells revealed that Fas indeed controls autoimmune T cells, but not T cells responding to strong antigenic stimulation. Thus, Fas-dependent elimination of APC is a major regulatory mechanism curbing autoimmune responses and acts in concert with Fas-mediated regulation of chronically activated autoimmune T cells.
Introduction
Naïve T cells are activated by two signals provided by APC: an antigen and a costimulatory signal induced by microbial activation of pattern-recognition receptors (PRR) (Janeway, 1989). As a result, activated T cells proliferate, clear an infection and disappear, while memory T cells persist. Negative regulation of activated T cells is ascribed to a T cell-autonomous activation-induced cell death (AICD) mechanism that involves tumor necrosis factor receptor family members including Fas (CD95/Apo1) (Nagata and Golstein, 1995). The importance of this mechanism has been established due to systemic autoimmunity in mice with a mutant fas gene (lpr, lymphproliferation) (Watanabe-Fukunaga et al., 1992) or Fas-ligand gene (gld, general lymphoproliferative disorder) (Lynch et al., 1994; Takahashi et al., 1994), as well as in humans with lymphoproliferative diseases (Fisher et al., 1995; Rieux-Laucat et al., 1995; Wu et al., 1996). In addition, studies of T cell-autonomous cell death in vitro (Brunner et al., 1995; Dhein et al., 1995) provided support for the paradigm of Fas-mediated AICD as a major regulator of T cell clonal contraction and autoimmunity. Since T cell clonal contraction elicited by a bacterial superantigen has been shown to be Fas-independent (Hildeman et al., 2002), the mechanisms of Fas involvement in autoimmunity became even more unclear. Moreover, the studies of systemic loss of Fas expression could not exclude the involvement of Fas-mediated death of cell types other than T cells in the prevention of autoimmunity. Earlier studies provided some evidence that Fas-negative non-T cells contribute to development of systemic autoimmunity: restoration of Fas expression by T cells abolished lymphoproliferation but not the production of autoantibodies (Fukuyama et al., 1998), while deletion of B cells from lpr/lpr mice led to diminished proliferation of T cells (Shlomchik et al., 1994). A reasonable explanation for these findings would be that the ‘other cell types’ are in fact APC. According to such a scenario, Fas-positive APC should be removed by activated T cells under normal circumstances, and the whole process is likely to work as a negative regulatory loop, driving the contraction of T cell responses. However, Fas-negative APC expressing autoantigens would sustain activation of self-reactive T cells. The disappearance of dendritic cells (DC) upon activation of T cells has been documented before (Ingulli et al., 1997; Wong and Pamer, 2003). Moreover, DC with a lifespan extended by transgenic overexpression of a viral caspase inhibitor (Chen et al., 2006) caused systemic autoimmunity. For such a regulatory mechanism to be specific, APC should be pre-conditioned for destruction during their maturation.
Thus, in order to test whether autoimmunity can be controlled by Fas-mediated destruction of APC, we sought the answers to three related questions: a. does PRR-induced maturation of APC predispose them to Fas-mediated destruction? b. does APC-specific inactivation of Fas signaling lead to systemic autoimmunity? and c. what is the real role of Fas-mediated AICD of T cells in autoimmunity and post-antigen clonal contraction?
Results
Fas expression by APC
The elimination of APC by activated T cells through Fas requires Fas expression by APC. It is also likely to require that Fas expression be inducible through PRR to ensure that elimination is specific for mature APC presenting cognate antigen. For these reasons, Fas expression by DC was tested both in vivo and in vitro. DC, freshly isolated from peripheral lymph nodes, contained a fraction of cells expressing a high level of Fas (Figure 1A), while injection of lipopolysaccharide (LPS) or unmethylated oligonucleotides (CpG) led to uniform up-regulation of Fas by DC (Figure 1B). Fas expression by non-stimulated DC was associated with CD11chi,CD11b+ DC, while the majority of CD11chiCD8+ DC were Fas-negative or Faslow (Figure 1C). Fas expression by plasmacytoid DC (pDC), defined as PDCA-1+, CD11clow, B220+ cells, was also inducible by LPS and CpG after an overnight exposure in vivo (Figure 1D). As expected, CD11chi, Fas+ cells expressed markers of activation such as the inducible costimulatory molecule CD86 (Figure 1E) and high levels of MHC class II (not shown).
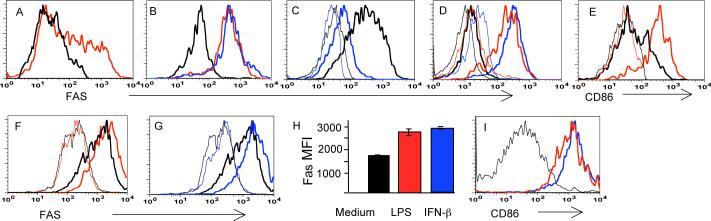
A. Some CD11chi cells in regional lymph nodes express Fas directly ex vivo (red line) compared to control CD11chi cells from a Fas knock-out (FasKO) mice (black line).
B. All CD11chi cells become Fas+ upon footpad injection (a day before staining) of LPS (red line) or CpG (blue line). Black line – CD11chi cells from a FasKO mouse injected with LPS.
C. Fas expression in unstimulated DC is primarily restricted to CD11b+ cells (thick black line) but not to CD8+ DC (thick blue line). Thin lines – same subsets from a FasKO mouse.
D. Plasmacytoid DC (PDCA-1+, CD11clo, B220+ cells) are Fas negative before activation (thick black line), but express Fas after overnight stimulation in vivo with LPS (thick red line) or CpG (thick blue line). Thin lines: corresponding populations in a FasKO mouse.
E. In vivo activated Fas+ DC express high levels of the costimulatory molecule CD86 (thick red line, gated on Fas+,CD11chi cells). Thick black line: CD86 expression by non-activated CD11chi cells; thin lines: respective negative controls.
F,G. Fas expression by myeloid BMDC (thick black line) is further up-regulated (thick colored lines) by overnight treatment in vitro with LPS (red) (F), or interferon ß (blue) (G). Thin lines: negative controls without anti-Fas antibodies. These plots are from the same experiment; thus the negative control curves are the same.
H. Summary data on Fas expression by BMDC stimulated by LPS or Type I interferon. MFI: mean fluorescence intensity±SE.
I. In vitro activated (by LPS, red line, or interferon ß, blue line) Fas+ BMDC express high levels of the co-stimulatory molecule CD86 compared to non-stimulated cells (black line).
Bone marrow-derived DC (BMDC) were found to express Fas and to further induce Fas expression after an overnight exposure to LPS or Type I interferon (Figure 1F-H, Supplemental Figure 1). Expression of Fas correlated with overall DC activation as determined by measuring expression of CD86 (Figure 1I). Thus, Fas expression by DC can be induced by triggering of PRR (Toll-like receptors) or by Type I interferon, the production of which is normally induced by a viral infection.
DC-specific deletion of Fas
To investigate the importance of Fas expression by APC and T cells for systemic autoimmunity, we produced mice in which Fas was deleted specifically in APC (DC or B cells) or in T cells (all T cells, or recently activated T cells). Exon IX of the Fas gene encoding the death domain was flanked with loxP recognition sites for the Cre recombinase (‘floxed’), similar to previously described mice (Hao et al., 2004) (see Supplemental Figure 2 showing the targeting strategy). The resulting mice (Fas knock-in, FasKI) were crossed to animals expressing Cre recombinase controlled by promoters of the following genes: CD11c (CD11c-Cre, ‘floxed’ genes deleted in DC), CD19 (CD19-Cre, deletion in B cells) (Rickert et al., 1997), p56lck (proximal Lck-Cre, deletion in all T cells) (Hennet et al., 1995), and granzyme B (GZB-Cre, deletion restricted to activated T cells)(Jacob and Baltimore, 1999).
The CD11c-Cre transgenic mice were produced using the CD11c genomic promoter and enhancer (Brocker et al., 1997). The Cre transgene was also co-expressed (via an IRES) with Green Fluorescent Protein (GFP) to report its expression by flow cytometery. Several transgenic strains were generated with different patterns of GFP expression. CD11c-Cre strain 4272 showed GFP expression in a fraction of DC (Figure 2A), while another strain (4097) had GFP expression in the majority of DC (Figure 2A). In strain 4097, all CD11chi cells expressed GFP (expected to have equimolar expression with Cre) in both CD11chi,CD11b+ cells and CD11chi,CD8+ cells (Figure 2B). Plasmacytoid DC (PDCA-1+, CD11clo, B220+) expressed a lower level of GFP (Figure 2C), as expected for CD11c-regulated expression. T and B cells did not express GFP (Figure 2D).
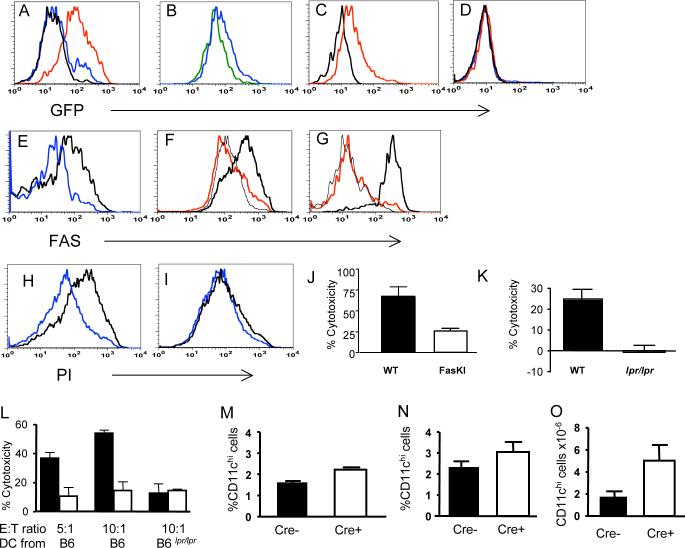
A. GFP expression (reflecting expression of Cre recombinase driven by the CD11c promoter) by lymph node DC from CD11c-Cre strains 4272 (blue line) and 4097 (red line). Black line - non-transgenic littermate.
B. GFP is expressed by CD11chi,CD11b+ cells (blue line) and CD1chi,CD8+ cells (green line). CD11c-Cre strain 4097 was used in B-D.
C. Plasmacytoid DC (PDCA-1+, CD11clo, B220+) express a low level of GFP (red line) as expected for CD11c-regulated expression. Black line: non-transgenic littermate.
D. Lack of GFP expression by T cells (CD4++CD8+ cells) (blue line) and by B cells (red line) in CD11c-Cre mice. Black line- control staining of total spleen from a non-transgenic mouse.
E. Fas expression is lost from GFP+ DC in the spleens of 4272 strain homozygous for FasKI (blue line) but not from GFP+ DC in heterozygous (FasKI/+) control mice (black line).
F. Fas expression is lost from the majority of LPS-treated BMDC of 4097 CD11c-Cre.FasKI mice (red line) compared to control CD11c-Cre DC (thick black line). BMDC from a FasKO mouse were used as negative control (thin black line).
G. Fas expression is lost from lymph node plasmacytoid DC in 4097 CD11c-Cre.FasKI mice. PDCA-1+ cells from a Cre+FasKI mouse (red line), a Cre+Fas-sufficient littermate (thick black line) and a FasKO mouse (black line) were compared 24 hrs after in vivo treatment with CpG.
H-J LPS-activated BMDC from control 4097 CD11c-Cre mice were sensitive to FasL mediated killing in vitro (H) compared to CD11c-Cre.FasKI mice (I). Propidium-iodide staining of CD11c+,GFP+ DC incubated overnight with control CT26 (blue line) or human FasL-transfected CT26−95L carcinoma cells (black line). J, % cytotoxicity was calculated using the formula (a-b/a)×100%, where a and b are % of live CD11c+GFP+ cells incubated with FasL-negative and FasL-positive CT26 cells, respectively. Mean from three experiments, p=0.03.
K. LPS-activated, peptide loaded splenic DC were killed in vivo in an antigen- and Fas-dependent manner by OT-1 T cells. Cytotoxicity (%) was calculated using the formula 100%×(a-b)/a, where ‘a’ is a ratio between DC incubated with SIINFEKL (labeled with a high dose of CFSE) and DC treated with irrelevant peptide (labeled with a low dose of CFSE) before injection of the mixture into mice, and ‘b’ is the ratio between the same groups after exposure to OT-1 T cells in vivo. Left bar – mixture of differentially labeled DC from B6 control mouse; right bar – mixture of DC from lpr/lpr mouse. Mean of four individual animals per group is shown.
L. Killing of activated BMDC in vitro by OT-1 T cells was Fas-ligand dependent. Equal numbers of BMDC labeled with two concentrations of CFSE were exposed to activated OT-1 (black bars) or FasL.KO OT-1 (open bars) T cells in the presence of specific peptide (CFSEhi cells) or without specific peptide (CFSElo cells), mixed and analyzed by flow cytometry. Cytotoxicity was calculated using the formula 100%×(a-b)/a, where ‘a’ is a ratio of CFSElo to CFSEhi BMDC incubated without T cells, and “b” is the ratio of the same cells incubated with T cells.
M-O. DCs accumulate in CD11c-Cre.FasKI mice with age. Six mo old strain 4272 CD11c-Cre-FasKI mice (M) show a larger % of CD11chi in the spleens of Cre+ mice (three animals of each type compared in the same experiment, p=0.006). In five mo old strain 4097 CD11c-Cre.FasKI mice (N) an increase in % of CD11c+ cells, p=0.1 and absolute number (O) of CD11c+ cells, p=0.035 were observed (four animals of each type were compared).
Although only a fraction of CD11c+ DC in strain 4272 expressed detectable levels of GFP, any effects on the immune system caused by Fas-elimination from DC were expected to be cell autonomous. Thus, it should not matter whether Fas was deleted in all or a fraction of DC. We therefore studied mice derived from both founders. Fas expression was eliminated from subsets of DC in both 4272 and 4097 CD11c-Cre strains crossed to FasKI mice (Figure 2E-G).
To show that the loss of Fas resulted in resistance of CD11c-Cre.FasKI DC to a death signal delivered by FasL, two types of experiments were performed. First, BMDC stimulated with LPS were exposed to CT26 carcinoma cells transfected with human FasL (CT26−95L). As expected, loss of Fas triggered by CD11c-Cre made DC resistant to FasL-mediated apoptosis in vitro (Figure 2H-J). Second, LPS-activated, specific peptide loaded splenic DC were labeled with 1μM fluorescent dye CFSE, mixed with control DCs (loaded with irrelevant peptide) labeled with a lower concentration of CFSE (0.1μM), and the mixture was injected into footpads of B6 mice. The next day, OT-1 T cells responsive to the ovalbumin (OVA)-derived peptide (SIINFEKL) presented by the MHC class I molecule Kb were injected i.v. into the same mice, and the ratio between differentially labeled DC in the popliteal lymph nodes was established 48 hrs later, allowing us to measure killing of SIINFEKL-loaded DC. Under these experimental conditions, 25−30% of B6-derived DC were killed, while there was no killing of DC obtained from B6lpr/lpr mice (Figure 2K). Thus, Fas-deficient DC were also resistant to killing in vivo by cognate OT-1 T cells. Importantly, killing of activated DC in vivo was antigen-specific. Because DC treated with high dose of LPS were shown to express FasL (Chen et al., 2006), we asked whether the actual killing of DC is performed by T cells, or whether the presence of T cells is required for suicide by FasL+ DC. Equal numbers of DC stained with CFSE were cultured with LPS and with (CFSEhi) or without (CFSElo) the SIINFEKL peptide, in the presence of activated OT-1 T cells or activated FasL.KO OT-1 T cells (Figure 2L). DCs were then mixed and analyzed by flow cytometry. Clearly, BMDC killing required FasL expression by T cells. Addition of non-activated OT-1 T cells did not lead to killing of DCs (not shown). Thus, the major contribution to DC elimination through Fas-mediated killing was delivered by activated antigen-specific T cells.
Abrogation of Fas-mediated apoptosis of DC was likely to result in their extended lifespan. Indeed, Fas-negative DC accumulated with age in the spleens of both 4272 and 4097 CD11c-Cre.FasKI mouse strains compared to Fas-sufficient littermates (Figure 2M-O).
Finally, to test the hypothesis that the lack of APC elimination by activated T cells enhances immune responses, we used mice carrying the lpr mutation crossed to mice lacking an adaptive immune system [mice carrying the scid mutation (Custer et al., 1985)]. When such mice are injected with spleen cells from normal MHC-matched, minor histocompatibility antigen-mismatched mice, the development of graft-vs.-host (GVH) response ensues. Recipient scid mice cannot respond to the graft, while their APC (DC, and possibly macrophages) can activate donor T cells. Using anti-nuclear antibodies (ANA) as a fast and reliable read-out for GVH development, we expected that ANA should develop more efficiently in scidlpr/lpr recipients than in Fas-sufficient scid recipients, because Fas-negative APC are more resistant to direct elimination by donor T lymphocytes. We found that, indeed, ANA were detectable much earlier in NOD.scidlprlpr recipients of B6g7 congenic splenocytes (Figure 3) than in Fas-sufficient NOD.scid animals (NOD mice were used simply because the appropriate combination of backgrounds was available). Although at the later time points ANA accumulated to saturation levels in both types of recipients (not shown), the APC resistant to Fas-mediated death clearly induced accelerated GVH.
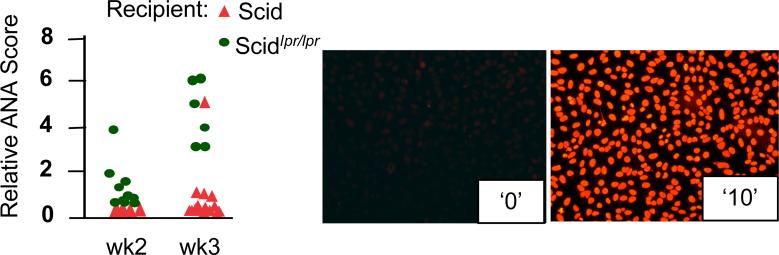
NOD.scid or NOD.scidlpr/lpr recipients were inoculated i.p. with 2×107 splenocytes of MHC-compatible B6g7 mice. ANA were detected by staining of HEp-2 cells with sera samples diluted 1:100. ANA values are shown for individual mice. Relative scale (examples shown on the right): 0 – normal 8 wk-old B6 mouse serum, 10 – serum of 1 yr old B6lpr/lpr mouse. See also Supplemental Figure 4 for explanation of ANA evaluation.
Thus, these findings support the idea of a negative regulatory loop based on Fas-mediated elimination of APC by T cells recognizing antigens presented by these APC.
Loss of Fas expression by APC leads to systemic immunity
Activated APC express costimulatory signals and may simultaneously express self-antigens. These two signals can trigger autoreactive T cells. Should a negative feedback loop of APC elimination through Fas be inactivated by deletion of Fas, autoimmunity is likely to develop. Thus, CD11c-Cre.FasKI mice were studied for signs of autoimmunity. Lack of Fas expression specifically by DC resulted in several manifestations of systemic autoimmunity: production of high titers of ANA, hyperimmunoglobulinemia, splenomegaly, and histological changes in spleen and liver. Some mice had elevated ANA levels as early as 8 wks of age, and by 12−16 wks of age, the majority of CD11c-Cre.FasKI mice showed high levels of ANA, unlike their Cre-negative FasKI littermates (Figure 4A, Supplemental Figure 5). In addition to production of ANA, total levels of both IgG (Figure 4B) and IgM (Figure 4B, Supplemental Figure 6) were elevated in mice lacking Fas expression in DC. These manifestations of autoimmunity were found to be very similar in both females and males. GFP-positive DC accumulating in CD11c-Cre.FasKI mice expressed high level of CD86 costimulatory molecules (Figure 4C), indicating that these cells have undergone maturation. The CD11c-Cre.FasKI mice developed splenomegaly (Figure 4D), associated with the loss of follicular architecture (Figure 4 E). The localization of DC was also abnormal: similar to previously reported changes in lpr/lpr mice (Fields et al., 2001) DC were spread throughout the periarteriolar lymphoid sheath (Figure 4E). In addition, polymorphic infiltrates were found around central veins in the liver. These infiltrates (Figure 4E) included DC and various leukocytes. Thus, Fas removal from DC led to their accumulation and to systemic autoimmunity.
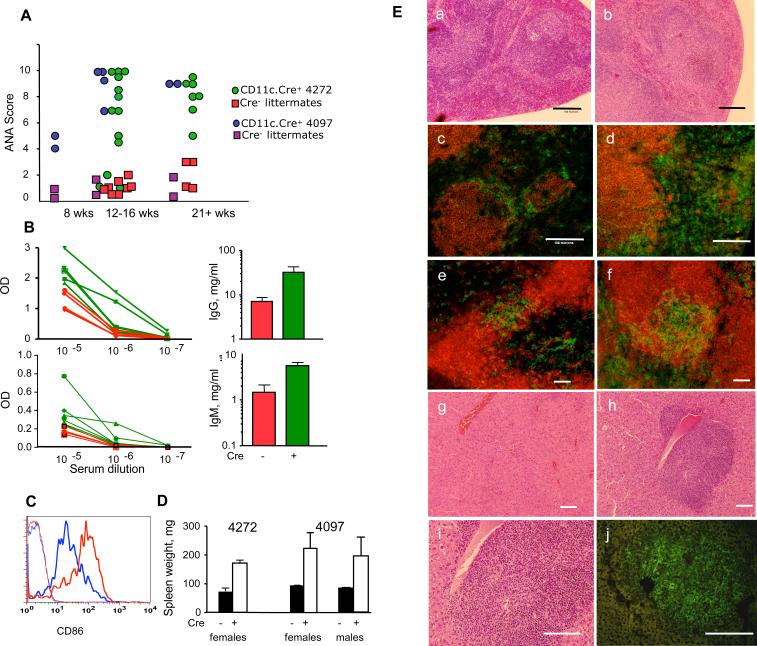
A. Cd11c-Cre.FasKI mice produce ANA. Sera from female mice of the indicated ages were tested at 1:100 dilution. Values are shown for individual mice. Cre-negative FasKI littermates were used as controls.
B. Hyperglobulinemia in Cd11c-Cre.FasKI mice. IgM and IgG detection by ELISA in sera of 4272 CD11c.Cre-FasKI mice (green lines and bars) or their Cre-negative littermates (red lines and bars). Left panels show the difference in relative concentration of Ig (OD in ELISA assay, lines represent individual mice); right panels show the difference in absolute concentrations of IgM or IgG. The p values (for absolute concentrations) were 0.05 and 0.04 for IgM and IgG, respectively. Results for strain 4097 can be found in Supplemental Figure 5.
C. DCs that accumulate in CD11c-Cre.FasKI mice with age are activated and express high level of CD86 in cells positive for GFP (red line). Blue line: DC from Fas-sufficient littermates; thin lines: negative controls (GFP+-Fas-sufficient mice).
D. CD11c-Cre.FasKI mice develop splenomegaly. Spleen weights were measured in six mo old strain 4272 CD11c-Cre.FasKI female mice (3 Cre-negative and 3 Cre-positive FasKI mice, p=0.004) and five mo old strain 4097 CD11c-Cre.FasKI female (4 control FasKI, 5 Cre+FasKI mice) and male (7 control FasKI, 6 Cre+ FasKI) animals. There was no significant difference between female and male Cre+FasKI spleen weights (p=0.75).
E. Pathological changes in spleens and livers of CD11c-Cre.FasKI mice (right column:b, d, f, h-j) compared to Fas-sufficient littermates (left column: a, c, e,g). Loss of splenic follicular architecture (b), expansion of DC in the spleen (d, CD8+ cells stained with anti-CD8-PE and DCs are counterstained with anti-CD11c-FITC in addition to GFP fluorescence in c and d), altered localization of DC in periarteriolar lymphoid sheath (f, B cells counterstained with anti-CD19-PE antibodies in e and f ); polymorphic infiltrates around central veins in the liver (h, i where i is an enlargement of h) containing CD11c+ cells (stained with anti-CD11c-FITC in addition to GFP fluorescence) (j). Cre-negative FasKI littermates show normal follicular structure (a), lower numbers of DC (c,e), and localization of DC within bridging channels (e) in the spleen and normal liver structure (g). Bar:100μm.
B cells function as effector cells producing antibodies, but also serve as another major APC subset. Removal of Fas specifically from B cells (Figure 5A) led to significant consequences. CD19-Cre.FasKI mice (both females and males) showed splenomegaly due to extensive lymphoproliferation (Figure 5B). Importantly, the accumulating cells were not composed exclusively of B cells, but also included T cells (Figure 5C). Both T cell and B cell subsets were polyclonal: T cells expressed a variety of T cell receptor Vß chains, while B cells carried diverse rearranged immunoglobulin heavy chains, and demonstrated a normal ratio of κ and λ light chains (data not shown). In addition to lymphoproliferation, the loss of Fas by B cells resulted in systemic autoimmunity as documented by production of ANA (Figure 5D, Supplemental Figure 5), development of hyperimmunoglobulinemia (Supplemental Figure 6), and, as previously reported, production of anti-DNA antibodies (Hao et al., 2004). Taken together, these observations suggest that Fas-negative B cells serving as APC fail to be eliminated by activated T cells and, thus, sustain survival of autoimmune T cells and produce autoantibodies.
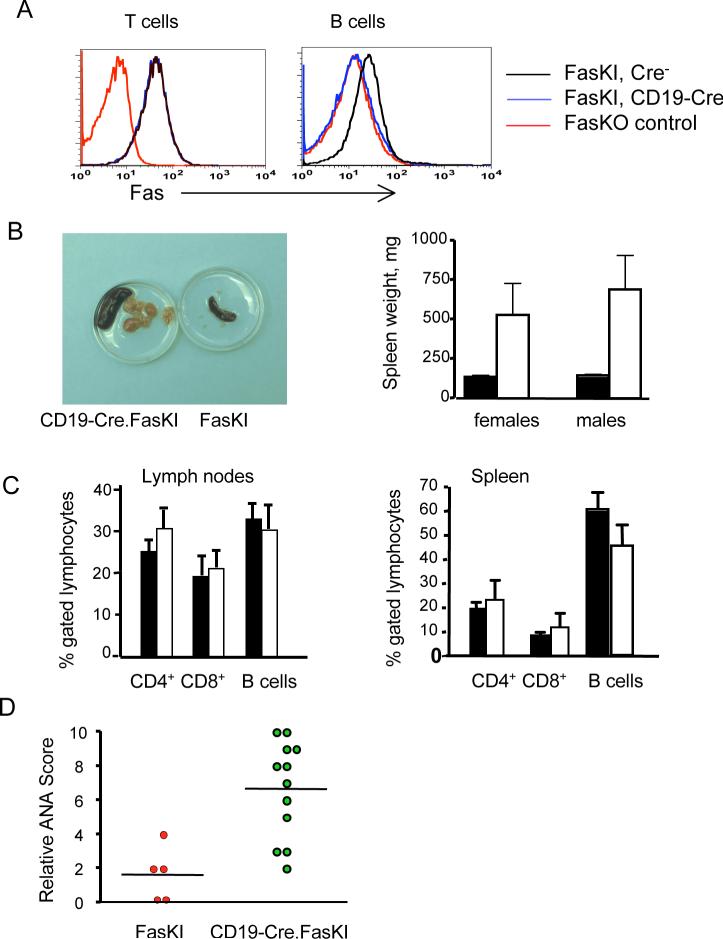
A. Flow cytometric analysis shows the specificity of Fas deletion by Cre in CD19-Cre.FasKI mice. T and B cells from Fas knock-out (FasKO) animals were used as negative controls.
B. Massive proliferation of T and B lymphocytes. Left panel: example of enlarged spleens and lymph nodes in a one year old CD19-Cre.FasKI mouse (left), compared to a Cre-negative littermate (right). Right panel: splenomegaly in >6 mo old CD19-Cre.FasKI female (n=7) and male (n=5) mice (white bars) compared to Cre-negative littermate females (n=5) and males (n=11) (black bar). Splenomegaly was not significantly different between females and males (p=0.6).
C. Both B and T cells expand in lymph nodes and spleens of CD19-Cre.FasKI mice. % of CD4+, CD8+, and B cells (defined as B220+CD3−) ±SE shown for FasKI controls (black bars) and CD19-Cre.FasKI littermates (white bars).
D. CD19-Cre.FasKI mice produce ANA. Dots represent sera obtained from individual 6+ mo old female mice tested at 1:100 dilution. Relative staining was 1.6±0.7 and 6.7±0.8 in Cre− and Cre+ FasKI mice respectively.
Fas-dependent regulation of T cells is limited to self-reactive T cells
We have found that the loss of Fas expression by APC led to systemic autoimmune reactions. This mechanism recapitulates some of the features of systemic immunity observed in lpr/lpr mice. Another hallmark feature of lpr/lpr phenotype is accumulation of double-negative T cells lacking co-receptors but expressing the B cell marker B220 (CD3+B220+ cells). These cells did not accumulate in CD11c-Cre.FasKI or CD19-Cre.FasKI mice (see below), indicating that their persistence must be a T cell-autonomous feature. Thus, we faced an obvious question of whether Fas receptor expressed by T cells truly plays the role it has been assigned in down-regulating normal immune responses (‘clonal contraction’) and in autoimmunity. To address these questions we used mice with selective Fas loss in all T cells or with such loss restricted to recently activated T cells. FasKI mice were crossed with Cre driven by the proximal Lck (Hennet et al., 1995) or Granzyme B (GZB) (Jacob and Baltimore, 1999) promoters, respectively.
First, we addressed the issue of Fas participation in clonal contraction of normal immune responses. We reasoned that should the T-cell-autonomous Fas-mediated AICD be the sole or major mechanism by which T cell clonal expansion is regulated in vivo, secondary T cell responses in mice lacking Fas expression by T cells should exceed such responses in mice with Fas-sufficient T cells. Several experimental systems were used. In one series of experiments, Lck-Cre.FasKI mice with T cell-specific deletion of Fas (Figure 6A) and the control Cre-negative FasKI mice were immunized with OVA and the response of CD4+ T cells was tested 11 days later by ELISPOT assay to measure the frequency of interferon-γ (IFNγ) secreting cells (Figure 6B). The responses of Lck-Cre+ FasKI mice were not higher than the responses of Cre-negative FasKI littermates, suggesting that Fas was not a factor down-regulating T cell responses. Similarly, in another system, Fas-negative, recently activated CD8+ T cells (in GZB-Cre.FasKI mice carrying the OT-1 T cell receptor transgene) did not demonstrate any advantage over Fas-sufficient cells in response to antigen delivered by Listeria monocytogenes (Lm-OVA) within the same test animal (Figure 6C). Fas-sufficient and Fas-deficient T cells also generated identical secondary responses to challenge with Lm-OVA (data not shown). Finally, similar observations were made in GZB-Cre.FasKI mice responding to the minor histocompatibility antigen H60 (Figure 6D). H60-specific responses of CD8+ cells in individual mice were traced by staining with specific tetramers. Neither the slopes of the contraction of primary responses, nor the magnitude of secondary responses (day 4 after secondary immunization) were different in Fas-sufficient and Fas-deficient mice.
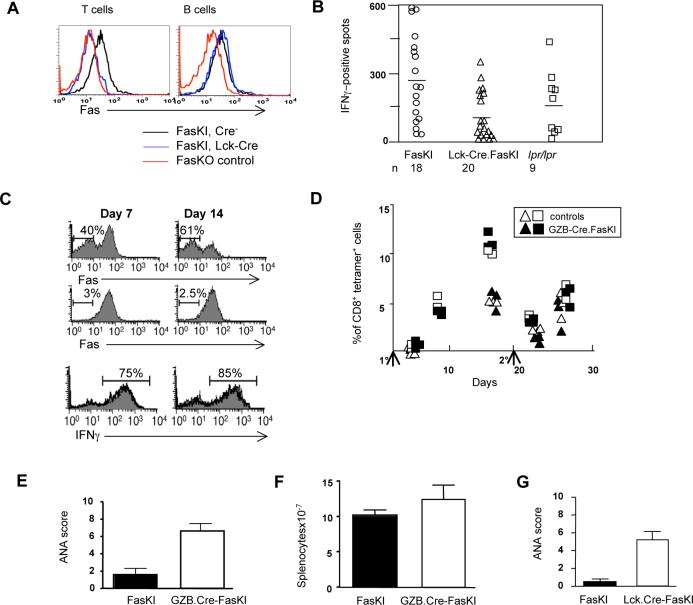
A. Flow cytometric analysis shows the specificity of Fas deletion by Cre in Lck-Cre.FasKI mice. T and B cells from Fas knock-out (FasKO) animals were used as negative controls.
B. Lck-Cre.FasKI, Cre-negative FasKI and B6lpr/lpr mice were immunized in the footpads with 50μg OVA mixed 1:1 with Complete Freund's Adjuvant. Regional lymph node cells were stimulated 11 days later in vitro overnight with OVA, and IFNγ secretion was detected by ELISPOT. Ordinate: mean number of dots (from triplicate wells) is shown adjusted to the frequency of CD4+ T cells determined by flow cytometric analysis on the day of the experiment. Symbols show individual mice.
C. GZB-Cre.FasKI.OT-1 transgenic Ly5.2 marked T cells and Ly5.1-marked OT-1 T cells were co-injected in Ly5.1+Ly5.2+ mice and their fate was followed after infection with Lm-OVA. Details of experimental design are shown in Supplemental Figure 7. T cells from GZB-Cre.FasKI.OT-1 donors (top panels) show significant loss of Fas 1−2 weeks after activation with OVA expressed by bacteria compared to OT-1 T cells (middle panels). The survival of activated cells from GZB-Cre.FasKI.OT-1 and wild-type OT-1 mice did not differ: Absolute numbers of OT-1vs GZB-Cre.FasKI.OT-1 T cells were 1.0×106 vs. 1.2×106 at day 7 and 1.7×105 vs. 1.6×105 on day 14, respectively. In addition, similar %s of IFNγ producing cells were found in both populations (bottom panels). Representative plots are shown. Bold line –GZB-Cre.FasKI.OT-1 T cells; shaded histogram – OT-1 T cells from the same adoptive host gated using Ly5 alleles.
D. Primary (1°) and secondary (2°) responses to minor histocompatibility antigen H60 are not different in GZB-Cre.FasKI mice compared to Cre-negative littermates. Note that there is no primary response at day 4 after immunization, thus only recall responses are compared at day 4 after secondary i.p. immunization with 2×107 C.B10 splenocytes. Ordinate: % of CD8+ T cells in PBL that are H60-tetramer-positive. Primary and secondary immunizations are marked by arrows. Data shown for individual mice traced in two separate experiments (triangles and squares, respectively). Black symbols – GZB-Cre.FasKI; open symbols – control mice.
E. ANA formation in GZB-Cre.FasKI mice. Sera from GZB-Cre.FasKI mice (n=11) or Cre-negative littermates (n=9) 6 mo of age or older were used at 1:100 dilution. Relative scores were 2.2± 0.4 and 6.3±0.8 in Cre− and Cre+ FasKI mice respectively.
F. Splenic cellularity in 8−10 mo old GZB-Cre. FasKI mice was not significantly greater than in control Cre-negative littermates. (p=0.215, 9 control and 19 Cre+ mice were used).
G. Lck-Cre.FasKI mice produce ANA. Sera obtained from individual 6+ mo old female mice (6 FasKI and 13 Lck-Cre.FasKI animals) were tested at 1:100 dilution. Relative scores were 0.5± 0.3 and 5.3±0.9 in Cre− and Cre+ FasKI mice respectively.
These results indicated that the loss of Fas by both Th1-type CD4+ T cells and CD8+ T cells does not provide them with a selective advantage over Fas-sufficient T cells, casting doubt on the role of T cell AICD as a major regulator of T cell clonal expansion in vivo. Importantly, the ratio of GZB-Cre.FasKI.OT-1 to OT-1 T cells recovered from the adoptive host in the co-transfer experiment was 1.07:1.0 on day 7 and 0.9:1.0 on day 14 post transfer, indicating that neither Fas− OT-1 T cells nor the recipient's own T cells responding to L.monocytogenes infection killed Fas-positive OT-1 T cells (Figure 6C). This result strongly argues against T cell ‘fratricide’(Crispe, 1994) as a negative regulatory mechanism.
Second, we asked whether Fas deficiency in T cells contributes to development of systemic autoimmunity. It takes over 6 months for B6lpr/lpr mice to develop the full autoimmune phenotype (lymphoproliferation, production of autoimmune antibodies and accumulation of CD3+B220+ cells), and some of these features are more pronounced in females (Theofilopoulos and Dixon, 1985; Warren et al., 1984a; Warren et al., 1984b). Thus, mice compared in these experiments were 6+ mo oldfemales. Sera from GZB-Cre.FasKImice were tested for the presence of ANA. Some animals showed high titers of ANA compared to littermates (Figure 6E), indicating that loss of Fas by activated T cells also leads to systemic autoimmunity. Mice lacking Fas in activated T cells demonstrated a slight but not significant increase in splenic cellularity (Figure 6F). Lck-Cre.FasKI mice also developed ANA (Figure 6G, Supplemental Figure 5), without splenomegaly (see Supplemental Figure 8). Furthermore, we found that only mice with T cell Fas deficiency, but not with APC Fas deficiency, accumulated CD3+(or Thy1.2+) B220+ double-positive cells (Figure 7 A, B). Accumulation of these cells in GZB-Cre.FasKI mice (but not in Cre-negative FasKI controls) suggests that they are indeed the progeny of previously activated T cells. Thy1.2+ cells expressing B220 were analyzed further (Figure 7 C, D). Levels of Fas expression were compared for Thy1.2+B220− cells (gate 1) and Thy1.2+B220+ cells either expressing co-receptors or not (gates 2a and 2b, respectively). T cells from GZB-Cre.FasKI mice had shown progressive loss of Fas expression by T cells that acquired B220 and lost co-receptor expression. Interestingly, in Cre-negative control FasKI mice Fas expression by Thy1.2+B220+,co-receptor-negative cells was also diminished, but never reached the level of the negative control (lpr/lpr mice).
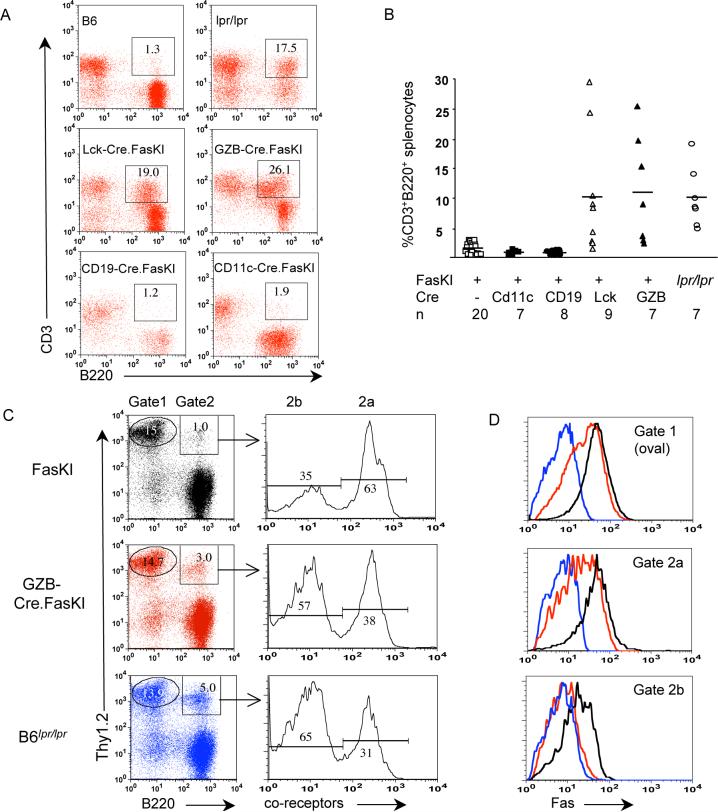
A. Examples of flow cytometric analyses showing the presence of CD3+B220+ cells in mice with Fas deletion in T cells (Lck-Cre.FasKI and GZB-Cre.FasKI) and APC (CD11c-Cre.FasKI and CD19-Cre.FasKI). All mice were 6+ months of age.
B. Elevated frequencies of CD3+B220+ splenocytes were found only in mice with T cell deletion of Fas including GZB-Cre.FasKI, which delete Fas upon activation only. All animals were over 6 months old. n – number of animals per group. The degree of variation in the numbers of CD3+B220+ cells in individual mice must reflect their origin from T cells activated randomly by autoimmune or environmental stimuli.
C,D. In GZB-Cre.FasKI mice, B220+ T cells lose expression of Fas. Splenocytes from Cre-negative FasKI, GZB-Cre.FasKI and B6lpr/lpr mice were stained with antibodies against Thy1.2 pan-T cell marker, B220, and a combination of anti-co-receptor antibodies (a mixture of anti-CD4 and anti-CD8 labeled with the same fluorochrome) (C). Gate 1 – Thy1.2+,B220− cells, Gate2 – Thy1.2+,B220+ cells. The latter were electronically separated into Thy1.2+,B220+, co-receptor+ cells (Gate 2a) and Thy1.2+,B220+, co-receptor− cells (Gate 2b). Gated cells were further analyzed for Fas expression (D). Line color reflects mouse genotype (shown in C).
Thus, Fas deficiency limited to T cells also leads to systemic autoimmunity, likely due to extended survival of autoreactive T cells.
Discussion
The genetic approach that we have taken by manipulation of Fas expression in two different types of immune cells, APC and T cells, allowed us to conclude that both of these subsets contribute to the Fas-dependent regulation of autoimmunity. Accumulation of DC in CD11c-Cre.FasKI mice supports the idea that Fas-negative DC have escaped apoptotic death. Similarly, in a recent report (Chen et al., 2006), systemic autoimmunity was detected in mice with DC overexpressing a transgenic caspase-blocking viral protein that extended their survival. Here we demonstrate directly that the mechanism responsible for DC elimination and preemption of autoimmunity is based on Fas signaling.
Importantly, freshly isolated DC were found not to express Fas uniformly, but Fas expression was induced by maturation-stimulating cues: LPS, CpG and Type I interferon. The requirement of DC activation through PRR to become sensitive to Fas and, hence, to removal by activated FasL-positive T cells, makes perfect sense for the negative feedback regulation and contraction of the T cell response. Fas deficiency in DC led to activation of other cell types (splenomegaly was detected that could not be explained by expansion of DC alone), but did not produce double-negative T cells. Thus, Fas deficiency limited to APC explains part of the phenotype observed in animals with systemic Fas loss, and it is by itself sufficient to cause autoimmunity. It is also likely that FasL is not the only regulator of APC death: perforin deficiency has been shown to cause familial histiocytosis (Stepp et al., 1999) and enhanced antigen response after multiple immunizations with DC (Yang et al., 2006). However, perforin-mediated death of DC can be blocked by maturation-induced upregulation of a serpin protease inhibitor (Medema et al., 2001). The cellular targets or timing for perforin- and FasL-mediated killing of APC may also differ. In this respect, it is worth noting that autoimmune reactions were observed in both CD11c-Cre.FasKI strains (4272 and 4097), despite significant differences in transgene expression. Thus, this mechanism is indeed cell-autonomous and minor APC populations resistant to one or another apoptotic mechanism can contribute to autoimmunity. Another important point is that while autoimmune reactions such as production of ANA are accelerated in B6.CD11c-Cre compared to B6lpr/lpr mice (no ANA could be detected in the latter at 8−10 wks of age, not shown), their development is still gradual. That emphasizes the random nature of autoimmune T cell activation.
It is currently unknown whether Fas-mediated APC death contributes primarily to regulation of CD4+ and/or CD8+ T cell responses, as CD4+ T cells need an extended stimulation by APC (Obst et al., 2005), while CD8+ T cells may need a single hit (Bevan and Fink, 2001; Mercado et al., 2000) or continuous stimulation (Curtsinger et al., 2005; Storni et al., 2003) to be fully activated.
Analysis of two mouse strains, with Fas deleted in all T cells or in activated T cells, revealed that Fas-negative T cells are also capable of inducing systemic autoimmunity. At the same time, we have established that Fas-negative T cells gain no survival advantage over Fas-positive T cells upon activation (Figure 4A-D). The paradox can be solved by a proposition that responses to strong stimuli do not require Fas on T cells for down-regulation, while prevention of systemic autoimmune reactions (when the supply of APC carrying weak but persistent auto-antigens is unlimited) requires both mechanisms: Fas-mediated negative regulation of chronically-activated T cells and Fas-mediated elimination of activated APC. The Fas-independence of the clonal contraction of T cells exposed to a strong stimulus, bacterial superantigen (Hildeman et al., 2002), has been previously established. We have found that responses to other more frequently occuring physiological stimuli (nominal antigens and pathogens inducing strong T cell responses, Figure 6) do not require Fas on T cells for clonal contraction, while responses to endogenous antigens did. These endogenous antigens are likely to be weak stimuli, simply because high avidity responding T cells are removed by central negative selection or tolerized in the periphery. Endogenous antigens persist, providing weak but chronic stimulation to T cells. Activation of APC by PRR ligands may increase the chances for T cell activation, also making such activation a random event. That explains the time-dependent development of autoimmune reactions in mice lacking Fas in T cells, as well as in mice lacking Fas in APC.
In our experiments, Fas deficiency in T cells did not lead to changes in animal viability or damage to internal organs as previously reported (Hao et al., 2004). Lck-Cre.FasKI and GZB-Cre. FasKI mice did not show significant loss of body mass or spleen mass (see Supplemental Figure 6), with GZB-Cre.FasKI showing a slight increase in splenic cellularity. In concordance with the study by the Rajewsky group (Hao et al., 2004), in our hands, CD4-Cre.FasKI mice did show weight loss and mild lymphopenia (Supplemental Figure 6). The reasons for the discrepancies found in the two systems are not yet clear (for discussion see Supplemental Figure 6).
Thus, Fas-mediated AICD has no functional significance for regulating the T cell responses to foreign antigens, but is likely a mechanism specifically involved in prevention of autoimmunity caused by chronic stimulation of T cells with weak signals. Fas-mediated removal of chronically activated T cells and activated APC presenting self antigens must act in concert to prevent systemic autoimmunity.
It is also intriguing to learn whether the ability of APC to undergo a timely death regulates such phenomena as latency of viral infections or organ-specific autoimmunity. Knowledge of the precise mechanisms of T cell-induced death of particular APC subsets would enable negative regulation of autoimmunity, better vaccine development and improvement of immune responses to pathogens and cancer.
Experimental Procedures
Mice
Mice with Exon IX of Fas flanked with LoxP sites (Gu et al., 1993) (Fas knock-in, FasKI) were produced as shown in Supplemental Figure 1. Animals with Cre recombinase controlled by the proximal Lck promoter B6.Cg-Tg(Lck-cre)548Jxm/J (Hennet et al., 1995) (Lck-Cre), by the CD19 promoter C.129P2-Cd19tm1(cre)Cgn/J (Rickert et al., 1997) (CD19-Cre), and by the granzyme B promoter B6;FVB-TgN(GZMB-Cre)1Jcb/J (Jacob and Baltimore, 1999) (GZB-Cre) as well as C57BL6/J and Fas-negative B6.129P2-Fastm1Osa/J (FasKO) mice were obtained from The Jackson Laboratory. Fas-ligand negative mice (Karray et al., 2004) were a generous gift from Saoussen Karray (Institut National de la Sante et de la Recherche Medicale, Hopital Necker, Paris, France) and were bred to OT-1 transgenic mice. To produce mice with Cre expressed under control of the CD11c promoter, the Cre recombinase gene from pBS185 plasmid (Gibco BRL) was introduced into the pIRES2-EGFP plasmid (BD Biosciences Clontech, Palo Alto, CA), excised as a Cre-IRES-GFP fragment and subcloned into a plasmid containing the 5.3 Kb genomic CD11c promoter/enhancer fragment(Brocker et al., 1997), (a generous gift from Klaus Karjalainen, Institute for Research in Biomedicine, Bellinzona, Switzerland). All mice were genotyped by PCR with primers amplifying the Cre transgene (5’- tgatgaggttcgcaagaacc-3’ and 5’-ccatgagtgaacgaacctgg-3’); and Fas (5’-gctgtgtctatcagtct-3’ and 5’-agagacccacctctaggtag-3’) generating 320bp wild-type and 400bp “floxed” allele products. OT-1 mice carrying a Vα2/Vβ5 transgene encoding an H-2Kb+OVA-specific T cell receptor (Hogquist et al., 1994) were bred to B6.SJL-Ptprca Pepcb/BoyJ mice carrying the Ly5.1 allele, while OT-1.FasKI.GzmB-Cre Tg inherited the Ly5.2 allele. B6(Ly5.1xLy5.2) mice were bred on site.
Antibodies and flow cytometric analysis
Labeled antibodies specific for Vβ5 (MR9−4), Vα2 (B20.1), CD8 (53−6.7), NK1.1, CD49b (DX5)(used in the experiment shown in Supplemental Figure 3), CD95 (Fas, Jo2), and Fas ligand (MFL3) were obtained from BD Biosciences Pharmingen (San Diego, CA). Anti-Ly5.1 (A20), anti-Ly5.2 (104) and anti-CD11c-APC were purchased from eBioscience (San Diego, CA). Anti PCDA-1 antibodies were from Miltenyi Biotech (Auburn, CA). Flow cytometric data were acquired using a FACSCalibur flow cytometer (Becton-Dickinson, Mountain View, CA). Data analysis was performed using CellQuest software (Becton-Dickinson) and FlowJo software (Tree Star Inc., Ashland, OR). Anti-nuclear antibodies were detected using HEp-2 substrate slides (RHI Gene Inc., Des Plains, IL) according to the manufacturer's instructions using sera diluted 1:100. Every slide included a standard normal serum from an 8 wk old B6 mouse (brightness=0) and positive control serum from a 1 yr old lpr/lpr mouse (brightness=10). See also Supplemental Figure 4.
Histology and immunohistochemistry
Tissues were fixed in picric acid/acetic acid/formalin and stained with hematoxylin/eosin. Fresh-frozen acetone-fixed tissue sections were stained with directly-coupled anti-CD11c-FITC, anti-CD8-PE and anti-CD19 PE (all from Pharmingen) and immediately photographed using a Spot CCD camera (Diagnostic Instruments, Sterling Heights, MA).
Listeria monocytogenes (Lm) infection
Frozen stocks of Lm engineered to express chicken ovalbumin (Lm-OVA) (Foulds et al., 2002) (a gift from Dr. Michael Bevan, University of Washington) were made by passage through B6 mice for 48 hours. For infection, a fresh aliquot of frozen Lm-OVA was grown in brain-heart infusion broth (Difco, Sparks, MD) for 3−4 hours at 37°C, diluted in sterile PBS and 2000 cfu injected i.v. in a 200μl volume.
T cell activation
Mice were immunized with 50 μg OVA in PBS mixed 1:1 with DIFCO Complete Freund's Adjuvant (BD Biosciences) into the hind footpads, and popliteal lymph node cells were stimulated 11 days later with 10 μg/ml OVA. For stimulation, 2×105 responders were mixed with 3×105 irradiated splenocytes from TCRß-negative B6 mice (B6.129P2-Tcrbtm1Mom/J, The Jackson Laboratory), incubated overnight in MultiScreen plates (Millipore, Bedford, MA), and IFNγ production detected using the mouse IFNγ ELISPOT pair from Pharmingen, according to the manufacturer's instructions. Spots from triplicate cultures were counted manually, and the numbers were normalized by the % of CD4+ T cells to compensate for possible loss of T cells. CD4+ T cells in the lymph nodes were enumerated by flow cytometric analysis of anti-CD4-stained cells on the day of T cell activation in vitro. CD8 cells did not contribute to IFNγ production under these experimental conditions. H60-reactive T cells were induced by i.p. injection of 2×107 CB10/J splenocytes and enumerated by staining with specific H60(LTFNYRNL)/Kb tetramers (a generous gift of Dr. Derry Roopenian, The Jackson Laboratory) used as described (Choi et al., 2002).
Activation of DC and killing assays
Bone marrow-derived DC were grown in the presence of GM-CSF as described (Inaba et al., 1992), and on day 5, DC were incubated overnight with 100ng LPS (Sigma, St. Louis, MO) or 500U/ml of recombinant interferon-ß (Pierce Biotechnology, Rockford, IL). To activate DC in vivo, mice were injected with 50 μl of PBS per footpad containing LPS (50μg) or 20μg of CpG (Coley Pharmaceutical Group, Wellesley, MA). For the in vitro killing assay, DC were activated with LPS as above and plated on top of 105 either CT26 carcinoma cell line or CT26 transfected with human FasL (CT26-CD95L) (Arai et al., 1997), a generous gift from Dr. Nabel, NIH/VRC, in a well of a 24 well plate. After 12 hrs incubation, cells were collected, stained with antiCD11c-APC and propidium iodide (PI) and analyzed by flow cytometry. For the in vivo killing assay, DCs were isolated by MACs technology using anti-CD11c magnetic beads (Miltenyi Biotech), according to the manufacturer's instructions, from collagenase/DNAse treated spleens of either B6 or B6lpr/lpr mice. DC were treated for 1hr at 37°C with 100ng/ml of SIINKEKL or with irrelevant peptide and 1μg/ml LPS, stained with 1μM (OT-1-treated) or 0.1μM (irrelevant peptide-treated) Carboxy-Fluoroscein Succinimidyl Ester (CFSE, Molecular Probes, Invitrogen, Carlsbad, CA), washed and injected as a 1:1 mixture (4×105 +4×105 cells) into footpads of B6 mice. Mice were injected 24 hrs later i.v. with 1.5×106 CD8+ OT-1 T cells purified by positive selection with magnetic beads. After 48 hrs, DC from collagenase/DNAse-treated popliteal lymph nodes were stained for CD11c and analyzed by flow cytometry. % cytotoxicity was estimated using the formula 100%×(a-b)/a, where ‘a’ and ‘b’ are the ratio between CFSElo and CFSEhi cells before footpad injection and after recovery from the lymph node, respectively. Similarly, for testing of cytoxicity in vitro, BMDC from B6 and B6lpr/lpr mice were labeled with CFSE as described above and cultured overnight in the presence of LPS (100ng/ml) and specific peptide (10ng/ml of SIINFEKL peptide) with or without OT-1 or FasL.KO OT-1 T cells. Cells (5×104 of labeled DC and variable numbers of T cells) were cultured in 0.5 ml of complete medium in 48 well plates in triplicates. DC from wells without peptide (CFSElo) were mixed with DC from well containing the peptide (CFSEhi), stained with anti-CD11c antibodies, and analyzed by flow cytometery. Cytotoxicity was calculated using the formula 100%×(a-b)/a, where “a” and “b” are the ratio between CFSElo and CFSEhi cells cultured without and with T cells, respectively.
ELISA for immunoglobulins
Relative concentrations of serum IgG and IgM were determined by titrating sera from Cre-FasKI and control Cre-negative littermates into wells of 96 well plates (Maxisorp, Nunc, Roskilde, Denmark) pre-covered with anti-mouse Fab antibodies (Sigma) and blocked with 20% FCS-PBS 2hrs at room temperature. Plates were incubated for 1 hr at room temperature, washed and incubated with either anti-mouse Fcμ or anti-Fcγ antibodies conjugated to alkaline phosphatase, washed and developed using Sigma-104 phosphatase substrate (Sigma). Dilutions of sera lower than 1:105 were saturating. Absolute concentrations of IgG and IgM in sera were determined by comparison with a standard mouse serum with defined Ig levels (Bethyl Laboratories, Montgomery, TX). Graphic presentation and statistical analysis of all data was performed using Unpaired Student's t test provided by GraphPad Prism version 4.00c for OS X, GraphPad Software (San Diego, CA).
Supplementary Material
01
2
Supplemental Figure 1. Fas expression by BMDC. Fas was expressed by BMDC from a B6 mouse and further upregulated by exposure to LPS or a type I Interferon. BMDC from a FasKO B6 mouse were used in the same experiment as controls. Thin lines: no anti-Fas antibody added. Although FasKO BMDC staining with anti-Fas was slightly shifted compared to ‘no antibody’ controls, it was definitely very small compared to the shift in B6 BMDC and was insensitive to treatments.
Supplemental Figure 2. Targeting of the Fas gene.
A. Overall strategy. Mice with Exon IX of the Fas gene flanked by loxP sites (floxed) are termed FasKI (Fas knock-in) mice. The final targeting construct contained homology regions (light blue boxes, 9.3Kb NotI/BamHI fragment on the 5’ end and 1.4Kb BamHI fragment on the 3’end), floxed Exon IX (dark blue box), floxed neo gene (green box), and the thymidine kinase gene (TK, gray box). About 200 clones were screened for proper recombination on both ends. Neo was removed by transient transfection of pIC-Cre plasmid with low Cre activity and the proper clones were identified by Southern blotting and PCR. Mice were then derived by blastocyst injection of targeted ES cells.
B. Southern hybridization analysis of ES cell DNA cut with Kpn I shows the presence of KI in ES clone D12. Hybridization with probe 1 (from intron VIII) shows the integrity of the insertion on the 5’end of FasKI. Hybridization with probe 2 highlights the 17Kb band reflecting the presence of the neo gene (intact 15 Kb genomic band from the untargeted allele and from feeder fibroblasts serves as an internal control). The 3.3 Kb band shows addition of the loxP site on the 5’ end of Exon IX. Probe 2 hybridizes with two bands because KpnI has a site corresponding to the middle of the probe. The 3.2Kb band is a genomic band from the untargeted allele and feeder cell DNA.
C. Removal of neo by transiently transfected Cre recombinase. Hybridization with KpnI-digested ES cell DNA shows deletion of neo in clones 1 and 2 (no 17kb band,*), but the 3.3kb band is present. The presence of KI minus neo was verified by PCR with two pairs of primers. Samples of “good” clones (on the left) containing FasKI (ki), and “bad” clones that have deleted FasKI and, thus, amplify the endogenous sequence only (e), along with DNA from the intact ES cell line (129) are shown. Bottom panel shows a typical genotyping PCR reaction of a litter derived from crossing two heterozygous FasKI mice. The top band (400bp) is a knock-in band; the bottom band (320bp) is a wild type (wt) band. ki- homozygous FasKI; wt- wild-type; h-heterozygous mouse. The litter shows Mendelian distribution of genotypes. Molecular markers: 100bp ladder, top band=500bp, bottom band=200bp. The absence of the wt allele in some animals proves that the gene was targeted correctly.
Supplemental Figure 3. Expression of CD11c-Cre-IRES-GFP transgene in NK cells.
Because CD11c can be found on NK cells, we investigated GFP expression by cells carrying NK cell markers in CD11c-Cre (4097) mice (left panels) and CD11c-Cre.FasKI mice (right panels). Shown is the % of CD11c+,GFP+ splenocytes among cells gated as DX5+ (top) or double-positive for DX5 and NK1.1 (bottom). No significant differences in % (and absolute numbers, not shown) were observed between the two strains. The total proportion of CD11c+,DX5+ cells in collagenase-treated spleens was 0.2% and 0.15% for CD11c-Cre and CD11c-Cre.FasKI, respectively.
Supplemental Figure 4. An explanation of how the ANA scoring system works.
ANA score was determined individually for each experiment with the same negative control sera from 8wk old B6 mice and positive control sera (lpr/lpr sera that gave a saturating staining) to minimize variation between experiments. The score depends on the brightness of staining. All nuclei test cells were stained, but with different intensity.
A. Shown are results of titration of two ANA-positive sera. A drop in brightness from 10 to 2 (5 times) corresponds to dilution by a factor of about 30 (on the top of the initial sera dilution of 1:100). Multiple digital images were ranked from dimmest to brightest and given a numeric brightness score (shown in the top left corners of images). Automated measurements are difficult due to the presence in some sera of antibodies to other cellular organels in addition to nuclear staining (B), or due to brighter staining of intranuclear compartments, such as nucleoli (C).
Supplemental Figure 5. Production of anti-nuclear antibodies in mice with deletion of Fas in DC, B cells and T cells.
ANA scores were determined in sera of female (red bars) and male (blue bars) mice of indicated ages. Black bars are respective Cre-negative FasKI mice. n, number of Cre−FasKI/Cre+FasKi mice in a group. Mean ANA scores are shown as a ratio of Cre− to Cre+ mice.
Analysis of ANA production in mice lacking Fas in DC, B cells and T cells revealed that: 1) mice with Fas deletion in APC showed higher titers of ANA compared to strains with Fas deletion in T cells [p values comparing ANA scores in older females of 4097 CD11c-Cre.FasKI, 4272 CD11c-Cre.FasKI, CD19-Cre.FasKI and Lck-Cre.FasKI were respectively 0.01, 0.01 and 0.08 (due to larger individual variation in CD19-Cre.FasKI mice)]; 2) there was no statistical difference between end-point (old) males and females in all types of Cre transgenic FasKI mice; and 3) there was a gender difference in young CD19-Cre.FasKI mice (p=0.02) with females producing more ANA.
While the output of autoreactive T cells is likely to be similar in all mice, the extended presentation of multiple autoantigens by persistent Fas-negative APC may give their T cells additional advantage over T cells with deletion of Fas, explaining the higher ANA concentrations in mice with Fas-negative APC.
It is possible (although more discrete kinetic studies are needed to confirm the point) that differences in autoimmune manifestations previously reported for B6lpr/lpr mice (Theofilopoulos and Dixon, 1985; Warren et al., 1984a; Warren et al., 1984b) are based on the properties of Fas-negative B cells.
Supplemental Figure 6. Hyperimmunoglobulinemia in mice lacking Fas in APC.
A. IgM and IgG hyperglobulinemia in 4097 CD11c-Cre.FasKI mice. Ig levels were determined by ELISA in female and male (5−6 animals per group) 20+ wk old mice. The differences between genders were not significant.
B. IgM and IgG detection by ELISA in sera of CD19.Cre-FasKI mice (blue lines and bars) or their Cre-negative littermates (red lines and bars). Left panels show the difference in relative concentration of Ig (OD in ELISA assay, lines represent individual female mice >25 wks of age); right panels show the difference in absolute concentrations (mg/ml±SE) of IgM or IgG (3−6 animals per group).
Supplemental Figure 7. Design of the experiment shown in Figure 4 B and C.
F1(Ly5.1×Ly5.2) B6 hosts received 5×105 CD8+ OT-1 wild-type cells and an equal number of CD8+ OT-1 GZB-Cre.FasKI cells. One day after transfer, recipients were immunized with 2000 cfu of Lm-OVA. Splenocytes from immunized mice were analyzed on d7 and d14 by flow cytometry for both donor populations distinguished by staining for Ly5.1+ (OT-1) or Ly5.2+ (OT-1 GZB-Cre.FasKI) or double Ly5.1+Ly5.2+ (host) T cells.
Supplemental Figure 8. Additional analysis of mice lacking Fas in T cells.
In our experiments, Fas deficiency in T cells did not lead to changes in animal viability or damage of internal organs as previously reported (Hao et al., 2004).
A. In female Lck-Cre.FasKI mice some loss of weight was observed, which became statistically significant only at one year of age. However, the same weight was found in age matched B6lpr/lpr females, indicating that the cause of the weight loss is not the attack on internal organs by T cells with up-regulated FasL [as previously suggested (Hao et al., 2004)] because lpr/lpr mice do not have a receptor for FasL. There was no significant loss of spleen mass or splenomegaly in Lck-Cre.FasKI mice, while B6lpr/lpr mice developed splenomegaly with the expected time course.
B. Purified T cells from Lck-Cre.FasKI mice (Fas-negative) were compared for FasL expression with purified T cells from Cre-negative littermates (Fas-positive). There was a very slight shift in FasL expression (red line in top panel), while FasKO T cells showed a shift in FasL expression (red line in bottom panel) restricted to a fraction of T cells compared to T cells from a B6 mouse (black line).
C. Lungs were carefully analyzed for the presence of fibrosis in groups of ten Lck-Cre.FasKI and ten Cre-negative littermates along with a smaller group of age-matched B6lpr/lpr mice. No signs of lung fibrosis were found in any of the animals when lungs were properly inflated with the fixative and Masson Trichrome staining protocol was used (L.G.Luna. Manual of histologic staining methods for the arms forces institute of pathology. 3rd edition. The Blakiston Division, McGraw-Hill Book Co., New York,1968). Blue staining of collagen fibrils was observed only around blood vessels and bronchi, but not within the alveolar tissue. Representative images from each group of mice at two magnifications (10x and 20x objectives, respectively) are shown.
D. We have also investigated CD4-Cre.FasKI animals that we have backcrossed to the NOD mouse strain. In concordance with the findings of the Rajewsky group for B6.CD4-Cre.FasKI mice, we have also found a body weight loss in NOD.CD4-Cre.FasKI mice (although not dramatic and not accompanied by a loss of viability) statistically significant in 30−35 wk old mice; many mice (but not all, p=0.07) have also shown loss of spleen mass. Spleen weight was recorded for some of these animals, while some had the splenic cellularity estimated. They were combined in one graph using a % of the mean value of control (weight or cellularity). Results were: 1.0±0.1 for control and 0.67±0.1 for Cre+ FasKI NOD mice. No lung pathology was observed in these mice either (not shown). Importantly, none of the Cre+ mice became diabetic, while control mice showed an incidence close to 80%. Thus, in these animals, only some features of the previously reported phenotype (Hao et al., 2004) were found: some loss of weight and signs of lymphopenia.
The reasons for the discrepancies found in these two systems are not yet clear. One possibility is that Fas deletion happens at different developmental stages in CD4-Cre and Lck-Cre FasKI mice, leading to different consequences in terms of up-regulation of FasL expression. One cannot ignore a possible interference with microflora that must be different in our animal facilities. Pathogens can change the environment leading to activation of T cells and exaggerated expression of FasL. That may explain why we observed no lung pathology. Additionally, another (and attractive) point to make is that Fas actually provides T cells with a survival signal. (The anti-apoptotic action of Fas is discussed in Park SM, Schickel R, Peter ME. Nonapoptotic functions of FADD-binding death receptors and their signaling molecules. Curr Opin Cell Biol. 2005.17:610). That would explain why NOD mice with Fas-negative T cells do not develop diabetes, and possibly why B6.CD4-Cre.FasKI mice had poor viability. In addition, T cells from Lck-Cre.FasKI mice and from lpr/lpr mice (Figure 6B) had a tendency for a lower response to OVA compared to control mice.
Thus, the role of Fas as a survival factor for T cells requires further investigation.
Acknowledgments
The authors are thankful to Dr. M. Weigert for valuable suggestions, Dr. Karjalainen for CD11c-promotor/enhancer construct, Dr. D. Roopenian for H60 tetramers, Dr. G. Nabel for FasL-expressing cell line, Dr. S. Karray for the gift of Fas-ligand negative mice, Mrs. J. Mach for technical assistance. This work was supported by JDRF grant 546 to A.V.C and NIH grants AI072627 to A.V.C. and AI044130 to P.J.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arai H, Chan SY, Bishop DK, Nabel GJ. Inhibition of the alloantibody response by CD95 ligand. Nat Med. 1997;3:843–848. [Abstract] [Google Scholar]
- Bevan MJ, Fink PJ. The CD8 response on autopilot. Nat Immunol. 2001;2:381–382. [Abstract] [Google Scholar]
- Brocker T, Riedinger M, Karjalainen K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J Exp Med. 1997;185:541–550. [Europe PMC free article] [Abstract] [Google Scholar]
- Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. [Abstract] [Google Scholar]
- Chen M, Wang YH, Wang Y, Huang L, Sandoval H, Liu YJ, Wang J. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. 2006;311:1160–1164. [Abstract] [Google Scholar]
- Choi EY, Christianson GJ, Yoshimura Y, Sproule TJ, Jung N, Joyce S, Roopenian DC. Immunodominance of H60 is caused by an abnormally high precursor T cell pool directed against its unique minor histocompatibility antigen peptide. Immunity. 2002;17:593–603. [Abstract] [Google Scholar]
- Crispe IN. Fatal interactions: Fas-induced apoptosis of mature T cells. Immunity. 1994;1:347–349. [Abstract] [Google Scholar]
- Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. [Abstract] [Google Scholar]
- Custer RP, Bosma GC, Bosma MJ. Severe combined immunodeficiency (SCID) in the mouse. Pathology, reconstitution, neoplasms. Am J Pathol. 1985;120:464–477. [Europe PMC free article] [Abstract] [Google Scholar]
- Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature. 1995;373:438–441. [Abstract] [Google Scholar]
- Fields ML, Sokol CL, Eaton-Bassiri A, Seo S, Madaio MP, Erikson J. Fas/Fas ligand deficiency results in altered localization of anti-double-stranded DNA B cells and dendritic cells. J Immunol. 2001;167:2370–2378. [Abstract] [Google Scholar]
- Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, Strober W, Lenardo MJ, Puck JM. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. [Abstract] [Google Scholar]
- Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J Immunol. 2002;168:1528–1532. [Abstract] [Google Scholar]
- Fukuyama H, Adachi M, Suematsu S, Miwa K, Suda T, Yoshida N, Nagata S. Transgenic expression of Fas in T cells blocks lymphoproliferation but not autoimmune disease in MRL-lpr mice. J Immunol. 1998;160:3805–3811. [Abstract] [Google Scholar]
- Gu H, Zou YR, Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993;73:1155–1164. [Abstract] [Google Scholar]
- Hao Z, Hampel B, Yagita H, Rajewsky K. T cell-specific ablation of Fas leads to Fas ligand-mediated lymphocyte depletion and inflammatory pulmonary fibrosis. J Exp Med. 2004;199:1355–1365. [Europe PMC free article] [Abstract] [Google Scholar]
- Hennet T, Hagen FK, Tabak LA, Marth JD. T-cell-specific deletion of a polypeptide N-acetylgalactosaminyl-transferase gene by site-directed recombination. Proc Natl Acad Sci U S A. 1995;92:12070–12074. [Europe PMC free article] [Abstract] [Google Scholar]
- Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, Marrack P. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16:759–767. [Abstract] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. [Abstract] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. [Europe PMC free article] [Abstract] [Google Scholar]
- Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J Exp Med. 1997;185:2133–2141. [Europe PMC free article] [Abstract] [Google Scholar]
- Jacob J, Baltimore D. Modelling T-cell memory by genetic marking of memory T cells in vivo. Nature. 1999;399:593–597. [Abstract] [Google Scholar]
- Janeway CA., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. [Abstract] [Google Scholar]
- Karray S, Kress C, Cuvellier S, Hue-Beauvais C, Damotte D, Babinet C, Levi-Strauss M. Complete loss of Fas ligand gene causes massive lymphoproliferation and early death, indicating a residual activity of gld allele. J Immunol. 2004;172:2118–2125. [Abstract] [Google Scholar]
- Lynch DH, Watson ML, Alderson MR, Baum PR, Miller RE, Tough T, Gibson M, Davis-Smith T, Smith CA, Hunter K, et al. The mouse Fas-ligand gene is mutated in gld mice and is part of a TNF family gene cluster. Immunity. 1994;1:131–136. [Abstract] [Google Scholar]
- Medema JP, Schuurhuis DH, Rea D, van Tongeren J, de Jong J, Bres SA, Laban S, Toes RE, Toebes M, Schumacher TN, et al. Expression of the serpin serine protease inhibitor 6 protects dendritic cells from cytotoxic T lymphocyte-induced apoptosis: differential modulation by T helper type 1 and type 2 cells. J Exp Med. 2001;194:657–667. [Europe PMC free article] [Abstract] [Google Scholar]
- Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. Early programming of T cell populations responding to bacterial infection. J Immunol. 2000;165:6833–6839. [Abstract] [Google Scholar]
- Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. [Abstract] [Google Scholar]
- Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med. 2005;201:1555–1565. [Europe PMC free article] [Abstract] [Google Scholar]
- Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317–1318. [Europe PMC free article] [Abstract] [Google Scholar]
- Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, de Villartay JP. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. [Abstract] [Google Scholar]
- Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994;180:1295–1306. [Europe PMC free article] [Abstract] [Google Scholar]
- Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, Henter JI, Bennett M, Fischer A, de Saint Basile G, Kumar V. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957–1959. [Abstract] [Google Scholar]
- Storni T, Ruedl C, Renner WA, Bachmann MF. Innate immunity together with duration of antigen persistence regulate effector T cell induction. J Immunol. 2003;171:795–801. [Abstract] [Google Scholar]
- Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. [Abstract] [Google Scholar]
- Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. [Abstract] [Google Scholar]
- Warren RW, Caster SA, Roths JB, Murphy ED, Pisetsky DS. The influence of the lpr gene on B cell activation: differential antibody expression in lpr congenic mouse strains. Clin Immunol Immunopathol. 1984a;31:65–77. [Abstract] [Google Scholar]
- Warren RW, Roths JB, Murphy ED, Pisetsky DS. Mechanisms of polyclonal B-cell activation in autoimmune B6-lpr/lpr mice. Cell Immunol. 1984b;84:22–31. [Abstract] [Google Scholar]
- Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. [Abstract] [Google Scholar]
- Wong P, Pamer EG. Feedback regulation of pathogen-specific T cell priming. Immunity. 2003;18:499–511. [Abstract] [Google Scholar]
- Wu J, Wilson J, He J, Xiang L, Schur PH, Mountz JD. Fas ligand mutation in a patient with systemic lupus erythematosus and lymphoproliferative disease. J Clin Invest. 1996;98:1107–1113. [Europe PMC free article] [Abstract] [Google Scholar]
- Yang J, Huck SP, McHugh RS, Hermans IF, Ronchese F. Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8+ T cells in vivo. Proc Natl Acad Sci U S A. 2006;103:147–152. [Europe PMC free article] [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.immuni.2007.03.016
Read article for free, from open access legal sources, via Unpaywall:
http://www.cell.com/article/S1074761307002488/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/101858581
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.immuni.2007.03.016
Article citations
Striking a balance: new perspectives on homeostatic dendritic cell maturation.
Nat Rev Immunol, 17 Sep 2024
Cited by: 1 article | PMID: 39289483
Review
CD14 is a decision-maker between Fas-mediated death and inflammation.
Cell Rep, 43(9):114685, 30 Aug 2024
Cited by: 0 articles | PMID: 39213151 | PMCID: PMC11471008
LTβR-RelB signaling in intestinal epithelial cells protects from chemotherapy-induced mucosal damage.
Front Immunol, 15:1388496, 30 May 2024
Cited by: 0 articles | PMID: 38873613
Neddylation is a novel therapeutic target for lupus by regulating double negative T cell homeostasis.
Signal Transduct Target Ther, 9(1):18, 15 Jan 2024
Cited by: 3 articles | PMID: 38221551 | PMCID: PMC10788348
Intravital imaging of the functions of immune cells in the tumor microenvironment during immunotherapy.
Front Immunol, 14:1288273, 06 Dec 2023
Cited by: 0 articles | PMID: 38124754 | PMCID: PMC10730658
Review Free full text in Europe PMC
Go to all (241) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Dendritic Cells Regulate Extrafollicular Autoreactive B Cells via T Cells Expressing Fas and Fas Ligand.
Immunity, 45(5):1052-1065, 25 Oct 2016
Cited by: 18 articles | PMID: 27793595 | PMCID: PMC5112117
Prevention of autoimmunity and control of recall response to exogenous antigen by Fas death receptor ligand expression on T cells.
Immunity, 29(6):922-933, 13 Nov 2008
Cited by: 25 articles | PMID: 19013083
Fas/CD95 prevents autoimmunity independently of lipid raft localization and efficient apoptosis induction.
Nat Commun, 7:13895, 23 Dec 2016
Cited by: 26 articles | PMID: 28008916 | PMCID: PMC5196435
Specific deletion of autoreactive T cells by adenovirus-transfected, Fas ligand-producing antigen-presenting cells.
Immunol Res, 26(1-3):235-246, 01 Jan 2002
Cited by: 11 articles | PMID: 12403361
Review
Funding
Funders who supported this work.
Juvenile Diabetes Research Foundation United States of America (1)
Grant ID: 546
NIAID NIH HHS (5)
Grant ID: AI044130
Grant ID: R01 AI072627
Grant ID: R01 AI044130
Grant ID: AI072627
Grant ID: R01 AI072627-01
NIDDK NIH HHS (1)
Grant ID: R01 DK053561-05
National Institutes of Health (2)
Grant ID: AI044130
Grant ID: AI072627




