Abstract
Free full text

Essential role for RGS9 in opiate action
Abstract
Regulators of G protein signaling (RGS) are a family of proteins known to accelerate termination of effector stimulation after G protein receptor activation. RGS9-2, a brain-specific splice variant of the RGS9 gene, is highly enriched in striatum and also expressed at much lower levels in periaqueductal gray and spinal cord, structures known to mediate various actions of morphine and other opiates. Morphine exerts its acute rewarding and analgesic effects by activation of inhibitory guanine nucleotide-binding regulatory protein-coupled opioid receptors, whereas chronic morphine causes addiction, tolerance to its acute analgesic effects, and profound physical dependence by sustained activation of these receptors. We show here that acute morphine administration increases expression of RGS9-2 in NAc and the other CNS regions, whereas chronic exposure decreases RGS9-2 levels. Mice lacking RGS9 show enhanced behavioral responses to acute and chronic morphine, including a dramatic increase in morphine reward, increased morphine analgesia with delayed tolerance, and exacerbated morphine physical dependence and withdrawal. These findings establish RGS9 as a potent negative modulator of opiate action in vivo, and suggest that opiate-induced changes in RGS9 levels contribute to the behavioral and neural plasticity associated with chronic opiate administration.
Regulators of G protein signaling (RGS) share a 130-aa RGS (GTPase-activating) domain, which binds to the GTP-bound form of Gαi or Gαq and accelerates the termination of effector stimulation (1-3). RGS proteins are thereby thought to repress the signaling efficacy of receptors coupled to these G proteins. In addition, many of the 25 mammalian RGS proteins known to date contain domains that provide them with additional anchoring or scaffolding properties (4-6). Thus, the net effect of RGS proteins on G protein-coupled receptor signaling in vivo may be complicated and difficult to ascertain from in vitro studies alone.
In brain, RGS proteins show distinct regional and cellular distributions (7). A prominent example is RGS9, which exists in two forms, RGS9-1 and RGS9-2, that are generated by alternative splicing (8, 9). These proteins differ at their C terminus only, with RGS9-1 containing 18 unique C-terminal amino acids and RGS9-2 containing 209 unique C-terminal amino acids. RGS9-1 is expressed solely in retina, where it is implicated in regulating phototransduction (9, 10). By contrast, RGS9-2 is expressed solely in brain, where it shows a distinctive pattern of expression in brain regions important for the actions of opiate drugs (7, 8). RGS9-2 is highly enriched in striatum (including the ventral striatum or nucleus accumbens, NAc), a region important for opiate reward, but is also present at much lower levels in periaqueductal gray and spinal cord, structures important for opiate analgesia (11).
We have demonstrated previously that RGS9-2 can negatively modulate opioid receptor function in cultured Xenopus melanophores in vitro (8, 12). These findings, coupled with the presence of RGS9-2 in opiate-responsive CNS regions, led us to hypothesize that RGS9-2 may be a critical negative regulator of opiate action in vivo and may play an important role in cellular adaptations that occur after chronic opiate administration. Consistent with this hypothesis, we show here that mice lacking RGS9 show a 10-fold increase in sensitivity to the rewarding effects of morphine, as well as increased morphine analgesia, delayed development of analgesic tolerance, and severe morphine dependence and withdrawal. These data support the view that changes in the duration of opioid receptor signaling cause robust alterations in sensitivity to opiate drugs and potently modify adaptations that occur with chronic opiate administration.
Materials and Methods
Animals. The creation of RGS9 knockout (KO) mice is described elsewhere (10). We used mice that were backcrossed into a C57BL/6 background for five to six generations. All experimental mice were generated through matings of heterozygous RGS9+/- mice. For all behavioral studies, 8-week-old wild-type (WT) (+/+) and KO (-/-) male littermates were used. In other studies, using Western blotting, 8-week-old C57BL/6 male mice from The Jackson Laboratory were used. Animals were housed in a temperature- and humidity-controlled environment with a 12-h light/12-h dark cycle and had free access to food and water.
Conditioned Place Preference. Place conditioning was performed as described (13), except that mice were conditioned to morphine or saline on alternate days for 6 days. Several visual and nonvisual (tactile) cues enable the animals to distinguish two side chambers of the apparatus. All conditioning and test sessions were performed under dim illumination. Mice did not show a preference for either side of the chamber at baseline. For herpes simplex virus (HSV) experiments, a shorter protocol was used so that the study could be completed within the time frame of maximal transgene expression (see ref. 14). Here, baseline preference was monitored 1 day after surgery at noon, and for the next 2 days mice were conditioned to the saline-paired side in the morning and the morphine-paired side in the afternoon. On day 4, mice were placed again in the central compartment at noon and allowed to move freely between the two chambers for 20 min.
Viral-Mediated Gene Transfer. HSV-LacZ, HSV-RGS9-2, and HSV-RGS4 constructs were generated as described (14, 15). Bilateral microinjections of the HSV vectors (0.8 μl) were delivered over 10 min into the NAc or dorsal striatum by stereotaxic surgery. The coordinates for the NAc for a 28-g mouse relative to bregma were: anterior-posterior (AP) = +1.7, lateral (L) = ±2.1, and dorsoventral (DV) = -4.5 mm at an angle of 10° from the midline; for dorsal striatum: AP = +1.7, L = ±0.20, and DV = -3.4 mm. Viral-mediated transgene expression was confirmed by β-galactosidase assays for HSVLacZ or by Western blot or in situ hybridization for the HSV-RGS vectors. The latter showed that HSV-RGS9-2 and HSVRGS4 mediate comparable levels of transgene expression. All experiments were performed between days 2 and 5 after HSV injection, when viral expression is maximal (14). Injection placement was confirmed for each animal at the end of the experiment. This method of viral-mediated gene transfer is associated with the same level of tissue damage as that seen with vehicle injections alone (see refs. 14 and 15).
Visual Acuity Tests. Visual acuity was measured by using the Morris water maze with a visible platform (13, 16). Distance traveled to reach the platform was monitored by using automated video tracking software from Noldus (ethovision). Visual acuity was also measured with the visual cliff test, performed exactly as described (17).
Morphine Analgesia and Tolerance. The tail flick test was carried out by using radiant heat that produces baseline tail flick latencies ranging from 2.5 to 3.5 sec. A cutoff time of 10 sec was used to minimize tissue damage. Baseline responses were determined for each mouse before drug injection. The hot plate test was performed on a platform heated to 52°C with a cutoff of 40 sec, or heated to 56°C with a cutoff of 30 sec, and the latency to paw lick or jump was recorded. A control response was determined for each animal before treatment. Morphine sulfate was injected s.c. and analgesia was monitored 30 min after morphine injection. The antinociceptive response was calculated as a percentage of maximal possible effect (MPE), where MPE = (test - control latency)/(cutoff - control) × 100 (18-20). For morphine tolerance, repeated morphine injections (15 mg/kg s.c.) were given daily for 4 days and analgesia was measured 30 min after each drug dose. A second morphine-tolerance test using morphine pellets (obtained from the National Institute on Drug Abuse, Bethesda) was also performed. Mice were implanted with a 25-mg pellet on day 1, and responses to 15 mg/kg morphine injected s.c. were monitored on days 1 and 3 after pellet implantation.
Morphine Withdrawal. Mice were injected i.p. with escalating morphine doses (20, 40, 60, 80, 100, and 100 mg/kg) every 8 h for 2.5 days. Two hours after the last morphine injection, naloxone (Sigma) 1 mg/kg was administered s.c. Withdrawal behaviors were then monitored for 30 min as described (21).
Conditioned Place Aversion. Conditioned place aversion was performed in the apparatus used for place preference. After habituation and measures of baseline preference, mice were injected with saline and placed in one of the apparatus. The same afternoon, mice were injected with 1 mg/kg naloxone s.c. and placed in the opposite compartment. After 2 training days, conditioned preference (aversion) was monitored for 20 min. Data are expressed as time in drug-paired side, post-versus preconditioning.
In Situ Hybridization. Brains and lumbar (L4-L6) spinal cords of 250-g Sprague-Dawley rats (Charles River Breeding Laboratories) were sectioned in a cryostat at 14 μm, and in situ hybridization was performed exactly as described (7, 8, 15). The riboprobe used represents a 250-nt sequence complementary to the RGS domain, common to RGS9-1 and RGS9-2. We used stringent conditions for hybridization, which result in highly specific recognition of these mRNAs (see refs. 7 and 8). The species recognized in the CNS is RGS9-2 mRNA, as confirmed by Northern blotting (7, 8).
Western Blotting and Dual-Label Immunohistochemistry. NAc, dorsal striatum, and periaqueductal gray were dissected as punches with a 12-gauge syringe needle from 1-mm-thick coronal sections of rat brain as described (15, 22). Spinal cord (L3-L6) was obtained by gross dissection, and the dorsal quadrants were separated from the ventral quadrants. The following anitibodies were used for Western blotting: a rabbit protein A-purified anti-RGS9 antibody (1:5,000 dilution) from Ted G. Wensel (Baylor College of Medicine, Houston), a rabbit affinity-purified anti-RGS7 antibody (1:5,000) from Phillip Jones (Wyeth-Ayerst Pharmaceuticals, Madison, NJ), a rabbit anti-RGS11 peptide antibody (1: 2,500) from Andrej Krummins (University of Texas Southwestern Medical Center), a rabbit anti-Gβ5 (C terminus) antibody (1:10,000) from William Simonds (National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda), and a rabbit anti-Gγ7 antibody (1:250) from Santa Cruz Biotechnology. The anti-RGS9 antibody was prepared against amino acids 226-484, common to RGS9-1 and RGS9-2, which include the RGS domain plus some flanking sequence. The specificity of this antibody is established: (i) it recognizes a single band of 76 kDa (RGS9-2) in crude brain extracts (RGS9-1 is not expressed in brain) (8, 15), and (ii) the band is absent in brain extracts from RGS9-/- mice (see Fig. 2).
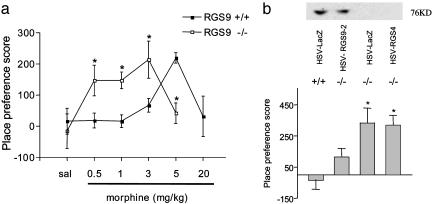
Regulation of morphine reward by RGS9-2. (a) RGS9 KO mice show ≈10-fold greater sensitivity to morphine over a full dose range in the place conditioning assay. Scores are calculated as the difference between the time spent in the drug-paired side post-versus preconditioning. Morphine was injected s.c. at 0, 0.5, 1, 3, 5, and 20 mg/kg (n = 6-10). Data are expressed as mean ± SEM. *, P < 0.05 between genotypes receiving the same treatment (ANOVA followed by probable least-squares difference post hoc test). (b) HSV-mediated overexpression of RGS9-2 (but not of LacZ or RGS4) in the NAc restores normal levels of RGS9-2 in this brain region as well as morphine place conditioning in RGS9 KO mice. Mice were conditioned with 3 mg/kg morphine s.c. (n = 4-6). *, P < 0.005 for HSV-LacZ in RGS9-/- mice and HSV-RGS4 in RGS9-/- versus HSV-LacZ in RGS9+/+ mice and HSV-RGS9-2 in RGS9-/- mice (ANOVA followed by probable least-squares difference post hoc test).
For immunohistochemical studies, 40-μm-thick coronal brain sections were processed with antibodies against tyrosine hydroxylase (1:5,000) or c-Fos (1:500) (Santa Cruz Biotechnology) exactly as described (21).
Opioid Receptor Assays. Levels of μ opioid receptor binding were measured as before (23) by incubating membranes (90-100 μg) with 2 nM [3H]naloxone in assay buffer for 90 min at 30°C. Naltrindole (0.5 nM) and norbinaltorphamine (2 nM) were included in the reaction mixture to block δ and κ opioid receptor sites, respectively. Nonspecific binding was determined by inclusion of 10 μM naltrexone. The incubation was terminated by vacuum filtration. Bound radioactivity was determined by liquid scintillation spectrophotometry at 45% efficiency for 3H.
[35S]Guanosine 5′-[γ-thio]triphosphate (GTP[γS]) binding was measured in 20-μm coronal brain sections in the presence or absence of 10 μM DAMGO (D-Ala2, MePhe4, Gly5-ol-enkephalin, a μ opioid agonist) according to published procedures (24).
Results
RGS9-2 Distribution and Regulation by Morphine. Previous studies have shown that RGS9-2 mRNA is expressed at very high levels in the NAc and dorsal striatum, with much lower levels seen in periaqueductal gray (7). This distribution pattern was replicated here, as shown in Fig. 1a, and was confirmed at the protein level by using Western blotting (Fig. 1b). Moreover, we found that RGS9-2 is expressed within the spinal cord, where it is enriched in the superficial laminae of the dorsal horn, a region important for opiate analgesia, as well as more diffusely in other areas. Confirming previous findings (8, 9), RGS9-1 was not detected in any CNS region examined (data not shown).
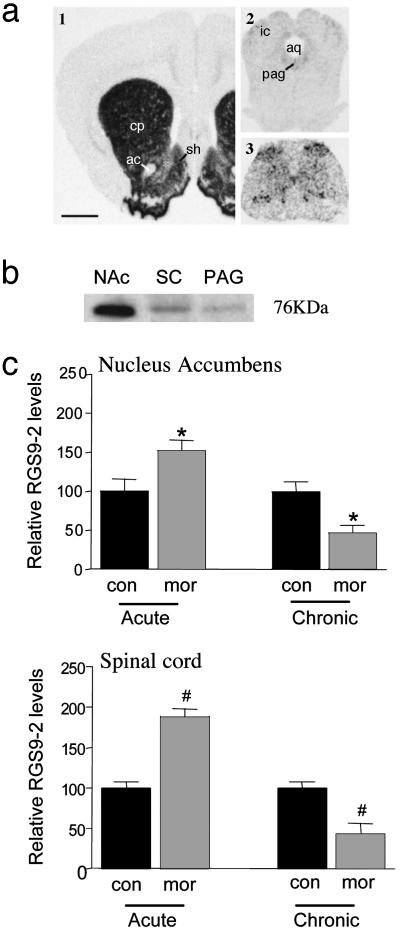
RGS9-2 distribution and regulation by morphine. Distribution of RGS9-2 mRNA (a) and protein (b) in the rat CNS is shown. The figure illustrates high levels of expression in the NAc (1) and much lower levels in periaqueductal gray (PAG) (2) and dorsal horn of the spinal cord (SC) (3). (c) Regulation of RGS9-2 by acute and chronic morphine (mor) in the mouse NAc and spinal cord, as determined by Western blotting. Acute, mice were used 2 h after a s.c. injection of 15 mg/kg morphine (n = 5); chronic, mice were used on day 6 after s.c. implantation of 25-mg morphine pellets on days 1 and 3 (n = 5). con, control. Data are expressed as mean ± SEM. *, P < 0.05; #, P < 0.001 (t test).
We next found that RGS9-2 expression is dynamically regulated in these various regions by acute and chronic morphine (Fig. 1c). Two hours after a single morphine injection, RGS9-2 levels were increased by ≈50% in the NAc and spinal cord of C57BL/6 mice. In contrast, chronic morphine decreased RGS9-2 levels by ≈50% in these regions. Similar effects were observed in the periaqueductal gray and dorsal striatum (data not shown).
In contrast, chronic morphine had no effect on levels of RGS7 or RGS11 in the NAc, two other members of the RGS7 subfamily (see ref. 6) expressed at appreciable levels in this brain region (RGS7, 88 ± 13% of control ± SEM; RGS11, 96 ± 7%; n = 3-5). Chronic morphine also failed to alter levels of the G protein subunit, Gβ5, in NAc (95 ± 16% of control, n = 5). There are reports in retina that RGS9-1 associates selectively with Gβ5 (10, 25-27), although it is not known whether this association holds for RGS9-2 in brain.
Influence of RGS9 on Morphine Reward. To understand the functional implications of RGS9-2 regulation by morphine, we studied behavioral responses to morphine in RGS9 KO mice (10). We first used the place conditioning paradigm, in which animals learn to prefer a drug-paired environment, to assess the rewarding effects of morphine. RGS9 KO mice showed a dramatic (10-fold) increase in sensitivity to morphine's rewarding effects (Fig. 2a). Whereas the lowest morphine dose at which WT littermates showed significant place conditioning was 5 mg/kg, mutant mice showed significant place conditioning to doses as low as 0.5 mg/kg. Morphine place conditioning shows an inverted-U dose-response function, with high doses of morphine unable to produce a significant preference (28). As shown in Fig. 2a, the loss of RGS9 caused a leftward shift over this entire dose-response to morphine.
The increased sensitivity to morphine seen in the RGS9 KO mice was not related to changes in opioid receptor coupling to G proteins per se: KO and WT littermates showed equivalent opiate-induced guanosine 5′-[γ-thio]triphosphate (GTP[γS]) binding in NAc sections. DAMGO (D-Ala2, MePhe4, Gly5-enkephalin), a μ opioid receptor agonist, induced GTP[γS] binding in NAc core (RGS9+/+ = 611 ± 35 nCi/g ± SEM, RGS9-/- = 604 ± 55 nCi/g; in NAc shell, +/+ = 661 ± 52 nCi/g, -/- = 643 ± 40 nCi/g; n = 6-7 per group; 1 Ci = 37 GBq). In addition, direct measure of μ opioid receptor levels by [3H]naloxone binding showed that receptor levels are not altered in NAc and dorsal striatum of mice lacking the RGS9 gene (RGS9+/+ = 115 ± 9 fmol/mg ± SEM, RGS9-/- = 120 ± 11; n = 5 per group). Because Gβ5 levels are dramatically down-regulated in retina of RGS9 KO mice (10), we determined whether a similar down-regulation occurs in brain. In contrast to retina, we found no difference in Gβ5 levels in NAc of RGS9 KO mice compared with WT littermates (112 ± 13% of control, n = 6). There also was no difference in levels of Gγ7, a G protein subunit enriched in striatum (29), in RGS9 KO mice (89 ± 9% of control, n = 3).
To investigate whether the enhanced sensitivity to morphine reward seen in the RGS9 KO mice is mediated by the loss of RGS9-2 from the NAc per se, we overexpressed RGS9-2 locally within this brain region of RGS9 mutant mice by using viral-mediated gene transfer. Indeed, bilateral overexpression of RGS9-2 in the NAc, which restores levels of the protein to near normal, completely reversed the phenotype seen in the mutants, whereas mutants infected with a control virus (encoding LacZ) remained more sensitive to morphine (Fig. 2b). As a control for RGS subtype specificity, we infected the NAc of RGS9 KO mice with an HSV vector expressing RGS4, a distinct RGS subtype present at appreciable levels in the NAc. RGS4 is regulated by opiates in the locus coeruleus but not in the NAc (22). Over-expression of RGS4 in the NAc of RGS9 KO animals failed to reverse the increased sensitivity to morphine seen in the mutants (Fig. 2b).
As a control of regional specificity of the HSV-RGS9-2 effect, we infected dorsal striatum of RGS9 KO or WT mice with HSV-RGS9-2 or HSV-LacZ. In striking contrast to results obtained for the NAc (Fig. 2b), we found that expression of RGS9-2 in dorsal striatum of RGS9 KO animals failed to reverse the enhanced sensitivity to morphine observed in the KO mice.
Place conditioning values for 3 mg/kg morphine ± SEM were: HSV-LacZ into RGS9+/+ mice = -9.25 ± 27 sec, HSV-RGS9-2 into RGS9-/- mice = 261 ± 67 sec, and HSV-LacZ into RGS9-/- mice = 193 ± 41 sec).
Influence of RGS9 on Morphine Analgesia and Tolerance. To evaluate the possibility that morphine's analgesic effects are also altered on loss of RGS9-2, we examined responses of mutant animals to hot plate and tail flick tests. RGS9 KO mice exhibited normal nociceptive thresholds compared with their WT littermates in the 52°C hot plate and tail flick tests (RGS9+/+ = 8.4 ± 0.6 sec ± SEM, RGS9-/- = 7.9 ± 0.6 sec in the hot plate test, and +/+ = 3.3 ± 0.7 sec, -/- = 3.1 ± 0.4 sec in the tail flick test). However, loss of RGS9 causes the animals to be more sensitive to morphine analgesia in both tests (Fig. 3 c and d). The dose of morphine (ED50) at which 50% of the RGS9-/- mice showed an analgesic response in the hot plate test was 5 mg/kg, whereas the dose of morphine at which 50% of the RGS9+/+ mice showed an analgesic response was 15 mg/kg. Morphine's duration of action is not affected by the loss of RGS9, because analgesic effects persisted for the same amount of time in the KO and WT mice (Fig. 3b).
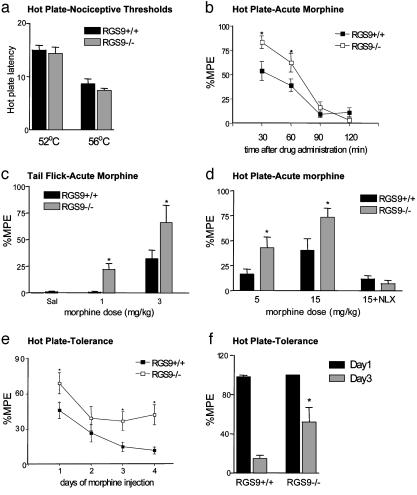
Regulation of analgesic responses to morphine by RGS9. (a) RGS9 KO mice (-/-) have normal nociceptive thresholds compared with their WT littermates (+/+) when tested in the 52°C and 56°C hot plate assay. (b) Analgesic responses to morphine 15 mg/kg s.c. in the hot plate test are greater in RGS9 KO mice; however, the duration of analgesia is not different. (c) Morphine dose-response in the tail flick test (n = 12-13 per group) is shifted to the left in KO vs. WT mice. (d) Similar enhancement in morphine-induced analgesia in RGS9 KO mice in the hot plate test (n = 7-9). Note the complete reversal of analgesia to morphine (15 mg/kg) by naloxone (1 mg/kg s.c.). (e) RGS9 KO mice show delayed tolerance to morphine compared with their WT littermates in the hot plate test. Responses to acute morphine (15 mg/kg) were monitored 30 min after drug administration for 4 days (n = 9-10). (f) A similar effect on morphine tolerance was observed when mice (n = 6 per genotype) were implanted with a 25-mg morphine pellet, and analgesic responses to acute morphine (15 mg/kg, s.c.) were monitored on days 1 and 3. Data are expressed as mean ± SEM. Responses in KO mice differed from responses in WT mice (*, P < 0.05), as determined by ANOVA and probable least-squares difference post hoc test.
Tolerance to morphine's analgesic effects develops after repeated morphine administration, a phenomenon that severely limits the clinical utility of opiate drugs in the treatment of pain. Strikingly, the RGS9 mutants show delayed development of morphine tolerance in response to chronic morphine administration compared with WT littermate mice (Fig. 3 e and f).
Influence of RGS9 on Morphine Physical Dependence and Withdrawal. Finally, we examined the ability of chronic morphine to induce physical dependence by assessing naloxone (an opioid receptor antagonist)-precipitated opiate withdrawal in RGS9 KO mice and their WT littermates. Mice lacking RGS9 exhibited dramatically enhanced opiate withdrawal, with all signs of withdrawal examined being more severe in the mutants (Fig. 4a). In addition, c-Fos induction during antagonist-precipitated morphine withdrawal, a molecular marker of withdrawal (e.g., refs. 18 and 30), is greater in the locus coeruleus and other brain areas in RGS9 KO mice (Fig. 4 c and d). RGS9 also appears to be involved in modulating the tone of endogenous opioidergic signaling. A low dose of naloxone, which had no effect in WT mice, induced a significant place aversion in opiate-naïve RGS9 KO mice (Fig. 4b).
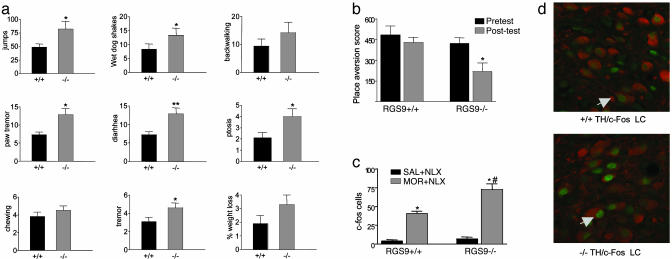
Regulation of morphine dependence by RGS9. (a) RGS9 KO mice show greater antagonist-precipitated morphine withdrawal compared with WT littermates (n = 8), an effect seen for all withdrawal behaviors examined. Data are expressed as mean ± SEM. *, P < 0.05; **, P < 0.01 between genotypes (ANOVA followed by PSTD test). (b) Opiate-naïve RGS9 KO mice are more sensitive to the aversive effects of naloxone (1 mg/kg s.c.) in the place aversion assay (n = 7-8). *, P < 0.05 between pre- and posttest values (paired t test). (c) Induction of c-Fos in the locus coeruleus 2 h after precipitation of morphine withdrawal was greater in mice lacking RGS9. Data represent the number of c-Fos-labeled cells in morphine-dependent or control KO (-/-) and WT (+/+) littermate mice (n = 4-5 per group). Data are expressed as mean ± SEM. *, P < 0.005 between treatments; #, P < 0.01 for the withdrawal group between genotypes (ANOVA followed by probable least-squares difference post hoc test). (d) Representative high-power confocal photomicrographs of sections through the locus coeruleus (LC) from +/+ and -/- mice undergoing withdrawal dual-labeled for c-Fos (green) and tyrosine hydroxylase (TH, red).
Discussion
Our findings demonstrate that deletion of the RGS9 gene causes a dramatic increase in sensitivity to the rewarding and analgesic effects of morphine. In addition, RGS9 KO mice develop tolerance to morphine's analgesic effects at a much slower rate than their WT littermates. These findings are consistent with recent observations that knockdown of RGS9 by using antisense oligonucleotides increases opiate analgesia and decreases analgesic tolerance (20) and with recent in vitro studies that show that RGS9 or the RGS domain modulates μ opioid receptor signaling (8, 12, 32). We also show here that RGS9 KO mice develop a greater degree of physical dependence on morphine. Finally, RGS9 appears to regulate tonic opioidergic signaling in brain, because opiate-naïve RGS9 KO mice are more sensitive to the aversive effects of naloxone.
Together, these behavioral data suggest that the ability of acute morphine to increase RGS9-2 levels in the NAc and in other regions where it is expressed (periaqueductal gray, dorsal horn of spinal cord, and dorsal striatum) may be a mechanism of acute tolerance. Conversely, the opposite effect of chronic morphine in down-regulating RGS9-2 levels may not only increase sensitivity to morphine but may also alter the animal's adaptations to repeated drug exposure. Thus, down-regulation of RGS9-2 by chronic morphine may contribute to a state of addiction by enhancing morphine reward, opposing the development of tolerance, and exacerbating the development of physical dependence. The mechanism by which morphine regulates RGS9-2 levels is not known, but may occur posttranslationally given the rapidity of changes in RGS9-2 levels seen after acute morphine. Our findings lend further support to the notion that regulation of RGS protein expression may be related to drug addiction, because RGS9-2 has been implicated recently in the actions of psychostimulants (15), and other RGS subtypes (i.e., RGS2 and -4) are regulated by chronic opiate exposure in other brain regions (22).
Our data on morphine reward suggest that prolonged μ receptor signaling, resulting from the loss of RGS9, causes a 10-fold increase in sensitivity to the rewarding effects of morphine, measured by the place preference paradigm. One possible confound in the use of RGS9 KOs in this paradigm is that loss of RGS9-1 in the retina alters phototransduction (10), which could impair the animals' vision. However, we found that RGS9 mutant and WT animals did not differ in the water maze using a visible platform (values for distance traveled to find the platform are RGS9+/+ = 469 ± 82 cm and RGS9-/- = 487 ± 135 cm, n = 10), and they also were indistinguishable in the visual cliff test (not shown). The place preference test was performed under dim illumination with no differences in lighting between the two chambers of the apparatus, and the chambers are distinguished by nonvisual as well as visual cues. Finally, the enhanced responsiveness to morphine was completely normalized upon overexpression of RGS9-2 in NAc. This latter finding definitively establishes that the 10-fold increase in morphine reward observed in the KOs is caused by the loss of RGS9-2 from NAc per se and is unrelated to RGS9-1 function in the retina.
The most straightforward explanation for the enhanced responsiveness to morphine seen in the RGS9 KO mice is increased signaling efficacy of opioid receptors in the absence of RGS9-2. This is consistent with the observation that RGS9-2 can dampen μ opioid receptor responses in vitro (8, 12). However, the loss of RGS9-2 might also enhance morphine responses via altered function of other G protein-coupled receptors involved in addiction and analgesia, such as the dopamine D2 (18, 31) and substance P (19) receptors. As with all constitutive KO animals, changes during development in signaling pathways that directly or indirectly affect responses to morphine should also be considered. However, there is evidence that such developmental changes do not contribute to the enhanced responsiveness to morphine in the RGS9 KOs. We found that levels of μ opioid receptor binding and G protein activation are not altered in the NAc of RGS9 KO mice. There also is no difference in levels of two G protein subunits, Gβ5 and Gγ7, in the mutants. The Gβ5 subunit is virtually absent in the retina of RGS9 KOs (10), and there is in vitro evidence that RGS9-1 may associate selectively with this subunit (25-27). In contrast to RGS9-1 and retina, however, levels of Gβ5 are normal in NAc of RGS9 KO mice. The reason for this difference is not known and requires additional investigation. The best evidence that developmental compensations are not responsible for the enhanced responsiveness to morphine in the RGS9 KO mice is the finding that this behavioral abnormality can be restored completely to normal on overexpression of RGS9-2 in NAc.
The regulation of morphine analgesia and tolerance by RGS9 warrants further comment. Previous studies have demonstrated an association between these phenomena and changes in opioid receptor desensitization and internalization (33, 34). In addition, mice with mutations in several other genes display abnormal tolerance to opiates, for example, GIRK-2 (a K+ channel), Gz (a G protein α subunit), and GluRε1 (an N-methyl-d-aspartate receptor subunit) (35-37). Results of the present study establish that other proteins, such as RGS9-2, which directly modulate G protein-coupled receptor signaling, also contribute to the process of analgesic tolerance. These findings raise the possibility that agents that inhibit RGS9-2 may be useful as adjuncts to reduce the development of tolerance in opiate treatment of chronic pain. The region of the CNS responsible for RGS9-2's modulation of opiate analgesia and tolerance remains unknown. RGS9-2 may be exerting this effect via the periaqueductal gray or dorsal horn of spinal cord, regions key for nociception and opiate analgesia. Alternatively, the NAc may be the critical site of RGS9-2 action, because this brain region expresses the highest levels of RGS9-2 and has been shown to modify nociception and opiate analgesia (14).
Taken together, the results of the present study establish RGS9-2 as a critical regulator of behavioral responses to opiates. RGS9-2 is required for normal responses to morphine's rewarding effects, acute and chronic analgesic effects, and induction of physical dependence. Further exploration of RGS9-2 modulation of opioid systems will shed light on the neural and molecular basis of opiate addiction, opiate analgesic tolerance, and other important features of opiate-induced behavioral plasticity.
Acknowledgments
We thank Amy M. Taylor for technical assistance, Cathy Steffen and Qindan Chen for assistance with mouse breeding and genotyping, and Dr. Craig Powell for helpful discussions and advice. This work was supported by grants from the National Institute on Drug Abuse and National Institute of Mental Health.
Notes
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: RGS, regulators of G protein signaling; HSV, herpes simplex virus; NAc, nucleus accumbens; KO, knockout.
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.2232594100
Read article for free, from open access legal sources, via Unpaywall:
https://www.pnas.org/content/pnas/100/23/13656.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.2232594100
Article citations
Regulator of G protein signaling 6 (RGS6) in dopamine neurons promotes EtOH seeking, behavioral reward, and susceptibility to relapse.
Psychopharmacology (Berl), 241(11):2255-2269, 10 Jun 2024
Cited by: 0 articles | PMID: 38856764
Direct modulation of G protein-gated inwardly rectifying potassium (GIRK) channels.
Front Physiol, 15:1386645, 06 Jun 2024
Cited by: 1 article | PMID: 38903913 | PMCID: PMC11187414
Review Free full text in Europe PMC
S-nitrosoglutathione reductase alleviates morphine analgesic tolerance by restricting PKCα S-nitrosation.
Redox Biol, 75:103239, 14 Jun 2024
Cited by: 0 articles | PMID: 38901102 | PMCID: PMC11253161
Genetic mouse models in opioid research: current status and future directions.
J Neural Transm (Vienna), 131(5):491-494, 04 Mar 2024
Cited by: 0 articles | PMID: 38436758
Review
The genetic variant SLC2A1 -rs1105297 is associated with the differential analgesic response to a glucose-based treatment in newborns.
Pain, 165(3):657-665, 13 Sep 2023
Cited by: 0 articles | PMID: 37703430 | PMCID: PMC10859852
Go to all (176) article citations
Other citations
Wikipedia
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A unique role of RGS9-2 in the striatum as a positive or negative regulator of opiate analgesia.
J Neurosci, 31(15):5617-5624, 01 Apr 2011
Cited by: 51 articles | PMID: 21490202 | PMCID: PMC3412365
Nucleus accumbens-specific interventions in RGS9-2 activity modulate responses to morphine.
Neuropsychopharmacology, 39(8):1968-1977, 24 Feb 2014
Cited by: 25 articles | PMID: 24561386 | PMCID: PMC4059906
RGS9-2 is a negative modulator of mu-opioid receptor function.
J Neurochem, 103(2):617-625, 23 Aug 2007
Cited by: 48 articles | PMID: 17725581
Reflections on: "A general role for adaptations in G-Proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function".
Brain Res, 1645:71-74, 29 Dec 2015
Cited by: 23 articles | PMID: 26740398 | PMCID: PMC4927417
Review Free full text in Europe PMC





