Abstract
Background
It is unclear whether the use of selective serotonin reuptake inhibitors (SSRIs) and other antidepressant drugs reduce the risk of suicide in people with depression. We explored the association between exposure to SSRIs and risk of suicide completion or attempt.Methods
We conducted a systematic review of observational studies that reported completed or attempted suicide in depressed individuals who were exposed to SSRIs compared with those who were not exposed to antidepressants. We assessed the overall risk of completed or attempted suicide.Results
Eight studies involving more than 200 000 patients with moderate or severe depression were included in the meta-analysis. Although exposure to SSRIs increased the risk of completed or attempted suicide among adolescents (odds ratio [OR] 1.92, 95% confidence interval [CI] 1.51-2.44), the risk was decreased among adults (OR 0.57, 95% CI 0.47-0.70). Among people aged 65 or more years, exposure to SSRIs had a protective effect (OR 0.46, 95% CI 0.27-0.79). Sensitivity analyses did not change these findings. In particular, for studies that used completed suicide as an outcome, exposure to SSRIs was associated with increased risk among adolescents (OR 5.81, 95% CI 1.57-21.51) and decreased risk among adults (OR 0.66, 95% CI 0.52-0.83) and older people (OR 0.53, 95% CI 0.26-1.06).Interpretation
Based on data from observational studies, use of SSRIs may be associated with a reduced risk of suicide in adults with depression. Among adolescents, use of SSRIs may increase suicidality.Free full text

Selective serotonin reuptake inhibitors and risk of suicide: a systematic review of observational studies
Associated Data
Abstract
Background
It is unclear whether the use of selective serotonin reuptake inhibitors (SSRIs) and other antidepressant drugs reduce the risk of suicide in people with depression. We explored the association between exposure to SSRIs and risk of suicide completion or attempt.
Methods
We conducted a systematic review of observational studies that reported completed or attempted suicide in depressed individuals who were exposed to SSRIs compared with those who were not exposed to antidepressants. We assessed the overall risk of completed or attempted suicide.
Results
Eight studies involving more than 200 000 patients with moderate or severe depression were included in the meta-analysis. Although exposure to SSRIs increased the risk of completed or attempted suicide among adolescents (odds ratio [OR] 1.92, 95% confidence interval [CI] 1.51–2.44), the risk was decreased among adults (OR 0.57, 95% CI 0.47–0.70). Among people aged 65 or more years, exposure to SSRIs had a protective effect (OR 0.46, 95% CI 0.27–0.79). Sensitivity analyses did not change these findings. In particular, for studies that used completed suicide as an outcome, exposure to SSRIs was associated with increased risk among adolescents (OR 5.81, 95% CI 1.57–21.51) and decreased risk among adults (OR 0.66, 95% CI 0.52–0.83) and older people (OR 0.53, 95% CI 0.26–1.06).
Interpretation
Based on data from observational studies, use of SSRIs may be associated with a reduced risk of suicide in adults with depression. Among adolescents, use of SSRIs may increase suicidality.
There is uncertainty about the safety of selective serotonin reuptake inhibitors (SSRIs), which may cause worsening of suicidal thoughts in vulnerable people.1,2 In 2005, a systematic review of published randomized controlled trials comparing SSRIs with another active treatment or placebo found an almost 2-fold increase in the odds of fatal and nonfatal suicide attempts among those exposed to SSRIs.3 No increase in risk was observed, however, when only fatal suicide attempts were included. Another systematic review,4 which included both published and unpublished randomized controlled trials submitted by pharmaceutical companies to the safety review of the Medicine and Healthcare products Regulatory Agency compared the use of SSRIs and placebo in adults with depression and other clinical conditions.4 This review showed no evidence of increased risk of completed suicide and only weak evidence of increased risk of self-harm.
More recently, the US Food and Drug Administration (FDA) performed a meta-analysis of individual patient data from 372 randomized placebo-controlled trials of antidepressants with a total of nearly 100 000 patients.5 This study reported that the incidence of reported suicidal behaviour was strongly related to age.5 The risk associated with antidepressant use relative to placebo was increased among patients aged 25 or fewer years, and it was reduced among patients aged 65 or more years.5 The risk among patients aged 25–64 years was neutral; however, risk was reduced when suicidal behaviour and ideation were considered together.5 Based on these findings, in May 2007 the FDA ordered that all antidepressant drugs carry an expanded black-box warning on their label that included information about increased risk of suicidal behaviour in young adults aged 18–24 years.6,7
A controversial point of the FDA analysis is that the included trials were not primarily designed to measure suicidality (a composite outcome that includes suicide ideas, preparatory acts, suicide attempts and deaths by suicide).5 Of all suicidality events, less than 30% were serious suicide attempts or deaths. Additionally, considering that suicidality was self-reported rather than observed by others in most clinical trials, it is possible that antidepressant treatment, particularly in younger individuals, enhanced communication about suicidality, which may have allowed them to become more articulate and open about their thoughts and actions. Alternatively, antidepressant treatment might have enhanced communication about suicidality in all age groups, but increased attention to adverse effects might have led to enhanced detection of suicidality in younger individuals.5
It is unlikely that individual randomized trials will be designed to primarily investigate the effect of antidepressant use on suicidality, and future systematic reviews of clinical trial data will not be able to overcome the limitations of the FDA analysis. Therefore, we sought to further explore the association between SSRI exposure and risk of completed or attempted suicide by conducting a systematic review and meta-analysis of observational studies. By including a large, broad spectrum of individuals followed under naturalistic circumstances, systematic reviews of observational studies may offer an added dimension in the evaluation of drug safety that is complementary to that provided by clinical trials.8,9 Additionally, observational studies may allow researchers to move from the controversial concept of suicidality to hard outcomes such as suicide attempt and completion. Specifically, we set out to quantify the risk of completed or attempted suicide among people in different age groups with depression after exposure to SSRIs.
Methods
Study selection and data collection
Included and excluded studies were collected following the Quality of Reporting of Meta-analysis (QUOROM) guidelines.10 Because the included studies were observational in design, we also followed the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines published by Stroup and colleagues11 for the meta-analysis of the design, performance and reporting of observational studies (Appendix 1, available at www.cmaj.ca/cgi/content/full/180/3/291/DC2).
We included observational cohort and case–control studies that reported data on completed or attempted suicide among people exposed to SSRIs and among those who were not exposed to antidepressants. We included studies that reported relative risk [RR] estimates suitable for re-analysis. Only studies that used International Classification of Disease (ICD, ninth or tenth revision) definitions of completed or attempted suicide were retained. This criterion corresponds to coding 1 (completed suicide) and 2 (suicide attempt) of the FDA classification of suicide events.5,12 We did not include the following suicide-related events: preparatory acts toward imminent suicidal behaviours, suicidal ideation, and self-injurious behaviour (intent unknown; FDA coding 3–5). Study participants were of either sex and any age with a diagnosis of major depression. Studies adopting proxy measures to identify patients with depression were included. As recommended by the MOOSE guidelines,11 we used broad inclusion criteria and performed sensitivity analyses to relate design features to the outcomes.
We identified relevant studies by searching MEDLINE and EMBASE using the search terms “antidepressive agents” or “antidepressive agents second generation” AND “suicide” or “suicide attempted” (Appendix 2, available at www.cmaj.ca/cgi/content/full/180/3/291/DC2). We searched the reference lists of relevant articles, including review articles, by hand for other relevant studies. The search covered the period from January 1990 to June 2008. We examined all titles and abstracts, and we obtained full texts of potentially relevant papers. Working independently and in duplicate, we read the papers and determined whether they met inclusion criteria. We resolved disagreement by consensus, and we extracted data independently in duplicate using a standardized form (Appendix 3, available at www.cmaj.ca/cgi/content/full/180/3/291/DC2). We did not exclude articles published in languages other than English.
We assessed study quality using a 10-point scale adapted from a recently published quality scale for observational studies (Appendix 4, available at www.cmaj.ca/cgi/content/full/180/3/291/DC2).13 A score of 7 or above indicated high quality, and a score of 6 or below indicated low quality. This threshold was derived from Etminan and colleagues.14 Scoring of quality assessment was performed independently by 2 of the authors (C.B. and E.E.). There was high concordance between the 2 authors (kappa 0.78, standard error 0.26), suggesting no evidence of systematic disagreement bias. As recommended by the MOOSE study group, the quality scores were included in the sensitivity analyses, but they were not used as weights in the analyses.11
Data synthesis
The outcome measure included in this analysis was completed or attempted suicide. Suicide attempts had to be sufficiently serious to have led to medical contact. A patient was considered to have made a suicide attempt if there were ICD-9 or ICD-10 diagnostic codes in his or her records for inpatient stay or outpatient visit. These codes included self-inflicted injury from poisoning, hanging, submersion, firearms, cutting or piercing, jumping from high places, or other means. For each study, we used the most adjusted RRs, odds ratios (ORs) or hazard ratios with the corresponding 95% confidence intervals (CIs).8,15
We visually inspected the graphs to investigate the possibility of statistical heterogeneity. This was primarily supplemented by use of the I2 statistic. This statistic provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. Where the I2 estimate was greater than or equal to 50%, we interpreted this as indicating the presence of high levels of heterogeneity.16
The results of studies were pooled, and an overall OR was obtained from fixed-and random-effects models. To maintain a conservative approach, the random-effects model was presented, because this takes into account any differences among studies even if there is no statistically significant heterogeneity.17 The possibility of publication bias was examined by use of the funnel plot method described by Egger and colleagues.18
Sensitivity analyses and metaregression
We performed sensitivity analyses to examine effect sizes when only the following types of studies were included: studies that used completed suicide as an outcome measure; studies that used a formal diagnosis of depression; studies that used an “external” control group (that is, we excluded studies that compared the risk of suicide during SSRI use with the risk during no antidepressant use in the same cohort); studies with a quality score of 7 or above; studies including data for both adolescents and adults; and studies that had a cohort design. Additionally, given the small number of included studies, we tested for the possible excessive influence of individual studies using a meta-analysis influence test that eliminated each of the included studies one at a time.
We used unrestricted maximum likelihood random-effects metaregression to determine whether there was a relation between age and risk of completed or attempted suicide.
Results
Based on the titles and abstracts of 1492 citations, we identified 33 potentially relevant studies (Figure 1). Of these, we excluded 4 studies because SSRI use was not the exposure of interest.19–22 We excluded 18 studies because the comparison group was not individuals who had not ben exposed to antidepressant treatment.21,23–39 Two additional studies were excluded because a diagnosis of major depression or a proxy measure to identify patients suffering from depression was not used,40,41 and 1 was excluded because it did not report data on SSRI use that was suitable for re-analysis.42 Thus, 8 studies were eligible for inclusion and were assessed for quality (Figure 1).43–50

Figure 1: Quality of Reporting of Meta-analysis (QUOROM) flow diagram of studies included in the meta-analysis of the risk of suicide with the use of selective serotonin reuptake inhibitors (SSRIs).
The included studies are summarized in Appendix 5 (available at www.cmaj.ca/cgi/content/full/180/3/291/DC2). All of the included studies were primarily designed to assess the risk of completed or attempted suicide among individuals exposed to SSRIs compared to who had not been exposed to antidepressants. Of the included studies, 6 had a cohort design and 2 were case–control studies. Five of the studies identified patients from Medicaid databases, and 3 used national registries. Five of the studies provided data on completed suicide, and 3 studies used attempted suicide as an outcome measure. All of the included studies used ICD-9 or ICD-10 codes to define completed and attempted suicide.
Among adolescents, exposure to SSRIs significantly increased the risk of completed or attempted suicide (random-effect OR 1.92, 95% CI 1.51–2.44, I2 0.0%) (Figure 2). Among adults, SSRI exposure significantly decreased the risk of completed or attempted suicide (random-effect OR 0.57, 95% CI 0.47–0.70, I2 52.5%). Among elderly people (aged 65 or more years), exposure to SSRIs had a significant protective effect (random-effect OR 0.46, 95% CI 0.27–0.79, I2 0.0%). The metaregression with age as a moderator and the risk of completed or attempted suicide as dependent variable suggested a promoting effect of SSRI exposure on the risk of suicide among adolescents and a protective effect among adults and elderly individuals (Figure 3). Although Egger's regression test did not suggest statistically significant asymmetry, visual inspection of the funnel plot suggested that there might be a lack of small studies that failed to show an excess risk associated with SSRI exposure (Appendix 6, available at www.cmaj.ca/cgi/content/full/180/3/291/DC2).
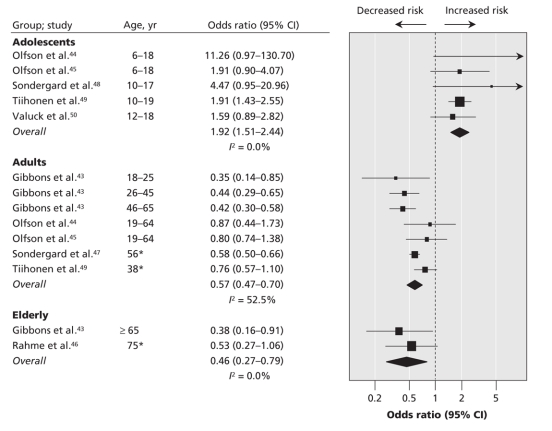
Figure 2: Random-effect meta-analysis of the risk of suicide attempt and completion associated with the use of selective serotonin reuptake inhibitors compared with no exposure to any antidepressants. *Mean age. Note: CI = confidence interval.

Figure 3: Metaregression of the effect of age on the log odds ratio for the risk of completed or attempted suicide associated with the use of selective serotonin reuptake inhibitors. Each circle represents 1 study. Circle size reflects the weight of the study in the metaregresison. Slope = –0.37, Q = 74.99, p < 0.001.
Sensitivity analyses, which were performed to investigate the strength of the results, did not change these findings (Table 1). Additionally, we observed little or no effect of eliminating each of the included studies from the analysis (Appendix 7, available at www.cmaj.ca/cgi/content/full/180/3/291/DC2).
Table 1

Two studies provided data suitable for analysis of the risk of completed or attempted suicide associated with individual antidepressant agents (Figure 4).44,49 Among adults, no individual antidepressant was significantly associated with completed or attempted suicide. Among adolescents, exposure to paroxetine (random-effect OR 1.77, 95% CI 1.05–2.99, I2 48.1%) and venlafaxine (random-effect OR 2.43, 95% CI 1.47–4.02, I2 0.0%) was significantly associated with increased risk (Figure 4).
Interpretation
We found that the relation between exposure to SSRIs and the risk of suicide is influenced by age. Exposure to SSRIs decreased the risk of suicide by over 40% among adults and decreased the risk by over 50% among elderly people. However, among adolescents, exposure to SSRIs almost doubled the the risk of suicide. These results are consistent with the main conclusion of the recent FDA meta-analysis of clinical trial data.5 However, our risk estimates were very similar to those obtained by the FDA only for the elderly and adolescent groups. Although the FDA reported a neutral effect of SSRIs (or a promoting effect among adults aged 18–25), we found a strong protective effect associated with SSRI treatment.
We tested the robustness of these results in several ways. First, sensitivity analyses showed similar results when more homogeneous subgroups of studies (i.e., outcome measure, diagnostic criteria, control group and quality score) were included. This effect was maintained when only studies with data simultaneously about adolescents and adults were retained. Second, we found little or no effect of eliminating each of the studies from the analysis, which suggests that no individual study had excessive influence on the main results. Third, despite the heterogeneity that was observed in terms of study designs and characteristics, low or moderate levels of statistical heterogeneity were generally observed.
Observational studies have limited ability to adjust for baseline differences and are prone to bias and confounding; thus, alternative explanations for the results of this analysis cannot be excluded.51 All of the included studies enrolled individuals with major depression or used proxy measures of major depression, and, therefore, confounding by indication should not have occurred.
Confounding by severity of illness cannot be excluded. However, this confounder would have to have varied systematically with age to explain the very different findings in adolescents and adults. Among adolescents, SSRI treatment is often reserved for very severe cases, and prescription of antidepressant drugs might have been triggered by suicidal ideas. Thus, the excess risk might be explained by confounding by severity. That is, adolescents who received SSRIs might have been more severely depressed (or more suicidal) than adolescents who did not receive SSRIs. In contrast, among adults SSRIs may be similarly prescribed in severe and less severe cases, and confounding by indication might not have occurred. In some studies, confounding by severity has been taken into account. For example, Olfson and colleagues44 limited their analysis to individuals who received inpatient treatment for depression, thus ensuring a fairly comparable level of illness severity. In 2 other studies, comparability between groups was increased by the comparison of the risk of suicide during SSRI use with the risk during no antidepressant use in the same cohort.46,49 Although these designs likely limited the confounding effect of the severity of illness, we cannot exclude the fact that residual confounding might have inflated the excess risk found among adolescents.
The incidence of depression is higher among women than among men; however, the reverse pattern is observed for suicide.52 Thus, it would have been interesting to investigate the effect of sex on the risk of suicide. Similarly, the timing of the attempted or completed suicide in relation to the onset of exposure is another moderator variable that would have been clinically useful to analyze, because the risk of death by suicide may not be significantly higher in the month after starting medication than in subsequent months.53 However, information about these variables was not homogeneously reported, and re-analyses of aggregate data cannot answer issues related to patient-level moderators of treatment effect. Re-analyses of data from individual patients may have the potential to address these issues.
Differences between individual drugs need confirmation. Only 2 of the included studies provided data on specific antidepressants, and confounding by indication might have affected our results in unpredictable ways. Additionally, it is not clear why the use of some antidepressants, such as paroxetine and venlafaxine, increases the risk of suicide more than others. Intriguingly, previous re-analyses of randomized studies, including the FDA study, reported similar differences between antidepressants.38,54–56 Differences in long-term efficacy and safety should be confirmed in trials of head-to-head comparisons.57 Such an evidence base would assist clinicians in making choices about optimal antidepressant treatment.
Conclusion
Data from observational studies should reassure doctors that prescribing SSRIs to patients with major depression is safe. However, children and adolescents should be followed very closely because of the possibility of increased of risk suicidal thoughts and suicide. Paroxetine and venlafaxine may be better avoided based on the increasing evidence from randomized and observational studies that the risks might outweigh the benefits for most adolescents.
@@ See related commentary by Gibbons and Mann, page 270
Footnotes
Une version française de ce résumé est disponible à l'adresse www.cmaj.ca/cgi/content/full/180/3/291/DC1
Funding: We are grateful to the Fondazione Cariverona, who provided a 3-year grant to the World Health Organization Collaborating Centre for Research and Training in Mental Health and Service Organization at the University of Verona.
This article has been peer reviewed.
Contributors: All of the authors contributed to the design of the systematic review, the coordination, data collection and management, statistical analyses, interpretation of the results and writing of the manuscript. All of the authors approved the final version submitted for publication.
Competing interests: None declared.
Correspondence to: Dr. Corrado Barbui, Department of Medicine and Public Health, Section of Psychiatry and Clinical Psychology, University of Verona, Policlinico GB Rossi, Piazzale Scuro 10, 37134 Verona, Italy; fax 39 045-8027498; [email protected]
REFERENCES
Articles from CMAJ : Canadian Medical Association Journal are provided here courtesy of Canadian Medical Association
Full text links
Read article at publisher's site: https://doi.org/10.1503/cmaj.081514
Read article for free, from open access legal sources, via Unpaywall:
https://www.cmaj.ca/content/cmaj/180/3/291.full.pdf
Free to read at www.cmaj.ca
http://www.cmaj.ca/cgi/content/full/180/3/291
Free to read at www.cmaj.ca
http://www.cmaj.ca/cgi/reprint/180/3/291.pdf
Free to read at www.cmaj.ca
http://www.cmaj.ca/cgi/content/abstract/180/3/291
Citations & impact
Impact metrics
Article citations
Psychotropic Medications Promote Time-Dependent Reduction of Suicidal Ideation in Mood Disorder: A Prospective Cohort Study.
J Korean Med Sci, 39(31):e226, 12 Aug 2024
Cited by: 0 articles | PMID: 39137811 | PMCID: PMC11319105
Perspective on adolescent psychiatric illness and emerging role of microRNAs as biomarkers of risk.
J Psychiatry Neurosci, 49(4):E282-E288, 01 Jul 2024
Cited by: 0 articles | PMID: 39209460 | PMCID: PMC11374446
Prevention of suicidal behavior with lithium treatment in patients with recurrent mood disorders.
Int J Bipolar Disord, 12(1):6, 09 Mar 2024
Cited by: 1 article | PMID: 38460088 | PMCID: PMC10924823
Review Free full text in Europe PMC
Risk and Protective Factors of Self-harm and Suicidality in Adolescents: An Umbrella Review with Meta-Analysis.
J Youth Adolesc, 53(6):1301-1322, 02 Apr 2024
Cited by: 0 articles | PMID: 38564099 | PMCID: PMC11045640
Review Free full text in Europe PMC
A Retrospective Case-Control Study on the Differences in the Effectiveness of Theta-Burst Stimulation Therapy for Depression with and without Antidepressant Medication.
J Clin Med, 13(2):399, 11 Jan 2024
Cited by: 1 article | PMID: 38256534 | PMCID: PMC10816069
Go to all (115) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The association between suicidality and treatment with selective serotonin reuptake inhibitors in older people with major depression: a systematic review.
JBI Database System Rev Implement Rep, 13(3):174-205, 17 Apr 2015
Cited by: 4 articles | PMID: 26447056
Review
[SSRIs (selective serotonin reuptake inhibitors) and suicidality in adults, adolescents and children].
Tijdschr Psychiatr, 51(6):387-393, 01 Jan 2009
Cited by: 6 articles | PMID: 19517368
Review
Risk of Suicidal Behaviors and Antidepressant Exposure Among Children and Adolescents: A Meta-Analysis of Observational Studies.
Front Psychiatry, 13:880496, 26 May 2022
Cited by: 18 articles | PMID: 35693956 | PMCID: PMC9178080
Review Free full text in Europe PMC
Use of selective serotonin reuptake inhibitors in children and adolescents.
Drug Saf, 27(13):991-1000, 01 Jan 2004
Cited by: 29 articles | PMID: 15471506
Review






