Abstract
Free full text

Antigenic heterogeneity in high- and low-virulence strains of Rickettsia rickettsii revealed by monoclonal antibodies.
Abstract
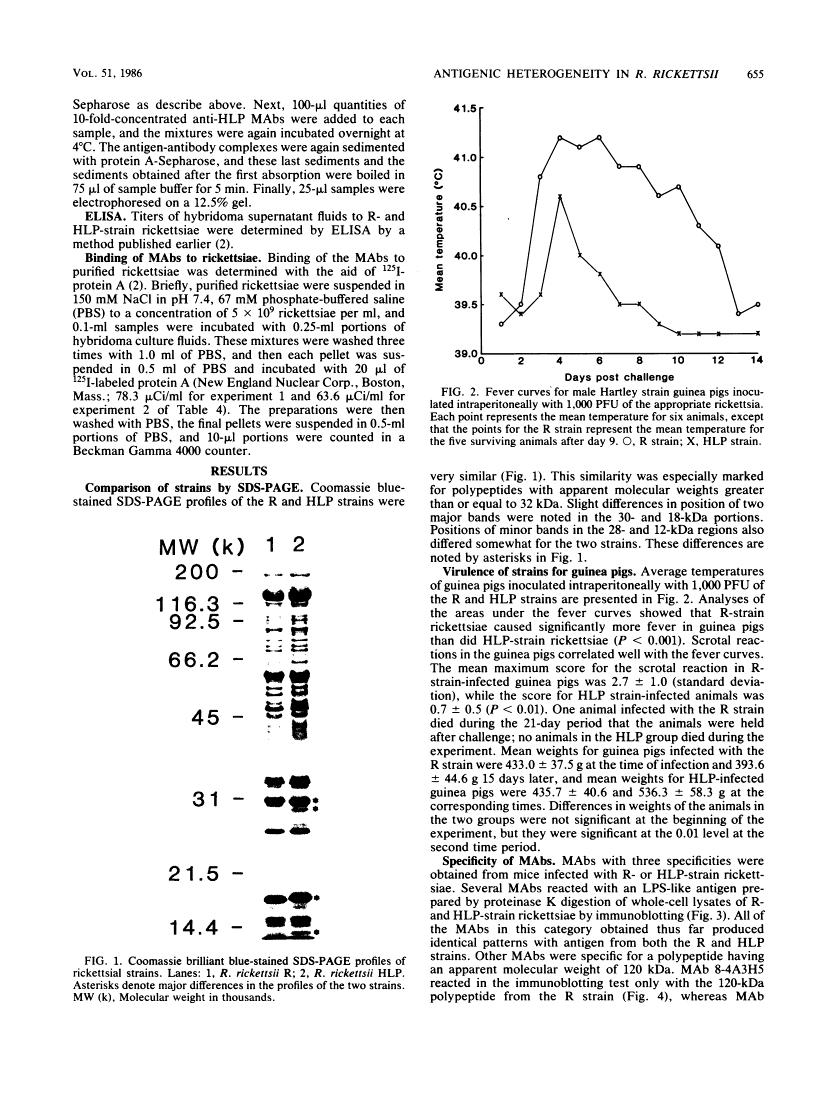
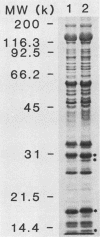
Previously it has been reported that strains of Rickettsia rickettsii that differ greatly in their ability to cause disease in guinea pigs are similar by serological and sodium dodecyl sulfate-polyacrylamide gel electrophoresis analyses. In this study, we used monoclonal antibodies to the virulent R and the relatively avirulent HLP strains to investigate strain differences which might account for the disparate behavior of the strains in guinea pigs. Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis profiles of the R and HLP strains were nearly identical for polypeptides with apparent molecular weights greater than 32 kilodaltons (kDa). All of the monoclonal antibodies to a lipopolysaccharide-like antigen reacted equally well with antigen from both strains by immunoblotting. None of the antibodies to the lipopolysaccharide-like antigen protected mice against challenge with viable rickettsiae. Some antibodies reacted with both 120- and 155-kDa polypeptides of both strains in radioimmune precipitation and immunoblotting tests, and other antibodies reacted only with the homologous strain. The monoclonal antibodies cross-reacted with the heterologous strain in the enzyme-linked immunosorbent assay essentially either completely or not at all. The ability of the monoclonal antibodies to the 120- and 155-kDa polypeptides to protect mice against the two strains was correlated with the ability of the antibodies to react with the antigens in the enzyme-linked immunosorbent assay and radioimmune precipitation or immunoblotting tests. These results demonstrate that R and HLP antigens which appear identical in molecular weight differ in their compositions of antigenic determinants.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.9M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker RL, Gerloff RK, Thomas LA, Mann RE, Bickel WD. Immunological properties of Rickettsia rickettsii purified by zonal centrifugation. Infect Immun. 1975 Jun;11(6):1203–1209. [Europe PMC free article] [Abstract] [Google Scholar]
- Anacker RL, List RH, Mann RE, Hayes SF, Thomas LA. Characterization of monoclonal antibodies protecting mice against Rickettsia rickettsii. J Infect Dis. 1985 Jun;151(6):1052–1060. [Abstract] [Google Scholar]
- Anacker RL, McCaul TF, Burgdorfer W, Gerloff RK. Properties of selected rickettsiae of the spotted fever group. Infect Immun. 1980 Feb;27(2):468–474. [Europe PMC free article] [Abstract] [Google Scholar]
- Anacker RL, Philip RN, Williams JC, List RH, Mann RE. Biochemical and immunochemical analysis of Rickettsia rickettsii strains of various degrees of virulence. Infect Immun. 1984 Jun;44(3):559–564. [Europe PMC free article] [Abstract] [Google Scholar]
- Anacker RL, Smith RF, Mann RE, Hamilton MA. New assay of protective activity of Rocky Mountain spotted fever vaccines. J Clin Microbiol. 1976 Sep;4(3):309–311. [Europe PMC free article] [Abstract] [Google Scholar]
- Batteiger B, Newhall WJ, 5th, Jones RB. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. [Abstract] [Google Scholar]
- BELL EJ, KOHLS GM, STOENNER HG, LACKMAN DB. NONPATHOGENIC RICKETTSIAS RELATED TO THE SPOTTED FEVER GROUP ISOLATED FROM TICKS, DERMACENTOR VARIABILIS AND DERMACENTOR ANDERSONI FROM EASTERN MONTANA. J Immunol. 1963 May;90:770–781. [Abstract] [Google Scholar]
- BELL EJ, PICKENS EG. A toxic substance associated with the rickettsias of the spotted fever group. J Immunol. 1953 May;70(5):461–472. [Abstract] [Google Scholar]
- BELL EJ, STOENNER HG. Immunologic relationships among the spotted fever group of rickettsias determined by toxin neutralization tests in mice with convalescent animal serums. J Immunol. 1960 Feb;84:171–182. [Abstract] [Google Scholar]
- BELL EJ, STOENNER HG. Spotted fever vaccine; potency assay by direct challenge of vaccinated mice with toxin of Rickettsia rickettsii. J Immunol. 1961 Dec;87:737–746. [Abstract] [Google Scholar]
- Burgdorfer W. A review of Rocky Mountain spotted fever (tick-borne typhus), its agent, and its tick vectors in the United States. J Med Entomol. 1975 Sep 25;12(3):269–278. [Abstract] [Google Scholar]
- Burgdorfer W, Sexton DJ, Gerloff RK, Anacker RL, Philip RN, Thomas LA. Rhipicephalus sanguineus: vector of a new spotted fever group rickettsia in the United States. Infect Immun. 1975 Jul;12(1):205–210. [Europe PMC free article] [Abstract] [Google Scholar]
- Hitchcock PJ, Brown TM. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. [Europe PMC free article] [Abstract] [Google Scholar]
- Hughes LE, Clifford CM, Gresbrink R, Thomas LA, Keirans JE. Isolation of a spotted fever group rickettsia from the Pacific Coast tick, Ixodes pacificus, in Oregon. Am J Trop Med Hyg. 1976 May;25(3):513–516. [Abstract] [Google Scholar]
- JACKSON EB, SMADEL JE. Immunization against scrub typhus. II. Preparation of lyophilized living vaccine. Am J Hyg. 1951 May;53(3):326–331. [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Levy NJ, Nicholson-Weller A, Baker CJ, Kasper DL. Potentiation of virulence by group B streptococcal polysaccharides. J Infect Dis. 1984 Jun;149(6):851–860. [Abstract] [Google Scholar]
- Markwell MA, Fox CF. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. [Abstract] [Google Scholar]
- Orskov F. Virulence factors of the bacterial cell surface. J Infect Dis. 1978 May;137(5):630–633. [Abstract] [Google Scholar]
- PARKER RR, PICKENS EG, LACKMAN DB, BELLE EJ, THRAIKILL FB. Isolation and characterization of Rocky Mountain Spotted Fever Rickettsiae from the rabbit tick Haemaphysalis leporis-palustris Packard. Public Health Rep. 1951 Apr 13;66(15):455–463. [Europe PMC free article] [Abstract] [Google Scholar]
- Philip RN, Casper EA. Serotypes of spotted fever group rickettsiae isolated from Dermacentor andersoni (Stiles) ticks in western Montana. Am J Trop Med Hyg. 1981 Jan;30(1):230–238. [Abstract] [Google Scholar]
- Philip RN, Casper EA, Burgdorfer W, Gerloff RK, Hughes LE, Bell EJ. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J Immunol. 1978 Nov;121(5):1961–1968. [Abstract] [Google Scholar]
- PRICE WH. The epidemiology of Rocky Mountain spotted fever. I. The characterization of strain virulence of Rickettsia rickettsii. Am J Hyg. 1953 Sep;58(2):248–268. [Abstract] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. [Europe PMC free article] [Abstract] [Google Scholar]
- STOENNER HG, LACKMAN DB, BELL EJ. Factors affecting the growth of rickettsias of the spotted fever group in fertile hens' eggs. J Infect Dis. 1962 Mar-Apr;110:121–128. [Abstract] [Google Scholar]
- Weiss E, Coolbaugh JC, Williams JC. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.51.2.653-660.1986
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC262399
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/reprint/51/2/653
Free to read at iai.asm.org
http://iai.asm.org/cgi/content/abstract/51/2/653
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/iai.51.2.653-660.1986
Article citations
Predicting the global potential distribution of two major vectors of Rocky Mountain Spotted Fever under conditions of global climate change.
PLoS Negl Trop Dis, 18(1):e0011883, 10 Jan 2024
Cited by: 0 articles | PMID: 38198451 | PMCID: PMC10805312
Identification of an autotransporter peptidase of Rickettsia rickettsii responsible for maturation of surface exposed autotransporters.
PLoS Pathog, 19(7):e1011527, 31 Jul 2023
Cited by: 5 articles | PMID: 37523399 | PMCID: PMC10414592
The Rickettsial Ankyrin Repeat Protein 2 Is a Type IV Secreted Effector That Associates with the Endoplasmic Reticulum.
mBio, 9(3):e00975-18, 26 Jun 2018
Cited by: 40 articles | PMID: 29946049 | PMCID: PMC6020290
Phylogeography of Rickettsia rickettsii genotypes associated with fatal Rocky Mountain spotted fever.
Am J Trop Med Hyg, 91(3):589-597, 23 Jun 2014
Cited by: 18 articles | PMID: 24957541 | PMCID: PMC4155566
Complementation of Rickettsia rickettsii RelA/SpoT restores a nonlytic plaque phenotype.
Infect Immun, 79(4):1631-1637, 07 Feb 2011
Cited by: 26 articles | PMID: 21300770 | PMCID: PMC3067566
Go to all (50) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Biochemical and immunochemical analysis of Rickettsia rickettsii strains of various degrees of virulence.
Infect Immun, 44(3):559-564, 01 Jun 1984
Cited by: 46 articles | PMID: 6427110 | PMCID: PMC263624
Reactivity of monoclonal antibodies to Rickettsia rickettsii with spotted fever and typhus group rickettsiae.
J Clin Microbiol, 25(1):167-171, 01 Jan 1987
Cited by: 98 articles | PMID: 2432081 | PMCID: PMC265851
Characterization of monoclonal antibodies protecting mice against Rickettsia rickettsii.
J Infect Dis, 151(6):1052-1060, 01 Jun 1985
Cited by: 65 articles | PMID: 3923129
Genomic comparison of virulent Rickettsia rickettsii Sheila Smith and avirulent Rickettsia rickettsii Iowa.
Infect Immun, 76(2):542-550, 19 Nov 2007
Cited by: 73 articles | PMID: 18025092 | PMCID: PMC2223442