Abstract
Background
It remains unknown whether diabetes mellitus (DM) is a risk factor for mild cognitive impairment (MCI).Objective
To investigate the association of DM with MCI using a population-based case-control design.Design
Population-based case-control study.Setting
Academic research.Participants
Our study was conducted, among subjects aged 70 to 89 years on October 1, 2004, who were randomly selected from the Olmsted County (Minnesota) population. Main Outcome Measure We administered to all participants a neurologic examination, the Clinical Dementia Rating Scale, and a neuropsychological evaluation (including 9 tests in 4 cognitive domains) to diagnose normal cognition, MCI, or dementia. We assessed history of DM, DM treatment, and DM complications by interview, and we measured fasting blood glucose levels. History of DM was also confirmed using a medical records linkage system.Results
We compared 329 subjects having MCI with 1640 subjects free of MCI and dementia. The frequency of DM was similar in subjects with MCI (20.1%) and in subjects without MCI (17.7%) (odds ratio [OR], 1.16; 95% confidence interval [CI], 0.85-1.57). However, MCI was associated with onset of DM before age 65 years (OR, 2.20; 95% CI, 1.29-3.73), DM duration of 10 years or longer (OR, 1.76; 95% CI, 1.16-2.68), treatment with insulin (OR, 2.01; 95% CI, 1.22-3.31), and the presence of DM complications (OR, 1.80; 95% CI, 1.13-2.89) after adjustment for age, sex, and education. Analyses using alternative definitions of DM yielded consistent findings.Conclusion
These findings suggest an association of MCI with earlier onset, longer duration, and greater severity of DM.Free full text

Duration and Severity of Diabetes Are Associated with Mild Cognitive Impairment
Abstract
Background
It remains unknown whether diabetes mellitus is a risk factor for mild cognitive impairment (MCI).
Objective
To investigate the association of diabetes mellitus with MCI using a population-based case-control design.
Design, Setting, and Participants
Our study was conducted in subjects aged 70 through 89 years on October 1, 2004, who were randomly selected from the Olmsted County, MN, population.
Main Outcome Measure
We administered to all participants the Clinical Dementia Rating Scale, a neurological exam, and a neuropsychological evaluation including 9 tests in 4 cognitive domains to diagnose normal cognition, MCI, or dementia. We assessed history of diabetes, diabetes treatment, and complications by interview and we measured fasting blood glucose. History of diabetes was also confirmed using a medical records-linkage system.
Results
We compared 329 patients with MCI to 1640 subjects free of MCI and of dementia. The frequency of diabetes was similar in subjects with MCI (20.1%) and in subjects without MCI (17.7%; odds ratio [OR], 1.16; 95% confidence interval [CI], 0.85-1.57). However, MCI was associated with onset of diabetes before age 65 years (OR, 2.20; 95% CI, 1.29-3.73), diabetes duration ≥10 years (OR, 1.76; 95% CI, 1.16-2.68), treatment with insulin (OR, 2.01; 95% CI, 1.22-3.31), and presence of complications (OR, 1.80; 95% CI, 1.13-2.89) after adjustment for age, sex, and education. Analyses using alternative definitions of diabetes yielded consistent findings.
Conclusions
These findings suggest an association between earlier onset, longer duration, and greater severity of diabetes and MCI.
Mild cognitive impairment (MCI) is a transitional stage between normal cognitive aging and dementia.1 In the absence of curative treatments for dementia, identification of subjects at increased risk of dementia and of modifiable risk factors may allow interventions that prevent progression from preclinical (MCI) to clinical disease (dementia). Several studies have suggested an association between diabetes mellitus and cognitive impairment,2-4 rapid decline in cognitive function,5,6 and dementia.7-9 In addition, diabetes has been associated with increased deposition and decreased clearance of beta amyloid.10-12 Poor glycemic control and chronic episodes of hypo- or hyperglycemia may lead to microangiopathy, neuronal loss, and cognitive impairment.13 Finally, diabetes is associated with increased cardiovascular risk and with macro- and microvascular cerebral disease,14 all of which may independently increase the risk of cognitive impairment. However, some studies have not confirmed the association.15-17
The inconsistency in findings may be due to differences in study design, source of study subjects, and differences in criteria for the diagnosis of diabetes or cognitive impairment. However, it may also be due to differences in the duration or severity of diabetes in study subjects. In this population-based case-control study, we investigated the association of diabetes and of markers of diabetes severity (ie, age at onset, duration, treatment type, and complications) with MCI.
METHODS
IDENTIFICATION OF CASES AND CONTROLS
Identification of cases and controls was performed as part of a population-based study to estimate the prevalence of MCI in Olmsted County, Minnesota. Details of the study design and participant recruitment are described elsewhere.18 The study protocol was approved by the Institutional Review Boards of the Mayo Clinic and Olmsted Medical Center. Briefly, we used the records-linkage system of the Rochester Epidemiology Project19 to construct a sampling frame of Olmsted County residents aged 70 through 89 years on October 1, 2004. A total of 9953 unique individuals were identified, and 5233 were randomly selected and evaluated for eligibility. We excluded subjects who died before they could be contacted (n=263) and subjects who were in hospice (n=56); subjects with previously diagnosed confirmed dementia (n=402) were identified by a screening of their medical record and were also excluded. Subjects who could not be contacted (n=114) were considered ineligible. Of the 4398 eligible subjects, 2719 (61.8%) agreed to participate in a face-to-face evaluation (n=2050) or by telephone (n=669). This case-control study was based on subjects who participated in the face-to-face evaluation. Subjects underwent a nurse evaluation and risk factors assessment (including the Clinical Dementia Rating Scale), a neurological evaluation, and a neuropsychological evaluation including 9 tests and covering 4 cognitive domains (memory, executive function, language, and visuospatial function).18 An expert panel of physicians, neuropsychologists, and nurses then reviewed all the information collected for each participant to reach a consensus diagnosis of cognitively normal, MCI, or dementia.
Cases
All subjects who participated in the face-to-face evaluation and were found to be affected by MCI were included as MCI cases (prevalent series of MCI cases). MCI was defined according to the following published criteria: cognitive concern by physician, patient, or nurse; impairment in 1 or more of the 4 cognitive domains; essentially normal functional activities; and not demented.1 Patients with MCI were classified as having amnestic MCI (a-MCI) if the memory domain was impaired or non-amnestic MCI (na-MCI) if there was no impairment in memory.
Controls
All subjects who participated in the face-to-face evaluation and were found to be cognitively normal were included as controls. A diagnosis of normal cognition was assigned according to published criteria.1,20 Thus, controls were all free of both MCI and dementia. A diagnosis of dementia was based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition criteria.21
MEASURE OF DIABETES
Diabetes was defined using 3 sources of information: 1) self-reports, 2) fasting blood glucose levels, and 3) medical index diagnoses.
Self-report of diabetes
As part of the nurse interview and risk factor assessment, participants were asked if they had ever been diagnosed as having diabetes or “borderline diabetes” by a doctor, or if they had ever had diabetic nerve problems in their legs or feet (neuropathy), ulcers or sores on their feet that were difficult to heal or that a doctor said were related to diabetes (neuropathy), eye problems or eye surgery attributed to diabetes (retinopathy), or kidney problems that had been attributed to diabetes (nephropathy). They were also asked about all medications used on a daily basis. Medication use was validated by reviewing the bottles of medications brought to the evaluation (subjects were instructed to bring these with them). Subjects who reported a physician diagnosis of diabetes, treatment for diabetes using oral anti-diabetic agents or insulin, or diabetic complications were classified as having diabetes.
Fasting blood glucose
Each participant underwent a blood draw, and a fasting blood glucose level was measured using a photometric rate reaction (Roche/Hitachi Modular Systems, Indianapolis, IN). The coefficient of variation of the test was 0.87 at a mean of 88 mg/dl and 0.63 at a mean of 289 mg/dl. A qualifying fasting blood glucose level for diabetes was defined as ≥126 mg/dl after a 10- to 12-hour fast for subjects evaluated in the morning,22 or a fasting blood glucose ≥114 mg/dl after a 4- to 6-hour fast for subjects evaluated in the afternoon.23 The latter cut-point was used because 4- to 6-hour fasting glucose levels measured in the afternoon are lower than the 10- to 12-hour fasting levels measured in the morning.23 Using a technique described in a previous study,23 we first determined the proportion of subjects with diabetes from a 10- to 12-hour fasting blood glucose measured in the morning and a cut-point of 126 mg/dl. We then determined the fasting blood glucose cut-point that would yield a similar proportion of subjects with diabetes from a 4- to 6-hour fasting blood glucose measured in the afternoon. We arrived at the same cut-point of 114 mg/dl reported by other investigators.23
Medical record ascertainment
Diabetes was also ascertained from the medical index of the records-linkage system serving Olmsted County.19 Persons were considered to have diabetes if they had at least 1 International Classification of Diseases code for diabetes (not otherwise specified), diabetes with and without mention of complications (neuropathy, retinopathy, nephropathy), and diabetes mellitus type 1 (International Classification of Diseases, Eighth Revision, Adapted Codes for Hospitals or Ninth Revision).24 Subjects who only had a code for hyperglycemia or borderline diabetes mellitus were considered unaffected. The date of first appearance of a diabetes code in the medical record was used to estimate the age at onset of diabetes.
MEASURE OF POTENTIAL CONFOUNDERS
Date of birth, education, cigarette smoking, and past medical history of hypertension, coronary heart disease (myocardial infarction, angina, coronary artery bypass grafting, or coronary revascularization), depression, and stroke or transient ischemic attack (TIA) were ascertained by interview. Surgeries for coronary heart disease were also ascertained by searching the surgical index of the records-linkage system.19 Current symptoms of depression were assessed through the Neuropsychiatric Inventory-Questionnaire administered to a study partner.25 DNA extraction and APOE genotyping was performed for each subject using standard methods.26
STATISTICAL ANALYSES
In the first set of case-control analyses, we defined diabetes as self-reported doctor’s diagnosis of diabetes, diabetes treatment, or diabetic complications. The associations of MCI with type of treatment for diabetes and diabetic complications were evaluated. We used logistic regression models with adjustment for age (expressed as a continuous variable), sex, and education (expressed as a continuous variable) because these 3 variables have been shown to be strongly associated with cognitive function. Potential confounding by smoking (ever vs never), hypertension, coronary heart disease, stroke or TIA, BMI (≥30 vs <30), current depression, and APOE genotype (ε4ε4 or ε3ε4 vs ε2ε2, ε2ε3, or ε3ε3) were examined with each variable entered separately in the models. Subjects with APOE genotype ε2ε4 were excluded because ε2 is considered protective while ε4 is considered a risk factor and because that genotype was rare (2.3%). The association of diabetes with MCI was also examined with these variables entered simultaneously in the model. Effect modification by these variables was examined in stratified analyses and also by including an interaction term for diabetes and the variable in the model.
In the second set of case-control analyses, the definition of diabetes was broadened by also including subjects who did not report a diagnosis of diabetes, diabetes treatment, or diabetic complications, but who had a qualifying fasting blood glucose level (≥126 mg/dl or ≥114 mg/dl). In sensitivity analyses, subjects were characterized as having diabetes if they had both a self-report of diabetes and were found to have at least 1 code for diabetes in the medical records-linkage system. For these sensitivity analyses, duration of diabetes was estimated using information on age at onset abstracted from the medical record.
RESULTS
CHARACTERISTICS OF STUDY SUBJECTS
Of the 2050 participants who were evaluated in person, 1969 subjects were found to be free of dementia and were included in this study. Eighty-one subjects were excluded: 1 subject had life-long impaired cognitive function not due MCI or dementia; 13 subjects did not complete the evaluation and therefore could not be assigned a diagnosis; and 67 subjects received a diagnosis of dementia from the evaluation. Subjects with MCI were significantly older, more likely to be men, and had a lower level of education than subjects without MCI (Table 1). MCI subjects were also more likely to have a history of stroke or TIA, an APOE ε3ε4/ε4ε4 genotype, and to be depressed.
Table 1
Demographic and Clinical Characteristics of Cases with MCI and Controls
| Variable | Cases with MCI n=329 n (%) | Controls n=1640 n (%) | Odds Ratio* | 95% CI | P Value |
|---|---|---|---|---|---|
| Age, years† | |||||
 70 through 79 70 through 79 | 103 (31.3) | 847 (51.6) | 1.00 | -- | -- |
 80 through 89 80 through 89 | 226 (68.7) | 793 (48.4) | 2.73 | 2.07-3.59 | <.001 |
| Sex, n (%) | |||||
 Women Women | 177 (41.6) | 830 (50.6) | 1.00 | -- | -- |
 Men Men | 192 (58.4) | 810 (49.4) | 1.67 | 1.30-2.13 | <.001 |
| Education, years | |||||
 <9 <9 | 48 (14.6) | 92 (5.6) | 2.65 | 1.78-3.96 | <.001 |
 9 through 12 9 through 12 | 140 (42.6) | 641 (39.1) | 1.42 | 1.09-1.84 | .008 |
 >12 >12 | 141 (42.9) | 907 (55.3) | 1.00 | -- | -- |
| Stroke or TIA, n (%) | 89 (27.1) | 205 (12.6) | 2.30 | 1.72-3.08 | <.001 |
| Coronary heart disease‡, n (%) | 121 (36.8) | 490 (29.9) | 1.17 | 0.91-1.52 | .23 |
| APOE ε3ε4 or ε4ε4§, n (%) | 89 (29.2) | 334 (21.9) | 1.55 | 1.17-2.05 | .002 |
| Depression, n (%) | 86 (27.0) | 182 (11.4) | 2.88 | 2.13-3.88 | <.001 |
| BMI ≥ 30, n (%) | 74 (23.3) | 457 (28.4) | 0.87 | 0.65-1.16 | .35 |
Hypertension , n (%) , n (%) | 234 (71.1) | 1154 (70.4) | 0.99 | 0.76-1.29 | .92 |
| Cigarette smoking¶, n (%) | |||||
 Never smoked Never smoked | 165 (50.2) | 805 (49.1) | 1.00 | -- | -- |
 Former smoker Former smoker | 152 (46.2) | 739 (45.1) | 1.02 | 0.79-1.31 | .91 |
 Current smoker Current smoker | 13 (4.0) | 66 (4.0) | 1.17 | 0.62-2.19 | .63 |
 Self-report or use of medications for hypertension.
Self-report or use of medications for hypertension.ASCERTAINMENT OF DIABETES FROM SELF-REPORT ONLY
Overall, 356 (18.1%) subjects were characterized as having diabetes based on self-report of a doctor’s diagnosis of diabetes, treatment for diabetes, or complications. Of these, 148 (41.6%) reported treatment for diabetes only, 85 (23.9%) reported treatment and complications, and 18 (5.0%) reported complications only. Of the 105 subjects (29.5%) who reported no treatment and no complications, 34 had at least 1 diagnostic code for diabetes in the medical record, 8 had a qualifying blood glucose level for diabetes, 24 had both; however, the remaining 39 subjects had no additional information.
There were no significant associations between diabetes and MCI overall, or MCI subtypes (Table 2, footnotes); however, there were significant associations with type of diabetes treatment and diabetic complications. The OR for treatment with insulin alone was significantly increased (OR, 2.05; 95% CI, 1.20-3.49). Subjects receiving both insulin and oral anti-diabetic agents (12 on metformin, 1 on pioglitazone) also had an elevated but non-significant OR (OR, 1.80; 95% CI, 0.48-6.71). The OR for any insulin treatment (insulin with or without an oral hypoglycemic agent) was significantly elevated (Table 2, model 1), but there was no significant association with oral hypoglycemic use only or with no treatment (Figure 1). There was also a significant association of MCI with presence of any diabetic complications. Specifically, the ORs were significantly elevated 2-fold for neuropathy and retinopathy, and 1.5-fold for nephropathy (Table 2, model 1), but the confidence intervals for the latter estimate included 1 (Figure 2). The estimates were all essentially the same after adjustment for vascular risk factors (Table 2, model 2) and depression (data not shown).
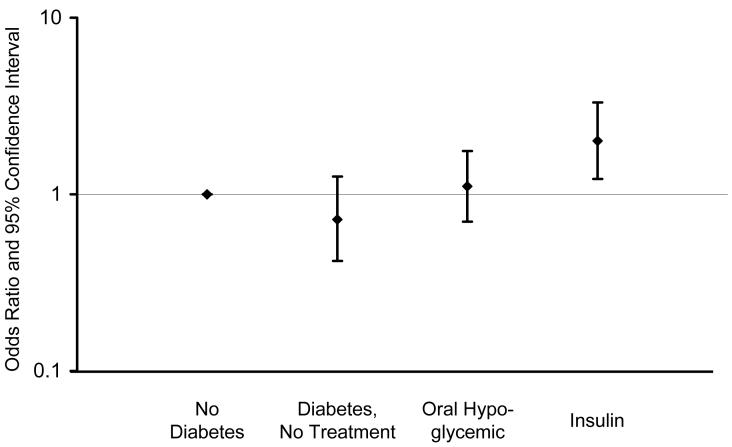
Odds ratios and 95% confidence intervals for the association of mild cognitive impairment with type of treatment for diabetes (no treatment, oral hypoglycemic agents, and insulin with or without an oral hypoglycemic agent) compared to subjects without diabetes (OR, 1.00).
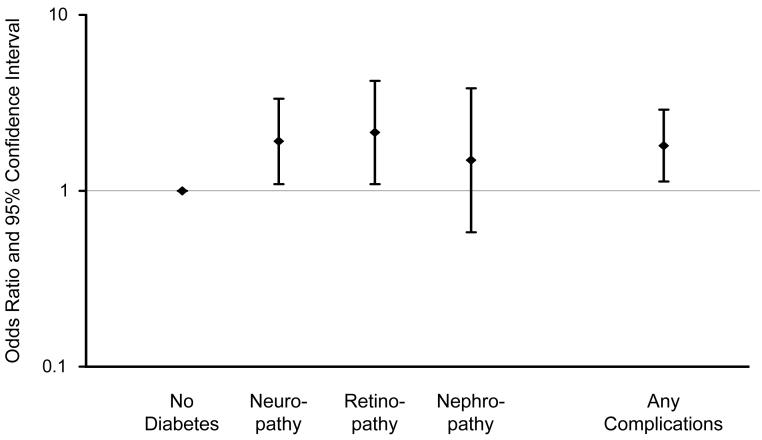
Odds ratios and 95% confidence intervals for the association of mild cognitive impairment with diabetic neuropathy, retinopathy, nephropathy, or any of the 3 complications. Subjects without diabetes served as the reference group (OR, 1.00).
Table 2
Case-Control Analyses for Diabetes Defined as Self-Report of Doctor’s Diagnosis, Treatment, or Complications of Diabetes
| Variable | Cases with MCI (n=329) n (%) | Controls (n=1640) n (%) | Model 1* | Model 2† | ||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | |||
| Diabetes | ||||||||
 No diabetes No diabetes | 263 (79.9) | 1349 (82.3) | 1.00 | -- | -- | 1.00 | -- | -- |
 Diabetes Diabetes | 66 (20.1) | 290 (17.7) | 1.16‡ | 0.85-1.57 | .34 | 1.20 | 0.86-1.67 | .28 |
| Treatment type§ | ||||||||
 Diabetes, no treatment Diabetes, no treatment | 15 (4.6) | 108 (6.6) | 0.72 | 0.41-1.26 | .24 | 0.75 | 0.41-1.35 | .34 |
 Oral hypoglycemic Oral hypoglycemic | 26 (7.9) | 118 (7.2) | 1.11 | 0.70-1.76 | .65 | 1.14 | 0.71-1.84 | .58 |
 Insulin Insulin | 25 (7.6) | 64 (3.9) | 2.01 | 1.22-3.31 | .006 | 2.14 | 1.25-3.66 | .005 |
| Complications§ | ||||||||
 No complications No complications | 39 (11.9) | 214 (13.1) | 0.93 | 0.64-1.35 | .69 | 0.97 | 0.66-1.44 | .89 |
 Any Complications Any Complications | 27 (8.2) | 76 (4.6) | 1.80 | 1.13-2.89 | .01 | 1.86 | 1.12-3.08 | .02 |
  Neuropathy Neuropathy | 19 (5.8) | 49 (3.0) | 1.91 | 1.09-3.34 | .02 | 1.87 | 1.02-3.42 | .04 |
  Retinopathy Retinopathy | 13 (4.0) | 30 (1.8) | 2.15 | 1.09-4.22 | .03 | 2.36 | 1.17-4.79 | .02 |
  Nephropathy Nephropathy | 6 (1.8) | 21 (1.3) | 1.49 | 0.58-3.82 | .40 | 1.58 | 0.61-4.13 | .35 |
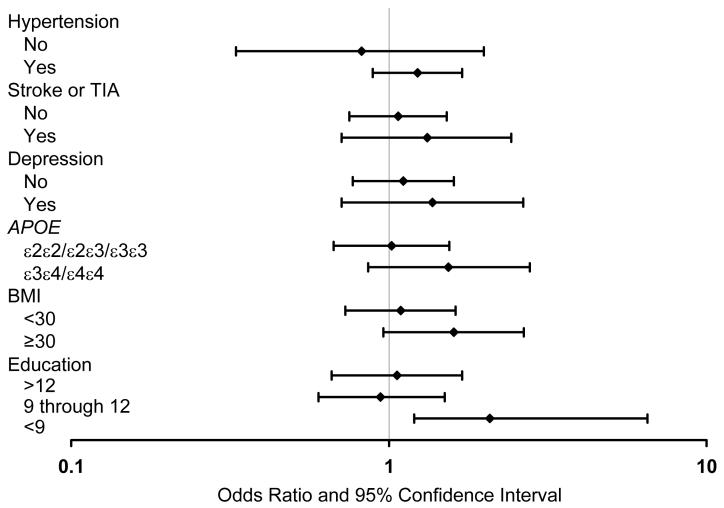
There were no statistically significant interactions between diabetes and demographic factors, clinical variables, or depression. However, for certain variables, the ORs were higher in subgroups of subjects exposed to variables that have been associated with cognitive impairment. Specifically, diabetes was significantly associated with MCI in subjects with <9 years of education (OR, 2.77; 95% CI, 1.17-6.57), but not in subjects with higher levels of education. The association of diabetes with MCI was also stronger in subgroups with APOE ε3ε4 or ε4ε4 genotype, hypertension, history of stroke or TIA, depression, and with a BMI ≥30 (after adjustment for age, sex, and education; Figure 3).
ASCERTAINMENT OF DIABETES FROM SELF-REPORT OR FASTING BLOOD GLUCOSE LEVEL
In this set of analyses, subjects were categorized as having diabetes based on self-report (n=356) or on having a fasting blood glucose level that met criteria for diabetes (n=54), for a total of 410 subjects with diabetes. The ORs for diabetes were slightly greater than those found using only self-report; however, they were not statistically significant (Table 3). The association of diabetes with MCI was marginally significant after adjustment for vascular risk factors (OR, 1.33; 95% CI, 0.98-1.81; P=.07) but did not change with adjustment for depression (data not shown). There was a significant association of diabetes with na-MCI (OR, 1.63; 95% CI, 1.01-2.63; P=.05) but not with a-MCI (Table 3, footnotes). The associations with treatment type or complications were essentially the same as in the analyses for diabetes defined by self-report only (Table 3).
Table 3
Case-Control Analyses for Diabetes Defined as Self-Report or as Abnormal Fasting Blood Glucose)
| Variable | Cases with MCI (n=329) n (%) | Controls (n=1640) n (%) | Model 1* | Model 2† | ||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | |||
| Diabetes | ||||||||
 No diabetes No diabetes | 249 (75.7) | 1309 (79.9) | 1.00 | -- | -- | 1.00 | -- | -- |
 Diabetes Diabetes | 80 (24.3) | 330 (20.1) | 1.24‡ | 0.93-1.66 | .14 | 1.33 | 0.98-1.81 | .07 |
| Treatment type§ | ||||||||
 Diabetes, no treatment Diabetes, no treatment | 29 (8.8) | 148 (9.0) | 0.99 | 0.64-1.52 | .96 | 1.10 | 0.70-1.72 | .67 |
 Oral hypoglycemic Oral hypoglycemic | 26 (7.9) | 118 (7.2) | 1.13 | 0.72-1.80 | .59 | 1.18 | 0.73-1.91 | .49 |
 Insulin Insulin | 25 (7.6) | 64 (3.9) | 2.05 | 1.25-3.39 | .005 | 2.23 | 1.30-3.81 | .004 |
| Complications§ | ||||||||
 No complications No complications | 53 (16.1) | 254 (15.5) | 1.06 | 0.76-1.49 | .72 | 1.16 | 0.81-1.65 | .41 |
 Any Complications Any Complications | 27 (8.2) | 76 (4.6) | 1.84 | 1.15-2.95 | .01 | 1.93 | 1.16-3.21 | .01 |
  Neuropathy Neuropathy | 19 (5.8) | 49 (3.0) | 1.91 | 1.09-3.34 | .02 | 1.87 | 1.02-3.42 | .04 |
  Retinopathy Retinopathy | 13 (4.0) | 30 (1.8) | 2.15 | 1.09-4.22 | .03 | 2.36 | 1.17-4.79 | .02 |
  Nephropathy Nephropathy | 6 (1.8) | 21 (1.3) | 1.49 | 0.58-3.82 | .40 | 1.58 | 0.61-4.13 | .35 |
SENSITIVITY ANALYSES INCLUDING ONLY DIAGNOSES OF DIABETES CONFIRMED BY MEDICAL RECORD
Of the 356 subjects with self-report of diabetes, 304 (85.4%) had at least 1 diagnostic code for diabetes from the records-linkage system. The frequency of diabetes was 17.6% in subjects with MCI and 15.0% in subjects without MCI (Table 4). The associations of diabetes and MCI were consistent with the primary and secondary analyses. In addition, we observed significant associations between age at onset and duration of diabetes and MCI. The adjusted ORs were significantly elevated 2-fold for subjects with diabetes onset before age 65, for subjects with duration of diabetes ≥10 years, for subjects treated with insulin, and for those with diabetic complications (Table 4, models 1 and 2).
Table 4
Case-Control Analyses for Diabetes Defined as Self-Report but also Confirmed by Medical Record Diagnosis
| Variable | Cases with MCI (n=329) n (%) | Controls (n=1640) n (%) | Model 1* | Model 2† | ||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | |||
| Diabetes | ||||||||
 No diabetes No diabetes | 271 (82.4) | 1393 (85.0) | 1.00 | -- | -- | 1.00 | -- | -- |
 Diabetes Diabetes | 58 (17.6) | 246 (15.0) | 1.22 | 0.88-1.68‡ | .23 | 1.28 | 0.91-1.81 | .16 |
| Age at onset§ | ||||||||
 <65 years <65 years | 21 (6.4) | 65 (4.0) | 2.20 | 1.29-3.73 | .004 | 2.19 | 1.25-3.82 | .006 |
 ≥65 years ≥65 years | 37 (11.2) | 181 (11.0) | 0.96 | 0.65-1.41 | .82 | 1.03 | 0.69-1.55 | .88 |
| Duration§ | ||||||||
 <10 years <10 years | 23 (7.0) | 147 (9.0) | 0.83 | 0.52-1.33 | .44 | 0.93 | 0.58-1.50 | .77 |
 ≥10 years ≥10 years | 35 (10.6) | 99 (6.0) | 1.76 | 1.16-2.68 | .008 | 1.80 | 1.14-2.84 | .01 |
Treatment type | ||||||||
 Diabetes, no treatment Diabetes, no treatment | 8 (2.4) | 65 (4.0) | 0.64 | 0.30-1.36 | .25 | 0.75 | 0.35-1.62 | .46 |
 Oral hypoglycemic Oral hypoglycemic | 25 (7.6) | 117 (7.1) | 1.10 | 0.69-1.75 | .68 | 1.13 | 0.70-1.83 | .62 |
 Insulin Insulin | 25 (7.6) | 64 (3.9) | 2.02 | 1.23-3.33 | .006 | 2.16 | 1.26-3.69 | .005 |
Complications | ||||||||
 No complications No complications | 32 (9.7) | 172 (10.5) | 0.96 | 0.64-1.45 | .85 | 1.05 | 0.68-1.60 | .84 |
 Complications Complications | 26 (7.9) | 74 (4.5) | 1.80 | 1.11-2.90 | .02 | 1.83 | 1.10-3.05 | .02 |
 The reference group included subjects without diabetes (same as first line in table).
The reference group included subjects without diabetes (same as first line in table).COMMENT
In this population-based case-control study, onset of diabetes before age 65 years, longer duration of diabetes, treatment of diabetes with insulin, and presence of diabetic complications were independently associated with MCI after accounting for age, sex, education, vascular risk factors, and depression. When we broadened the definition of diabetes to include subjects with a fasting blood glucose level that met criteria for diabetes, we observed marginally significant associations of MCI with diabetes overall. Our findings suggest that diabetes duration and severity, as measured by type of treatment and presence of diabetic complications, may be important in the pathophysiology of cognitive impairment in diabetics. In contrast, late onset of diabetes, short duration, or well-controlled diabetes, may have a lesser impact. Long duration of diabetes may be associated with greater cerebral macrovascular disease, clinical cerebral infarctions, and sub-clinical infarctions that may impair cognitive function.27,28 This is consistent with other studies in which vascular disease in mid-life predicted late life cognitive impairment or dementia.29
Severe diabetes is more likely to be associated with chronic hyperglycemia, which in turn, increases the likelihood of cerebral microvascular disease,30 and may contribute to neuronal damage, brain atrophy,31-33 and cognitive impairment. The 2-fold increased risk of MCI in subjects with diabetic retinopathy in the present study supports the potential effects of diabetes on cerebral microvascular disease and on the pathogenesis of MCI.
Alternative mechanisms besides vascular disease may be involved in the pathogenesis of cognitive impairment in diabetics. It has been hypothesized that defects in insulin action may increase β-amyloid aggregation.11,12,34 In type 2 diabetes, insulin therapy may inhibit synaptic activity in the brain,35 decrease insulin degrading enzyme production, promote the development of amyloid plaques,11,12 and may increase production of advanced glycation end-products associated with Alzheimer’s disease pathology.10-12 Recurrent or chronic hypoglycemia caused by treatment with insulin may also contribute to permanent cognitive impairment.36,37 In the present study, the association of diabetes with MCI persisted after adjustment for vascular risk factors; this supports the hypothesis that additional pathologic mechanisms independent of vascular disease contribute to MCI in diabetics.
Differences in the association of diabetes across MCI subtypes raise questions regarding the role of diabetes in the etiology and prognosis of MCI subtypes. When fasting blood sugars were taken into account, we observed a significant association of diabetes with na-MCI, but not with a-MCI. Other investigators have reported stronger associations between vascular risk factors and na-MCI, suggesting that vascular risk factors may increase the risk of na-MCI. Na-MCI may be a prodromal stage for vascular dementia38 or other non-degenerative dementias,39 whereas a-MCI may be a prodromal stage for neurodegenerative dementias such as Alzheimer’s disease. This hypothesis, however, is disputed by other studies that have found no difference in the association of vascular risk factors across MCI subtypes.40
We observed no significant interactions of diabetes with APOE ε4 genotype or with depression. However, in our stratified analyses, the ORs for diabetes were stronger in the strata of subjects exposed to variables that have been reported to be associated with cognitive impairment or dementia. We may not have had sufficient power to detect significant interactions in these stratified analyses.
There are several strengths of this study. Participants were randomly selected from the community, thus the potential for selection bias was reduced in comparison to studies performed among subjects seen in referral practices or memory clinics. Availability of fasting blood glucose levels enabled us to identify subjects with undiagnosed or unreported diabetes and thereby to reduce potential misclassification. In addition, using the medical records-linkage system of the Rochester Epidemiology Project we validated the self-report of diabetes and performed sensitivity analyses; these results confirmed our primary analyses.
There are also potential limitations of our study. A comparison of participants and non-participants showed lower participation in older men, subjects with lower education, and subjects with diabetes.18 This under-representation of subjects with diabetes may have precluded our ability to detect a significant association between diabetes overall and MCI. Active follow-up of participants by repeated interviews and examinations and passive follow-up of non-participants through the medical records-linkage system of the Rochester Epidemiology Project will enable us to determine whether these baseline differences are associated with differences in dementia incidence. Since MCI is typically not diagnosed in routine clinical practice, we may be unable to assess the impact on MCI incidence. Due to the cross-sectional design of the present study, we cannot be sure that diabetes preceded MCI. Finally, these findings were based on a primarily Caucasian sample representative of the Olmsted County community; therefore, extrapolation of findings to ethnic groups not represented in our study should be performed with caution.
ACKNOWLEDGMENT
Funding/Support: The study was supported by grants P50 AG16574, U01 AG06786, K01 AG028573, K01 MH68351, and R01 AR30582 from the National Institutes of Health and by the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program. The sponsors of this study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1001/archneur.65.8.1066
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2630223?pdf=render
Free to read at archneur.ama-assn.org
http://archneur.ama-assn.org/cgi/content/abstract/65/8/1066
Subscription required at archneur.ama-assn.org
http://archneur.ama-assn.org/cgi/content/full/65/8/1066
Subscription required at archneur.ama-assn.org
http://archneur.ama-assn.org/cgi/reprint/65/8/1066.pdf
Citations & impact
Impact metrics
Article citations
Cognitive impairment and associated factors among patients with diabetes mellitus in Africa: a systematic review and meta-analysis.
Front Endocrinol (Lausanne), 15:1386600, 17 Jul 2024
Cited by: 0 articles | PMID: 39086905 | PMCID: PMC11288936
Review Free full text in Europe PMC
Targeting redox imbalance in neurodegeneration: characterizing the role of GLP-1 receptor agonists.
Theranostics, 13(14):4872-4884, 04 Sep 2023
Cited by: 9 articles | PMID: 37771773 | PMCID: PMC10526673
Review Free full text in Europe PMC
Association between glycemic status and the risk of acute pancreatitis: a nationwide population-based study.
Diabetol Metab Syndr, 15(1):104, 19 May 2023
Cited by: 2 articles | PMID: 37208706 | PMCID: PMC10197233
Diabetes mellitus type 2 as an underlying, comorbid or consequent state of mental disorders.
World J Diabetes, 14(5):481-493, 01 May 2023
Cited by: 1 article | PMID: 37273248 | PMCID: PMC10236997
Review Free full text in Europe PMC
Cognitive Decline in Early and Premature Menopause.
Int J Mol Sci, 24(7):6566, 31 Mar 2023
Cited by: 17 articles | PMID: 37047549 | PMCID: PMC10095144
Review Free full text in Europe PMC
Go to all (112) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Accelerated progression from mild cognitive impairment to dementia in people with diabetes.
Diabetes, 59(11):2928-2935, 16 Aug 2010
Cited by: 113 articles | PMID: 20713684 | PMCID: PMC2963552
Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study.
Arch Gen Psychiatry, 65(10):1193-1198, 01 Oct 2008
Cited by: 227 articles | PMID: 18838636 | PMCID: PMC2575648
Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging.
Neurology, 75(10):889-897, 01 Sep 2010
Cited by: 420 articles | PMID: 20820000 | PMCID: PMC2938972
[Cognitive deficit: another complication of diabetes mellitus?].
Arq Bras Endocrinol Metabol, 52(7):1076-1083, 01 Oct 2008
Cited by: 1 article | PMID: 19082295
Review
Funding
Funders who supported this work.
NIA NIH HHS (8)
Grant ID: P50 AG016574-10
Grant ID: U01 AG06786
Grant ID: P50AG16574
Grant ID: U01 AG006786-23
Grant ID: K01 AG028573
Grant ID: K01 AG028573-03
Grant ID: U01 AG006786
Grant ID: P50 AG016574
NIAMS NIH HHS (3)
Grant ID: R01 AR030582
Grant ID: R01 AR030582-43
Grant ID: R01 AR30582
NIMH NIH HHS (3)
Grant ID: K01 MH068351-07
Grant ID: K01 MH068351
Grant ID: K01 MH68351