Abstract
Free full text

Tn5-induced mutations affecting virulence factors of Bordetella pertussis.
Abstract
Transposon Tn5 was used to isolate mutants of Bordetella pertussis. Strains with Tn5 insertions were screened for loss of virulence-associated factors, including filamentous hemagglutinin, hemolysin, and pertussis toxin. Several mutants deficient for hemolysin production were obtained. All produced dermonecrotic toxin, pertussis toxin, and filamentous hemagglutinin, but were found to vary with respect to adenylate cyclase production. One hemolysin mutant had no detectable adenylate cyclase activity; others had 0.6% or 16% wild-type activity, whereas a fourth seemed to be unaffected in terms of adenylate cyclase activity. Mutants deficient in the ability to hemagglutinate sheep erythrocytes were also isolated. These mutants either failed to synthesize or produced reduced amounts of three protein species of 200,000, 130,000, and 100,000 daltons, all of which reacted with antiserum to filamentous hemagglutinin. Pertussis toxin mutants were identified by screening culture supernatants for failure to induce a clustered growth pattern in Chinese hamster ovary cells, and identification was confirmed by the standard histamine-sensitizing assay in mice. These mutants will be useful to determine the relative contribution of each virulence factor to pathogenicity as well as to determine the identity of the antigens important in protective immunity.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.5M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
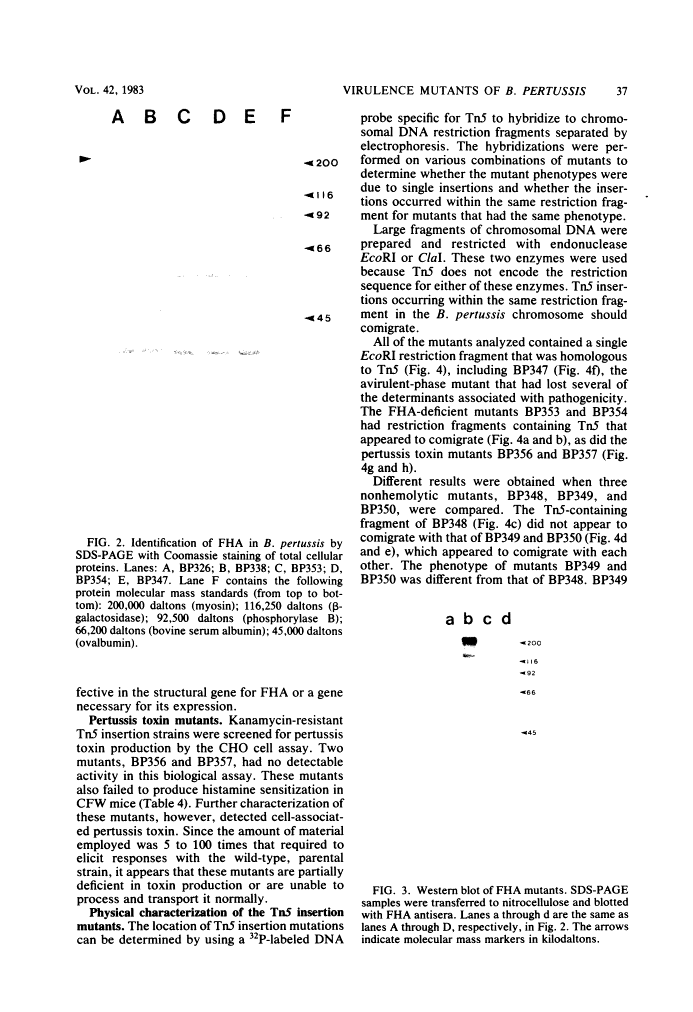
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann BJ, Low KB. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. [Europe PMC free article] [Abstract] [Google Scholar]
- Bassford P, Beckwith J. Escherichia coli mutants accumulating the precursor of a secreted protein in the cytoplasm. Nature. 1979 Feb 15;277(5697):538–541. [Abstract] [Google Scholar]
- Berg DE, Weiss A, Crossland L. Polarity of Tn5 insertion mutations in Escherichia coli. J Bacteriol. 1980 May;142(2):439–446. [Europe PMC free article] [Abstract] [Google Scholar]
- Burnette WN. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. [Abstract] [Google Scholar]
- Campbell A, Berg DE, Botstein D, Lederberg EM, Novick RP, Starlinger P, Szybalski W. Nomenclature of transposable elements in prokaryotes. Gene. 1979 Mar;5(3):197–206. [Abstract] [Google Scholar]
- Chumley FG, Menzel R, Roth JR. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. [Europe PMC free article] [Abstract] [Google Scholar]
- Cody CL, Baraff LJ, Cherry JD, Marcy SM, Manclark CR. Nature and rates of adverse reactions associated with DTP and DT immunizations in infants and children. Pediatrics. 1981 Nov;68(5):650–660. [Abstract] [Google Scholar]
- Cohen ML, Falkow S. Protein antigens from Staphylococcus aureus strains associated with toxic-shock syndrome. Science. 1981 Feb 20;211(4484):842–844. [Abstract] [Google Scholar]
- Confer DL, Eaton JW. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982 Sep 3;217(4563):948–950. [Abstract] [Google Scholar]
- Cowell JL, Hewlett EL, Manclark CR. Intracellular localization of the dermonecrotic toxin of Bordetella pertussis. Infect Immun. 1979 Sep;25(3):896–901. [Europe PMC free article] [Abstract] [Google Scholar]
- Figurski DH, Helinski DR. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. [Europe PMC free article] [Abstract] [Google Scholar]
- Hewlett EL, Manclark CR, Wolff J. Adenyl cyclase in Bordetella pertussis vaccines. J Infect Dis. 1977 Aug;136 (Suppl):S216–S219. [Abstract] [Google Scholar]
- Hewlett EL, Roberts CO, Wolff J, Manclark CR. Biphasic effect of pertussis vaccine on serum insulin in mice. Infect Immun. 1983 Jul;41(1):137–144. [Europe PMC free article] [Abstract] [Google Scholar]
- Hewlett EL, Sauer KT, Myers GA, Cowell JL, Guerrant RL. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect Immun. 1983 Jun;40(3):1198–1203. [Europe PMC free article] [Abstract] [Google Scholar]
- Hewlett EL, Urban MA, Manclark CR, Wolff J. Extracytoplasmic adenylate cyclase of Bordetella pertussis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1926–1930. [Europe PMC free article] [Abstract] [Google Scholar]
- Hewlett E, Wolff J. Soluble adenylate cyclase from the culture medium of Bordetella pertussis: purification and characterization. J Bacteriol. 1976 Aug;127(2):890–898. [Europe PMC free article] [Abstract] [Google Scholar]
- Hull RA, Gill RE, Hsu P, Minshew BH, Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. [Europe PMC free article] [Abstract] [Google Scholar]
- KASUGA T, NAKASE Y, UKISHIMA K, TAKATSU K. Studies on Haemophilus pertussis. I. Antigen structure of H. pertussis and its phases. Kitasato Arch Exp Med. 1953 Nov;26(2-3):121–133. [Abstract] [Google Scholar]
- Katada T, Ui M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc Natl Acad Sci U S A. 1982 May;79(10):3129–3133. [Europe PMC free article] [Abstract] [Google Scholar]
- Kawai Y, Moribayashi A. Characteristic lipids of Bordetella pertussis: simple fatty acid composition, hydroxy fatty acids, and an ornithine-containing lipid. J Bacteriol. 1982 Aug;151(2):996–1005. [Europe PMC free article] [Abstract] [Google Scholar]
- Kawai Y, Moribayashi A, Yano I. Ornithine-containing lipid of Bordetella pertussis that carries hemagglutinating activity. J Bacteriol. 1982 Nov;152(2):907–910. [Europe PMC free article] [Abstract] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Miller DL, Ross EM, Alderslade R, Bellman MH, Rawson NS. Pertussis immunisation and serious acute neurological illness in children. Br Med J (Clin Res Ed) 1981 May 16;282(6276):1595–1599. [Europe PMC free article] [Abstract] [Google Scholar]
- Peppler MS. Isolation and characterization of isogenic pairs of domed hemolytic and flat nonhemolytic colony types of Bordetella pertussis. Infect Immun. 1982 Mar;35(3):840–851. [Europe PMC free article] [Abstract] [Google Scholar]
- Pittman M. Pertussis toxin: the cause of the harmful effects and prolonged immunity of whooping cough. A hypothesis. Rev Infect Dis. 1979 May-Jun;1(3):401–412. [Abstract] [Google Scholar]
- Pittman M, Furman BL, Wardlaw AC. Bordetella pertussis respiratory tract infection in the mouse: pathophysiological responses. J Infect Dis. 1980 Jul;142(1):56–66. [Abstract] [Google Scholar]
- Robinson A, Irons LI. Synergistic effect of Bordetella pertussis lymphocytosis-promoting factor on protective activities of isolated Bordetella antigens in mice. Infect Immun. 1983 May;40(2):523–528. [Europe PMC free article] [Abstract] [Google Scholar]
- Rothstein SJ, Jorgensen RA, Postle K, Reznikoff WS. The inverted repeats of Tn5 are functionally different. Cell. 1980 Mar;19(3):795–805. [Abstract] [Google Scholar]
- Ruvkun GB, Ausubel FM. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. [Abstract] [Google Scholar]
- Tamura M, Nogimori K, Murai S, Yajima M, Ito K, Katada T, Ui M, Ishii S. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry. 1982 Oct 26;21(22):5516–5522. [Abstract] [Google Scholar]
- Watanabe M, Nakase Y. Mutant of Bordetella pertussis which lacks ability to produce filamentous hemagglutinin. Infect Immun. 1982 Mar;35(3):1018–1023. [Europe PMC free article] [Abstract] [Google Scholar]
- Weiss AA, Falkow S. Plasmid transfer to Bordetella pertussis: conjugation and transformation. J Bacteriol. 1982 Oct;152(1):549–552. [Europe PMC free article] [Abstract] [Google Scholar]
- Weiss AA, Falkow S. Transposon insertion and subsequent donor formation promoted by Tn501 in Bordetella pertussis. J Bacteriol. 1983 Jan;153(1):304–309. [Europe PMC free article] [Abstract] [Google Scholar]
- Wolff J, Cook GH, Goldhammer AR, Berkowitz SA. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3841–3844. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.42.1.33-41.1983
Read article for free, from open access legal sources, via Unpaywall:
https://iai.asm.org/content/iai/42/1/33.full.pdf
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/reprint/42/1/33
Free to read at iai.asm.org
http://iai.asm.org/cgi/content/abstract/42/1/33
Citations & impact
Impact metrics
Article citations
Eosinophils as drivers of bacterial immunomodulation and persistence.
Infect Immun, 92(9):e0017524, 15 Jul 2024
Cited by: 0 articles | PMID: 39007622 | PMCID: PMC11385729
Review Free full text in Europe PMC
Biosynthesis of the Inner Core of Bordetella pertussis Lipopolysaccharides: Effect of Mutations on LPS Structure, Cell Division, and Toll-like Receptor 4 Activation.
Int J Mol Sci, 24(24):17313, 09 Dec 2023
Cited by: 0 articles | PMID: 38139140 | PMCID: PMC10743493
RNase III and RNase E Influence Posttranscriptional Regulatory Networks Involved in Virulence Factor Production, Metabolism, and Regulatory RNA Processing in Bordetella pertussis.
mSphere, 6(4):e0065021, 18 Aug 2021
Cited by: 3 articles | PMID: 34406853 | PMCID: PMC8386462
Bordetella pertussis Can Be Motile and Express Flagellum-Like Structures.
mBio, 10(3):e00787-19, 14 May 2019
Cited by: 8 articles | PMID: 31088927 | PMCID: PMC6520453
Analysis of the In Vivo Transcriptome of Bordetella pertussis during Infection of Mice.
mSphere, 4(2):e00154-19, 17 Apr 2019
Cited by: 18 articles | PMID: 30996109 | PMCID: PMC6470212
Go to all (272) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis.
J Infect Dis, 150(2):219-222, 01 Aug 1984
Cited by: 144 articles | PMID: 6088647
Use of the promoter fusion transposon Tn5 lac to identify mutations in Bordetella pertussis vir-regulated genes.
Infect Immun, 57(9):2674-2682, 01 Sep 1989
Cited by: 58 articles | PMID: 2569447 | PMCID: PMC313511
Genetic studies of the molecular basis of whooping cough.
Dev Biol Stand, 61:11-19, 01 Jan 1985
Cited by: 10 articles | PMID: 2872097
Molecular aspects of Bordetella pertussis pathogenesis.
Int Microbiol, 2(3):137-144, 01 Sep 1999
Cited by: 39 articles | PMID: 10943406
Review