Abstract
Purpose of review
This review compares the fate of monocyte-derived cells that enter atherosclerotic plaques with those that accumulate at other sites of inflammation.Recent findings
Resolution of inflammatory reactions involves emigration of monocyte-derived cells out of the inflamed site through nearby lymphatic vessels. However, this emigratory process associated with resolution is impaired in atherosclerosis. The mechanism for impeded emigration from plaques in vivo remains to be determined, but multiple factors are likely involved, including specialized properties of artery walls and a negative impact of lipid mediators on monocyte-derived cell migration.Summary
Impaired egress would be expected to compound macrophage accumulation within plaques, contribute to build-up of necrotic pools, and explain in part the reticence of many plaques to regress, or resolve. Restoration of the capacity of monocyte-derived cells to leave plaques would, by contrast, be expected to facilitate regression, but it remains to be determined whether restoring egress may sometimes provoke unwanted outcomes as well.Free full text

Emigration of monocyte-derived cells to lymph nodes during resolution of inflammation and its failure in atherosclerosis
Abstract
Purpose of review
This review compares the fate of monocyte-derived cells that enter atherosclerotic plaques with those that accumulate at other sites of inflammation.
Recent findings
Resolution of inflammatory reactions involves emigration of monocyte-derived cells out of the inflamed site through nearby lymphatic vessels. However, this emigratory process associated with resolution is impaired in atherosclerosis. The mechanism for impeded emigration from plaques in vivo remains to be determined, but multiple factors are likely involved, including specialized properties of artery walls and a negative impact of lipid mediators on monocyte-derived cell migration.
Summary
Impaired egress would be expected to compound macrophage accumulation within plaques, contribute to build-up of necrotic pools, and explain in part the reticence of many plaques to regress, or resolve. Restoration of the capacity of monocyte-derived cells to leave plaques would, by contrast, be expected to facilitate regression, but it remains to be determined whether restoring egress may sometimes provoke unwanted outcomes as well.
Introduction
Atherosclerosis is a chronic inflammatory disease affecting great arteries. From early stages, the inflammatory infiltrate in the disease is dominated by monocytes that accumulate between arterial endothelium and the internal elastic lamina [1]. Following engulfment of lipoproteins by these phagocytes and their differentiation into cholesterol-laden foam cells, progression of atherosclerosis is characterized by remodeling of the intimal, medial, and adventitial compartments of the arteries over decades in humans. These inflammatory plaques rarely fully resolve, where full resolution is defined as a return to homeostasis in the vessel wall. However, experimental and clinical evidence that at least partial resolution, or plaque regression, can be achieved is encouraging [2••]. Mechanistic insight into how plaque regression occurs may point the way to development of therapeutics that would particularly facilitate regression. Here, I will discuss the view that plaque progression is characterized by a failure of normal migratory processes that bring about resolution of inflammation, and that regression of plaques would require a restoration of these mechanisms.
Resolution of acute inflammation: monocyte-derived cells emigrate through local lymphatic vessels and terminate in draining lymph nodes
Acute inflammatory responses are often transient and a return to homeostasis is achieved within days to weeks [3–6]. The sequence of events from initiation to resolution in these responses includes efficient clearance of the large number of early infiltrating neutrophils [7,8], such that at any given time, few neutrophil-derived corpses can be found at an inflammatory focus even when the magnitude of dying neutrophils is substantial. Dying neutrophils are engulfed by activated, resident macrophages, and infiltrating monocyte-derived cells. Interaction between these mononuclear phagocytes and apoptotic neutrophils triggers the release of anti-inflammatory mediators that actively promote resolution and a return to homeostasis [8]. The fate of monocyte-derived cells that engulf the short-lived neutrophils does not predominantly involve local death; instead, these cells emigrate from the inflammatory site, enter lymphatics, and accumulate in draining lymph nodes [6].
Collection of lymph through cannulation reveals that the major myeloid cell type that enter lymphatic vessels are antigen-presenting dendritic cells, rather than classical macrophages [9,10]. Accordingly, the phenotype of monocyte-derived cells that leave acute inflammatory sites shows features akin to dendritic cells [11], and they mobilize to the T cell zone of the lymph node [11], which is rich in dendritic cells but not macrophages [12]. Lysosomes of lymph-borne dendritic cells often contain debris derived from dead cells [9], apparently engulfed by the dendritic cells within peripheral tissue prior to entry into lymphatics [13]. Thus, dendritic cells may play a substantial role in the removal of dying cells from tissues, as they prepare to present antigens from their phagocytic cargo to T cells in the downstream lymph node. The arrival of dendritic cells, or much more rarely classical macrophages, to lymph nodes from the afferent lymph is a terminal event in their migration, as these cell types are either absent or very scarce in efferent lymph [10].
The course of events involved in the development of atherosclerotic plaques contrasts in several major respects with the sequence of events that define classical acute inflammatory responses. Though a small number of neutrophils enter atherosclerotic plaques [14], a neutrophil-dominant phase does not characterize early plaques. Instead, cells that simultaneously bear features of macrophages and dendritic cells appear to be the first cells that arrive at the site where plaques form in humans or experimental animals [15,16]. These cells are provoked to accumulate in low numbers, with dendrites spread, in regions of the arterial tree where laminar flow patterns are altered by curvatures or branch-points in the arteries [16]. Further accumulation of leukocytes does not occur in these foci unless hypercholesterolemia presents in the animal, in which case more monocytes are recruited to the same regions, and plaques begin to build.
Little consideration seems to have been given to the possibility that the absence of a typical neutrophil phase in atherosclerosis, which may result in diminished generation of molecules like resolvins [8] that promote resolution of inflammation, might negatively impact on the course of disease. Nonetheless, it is clear that atherosclerotic plaques fail to remove dying cells appropriately [17]. Ultimately, the accumulation of dead cell debris within atherosclerotic plaques leads to the formation of so-called necrotic cores that form a cholesterol-rich lipid pool. These pools destabilize plaques, especially so in humans when the fibrous cap overlying them thins to less than 65 microns and thereby becomes prone to rupture [18]. The cause of death among foam cells that are thought to primarily comprise the necrotic core is of great importance to understand. However, it may not be so much the death, but rather the failure to clear the dead cells, that goes strikingly awry in atherosclerosis. High rates of death among leukocytes characterize all inflammatory sites, even those that resolve as discussed, but only a few inflammatory diseases, including atherosclerosis and some infectious diseases like tuberculosis readily develop substantial necrotic foci. Factors that may contribute to the failure of dead cells to be appropriately removed by other, living macrophages or dendritic cells within plaques include competition for uptake of dying cells by oxidized LDL [17] and competition by the high levels of lysophosphatidylcholine (LPC) derived from modified LDL in plaques [19] with the generation of LPC gradients that may direct monocyte-derived cells toward dying cells for engulfment [20,21]. In addition, given that dendritic cells transport dead cell debris to lymph nodes [9,13], the process of emigration by monocyte-derived dendritic cell-like cells into lymph, which normally characterizes resolution of inflammation, may break down in atherosclerosis.
Do monocyte-derived cells emigrate out of plaques or get trapped within them?
To examine the potential of monocyte-derived cells to egress from plaques, my colleagues and I previously employed a method that would experimentally isolate this step from other events such as monocyte entry into plaques. Specifically, we took advantage of a surgical procedure in which a segment of the aorta containing plaques ectopically replaces a portion of the aorta in a recipient mouse [22–24]. If the recipient mouse is hypercholesterolemic, then the plaques in the newly transferred portion of the aorta progress and continue to accumulate foam cells. However, if the recipient is an atherosclerosis-resistant mouse without dyslipidemia, lesions in the transferred segment of the aorta rapidly regress. ApoE KO or wild-type mice, bearing distinct congenic alleles at the CD45 locus, were used as donors and recipients, such that the congenic allele distinguished the migratory fate of already recruited immune cells within lesions from those newly entering lesions during the course of the assay following transplantation [24]. CD45+ immune cells transferred with the aorta, already localized to lesions or to adventitial inflammatory sites, dispersed from the aorta to lymphoid tissues when the recipient was a normocholesterolemic wild-type mouse, but did so very rarely in apoE KO recipients [24].
Leukocytes that left the aortic wall in this study accumulated in at least two sites: the iliac lymph node, which drained the region of the transplanted aorta via afferent lymphatics, and the hepatic lymph node. These cells expressed MHC II and most were positive for both CD11c and CD115, indicating that they were not lymphocytes but instead monocyte-derived cells [24]. During the time points that were examined, lesion-derived cells were not found in the blood, possibly due to rapid clearance. The spleen contained interfering cells with high autofluorescence, making it difficult to draw conclusions on the extent of migration to spleen. Given that arrival in lymph nodes is a dead-end in the migration of nonlymphoid cells like monocyte-derived dendritic cells, the accumulation of these cells in the iliac lymph node indicates that they left the aortic wall via afferent lymphatic vessels. On the contrary, their accumulation in the hepatic lymph node is best interpreted as emigration from the aortic wall via an initial blood route that, upon arrival of the cells to the liver, led to transit from the liver sinusoids to the liver lymphatics that ultimately drain to the hepatic lymph node.
Together, the findings suggest that egress of immune cells from the aortic wall is minimal during progressive disease, consistent with the conclusion that the emigration of mononuclear phagocytes that normally characterizes resolution of inflammation is indeed impaired in atherosclerosis. However, conditions of disease regression favor emigration, and lesional phagocytes leave plaques by one of two routes. They may enter lymphatics and then ferry to the draining lymph node, or they alternatively migrate across arterial endothelium in the ablumenal-to-lumenal direction for direct re-entry into the bloodstream, where some of these cells are later collected in the hepatic lymph node.
Return to the bloodstream is at best very minimal during the emigration of monocyte-derived cells from other sites of inflammation [25]. Thus, at first glance, the transit of aorta-derived cells to the iliac lymph node, rather than the hepatic lymph node, seems more consistent with expectations. However, elasticized arteries retain properties unlike those of most other tissues where lymphatic emigration of monocyte-derived cells during inflammation has been documented. Specifically, the afferent lymphatics in the aorta and other arteries are located exclusively in the adventitia and are separated from the intimal plaque containing foam cells by the elasticized medial smooth muscle cell layers. The elasticized media is a formidable barrier to leukocyte trafficking, so much so that the medial layer of great arteries has been called an immunoprivileged site [26] (Fig. 1). Indeed, it is rare to find leukocytes in arteries localized within the medial layer, although in human arteries burdened with atherosclerotic plaques, phagocytes are observed in the medial layer at a greater frequency than in normal arteries [27•]. Thus, some plaque cells may access the adventitial lymphatics by traversing the media, but alternative routes of trafficking out of plaques such as direct re-entry into blood may be more favorable (Fig. 1). From this perspective, the descriptions first made by Gerrity [28] of foam cells retraversing the aortic endothelium, apparently en route to the circulation, are intriguing and consistent with the specialized anatomy of elasticized arteries that may render typically unlikely routes of trafficking major pathways utilized for egress.
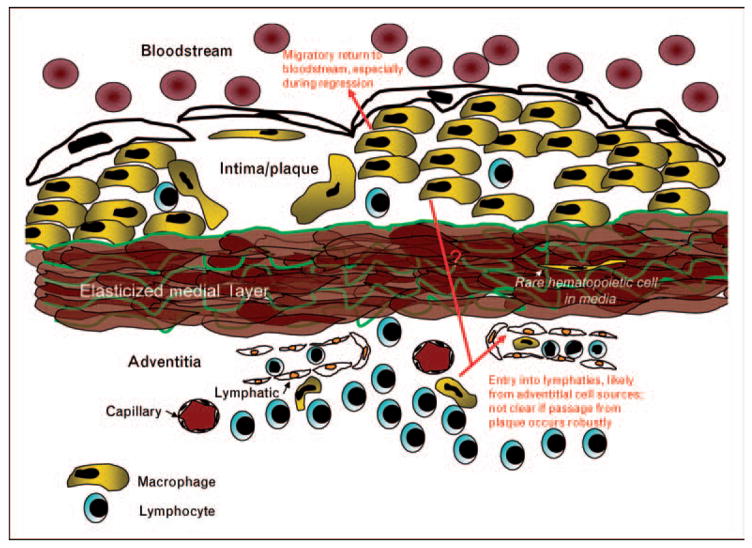
Monocyte-derived cells typically exit tissues through lymphatic vessels, but elasticized medial layers comprise an imposing barrier to leukocyte trafficking, and accordingly, few leukocytes are found in the medial layers beneath plaques. The relative inaccessibility of the lymphatic network may favor egress of monocyte-derived cells through the overlying arterial endothelium, returning the escaping phagocyte to the bloodstream. Escape of monocyte-derived cells in general appears to be rare in plaques that progress to greater size and complexity, and instead this migratory process may mainly characterize regressing, or resolving, plaques.
Shortly, following publication of the study discussed earlier [24], two bodies of work highlighted the robust accumulation of leukocytes in the adventitia just beneath plaques [29,30], which had not previously been reported in mouse models of atherosclerosis. The cellular composition of the adventitial response is distinct from the intimal inflammatory reaction, being largely dominated by B and T lymphocytes. Though macrophages and dendritic cells make up a smaller portion of the adventitial inflammation than they do in the intima, emigrated congenically identified CD45+ phagocytes from the transplanted aortae detected in the iliac lymph nodes [24] may very well have been cells that were originally localized within the adventitial tissue rather than the intima, already within accessible range of the lymphatics (Fig. 1). By contrast, the accumulation of cells in the hepatic lymph node much more likely derived from intimal plaques. Thus, a caveat of the surgical approach to trace migratory fate is that the behavior of adventitial cells carried along in the transplant cannot be readily distinguished from intimal plaque cells and the surgically induced remodeling of the tissue, including blood and lymphatic vessels in the adventitia and altered flow properties within the lumen of the aortic graft, may substantially modify the outcome quantitatively or qualitatively. CCR7 is a GPCR required for immune cell trafficking through afferent lymph [31,32]. Its role in egress from plaques has been described using the surgical model of atherosclerosis regression [33]. It will be important to determine in the future if CCR7 is a mediator primarily of the movement of cells from the adventitia to nearby lymphatics or whether it also directly mediates the egress of cells from the plaque intima.
Recently, we have designed an alternative technique to monitor specifically the migratory behavior of phagocytes within plaques that avoids the caveats of the surgical approach. The principle of the approach rests on tracking whether a nondegradable label brought into plaques by monocytes persists or disappears from plaques over time (Fig. 2). The method first takes advantage of the high phagocytic/macropinocytic capacity of monocytes by labeling them with fluorescent latex beads intravenously [34,35•]. The technique is akin to a pulse-chase experiment, in which the prolonged ‘pulse’ phase is comprised the period of time in which monocytes carry the bulk of the label in the circulation and could be recruited to tissues, including but not limited to atherosclerotic plaques. Because latex-labeled monocytes undergo an expected turnover from the circulation, the ‘pulse phase’ in mice with atherosclerosis lasts for about 5 days [35•]. Following this period, during the ‘chase phase,’ one can quantify the accumulation of label within plaques over time. If no phagocytes egress from plaques, then the quantity of label would persist indefinitely at the plateau reached after the pulse phase. However, if phagocytes leave plaques, some of the labeled phagocytes may be among them, and a loss of label would necessarily indicate egress from plaques, as the latex beads are not degraded and they could not be lost from plaques if the monocyte-derived cell that brought the label into the plaque died. Ongoing application of this approach in my laboratory suggests that the overall conclusions drawn from the surgical model will be upheld, including the conclusion that emigration from plaques does not occur during disease progression, but can be induced by atheroprotective therapies.
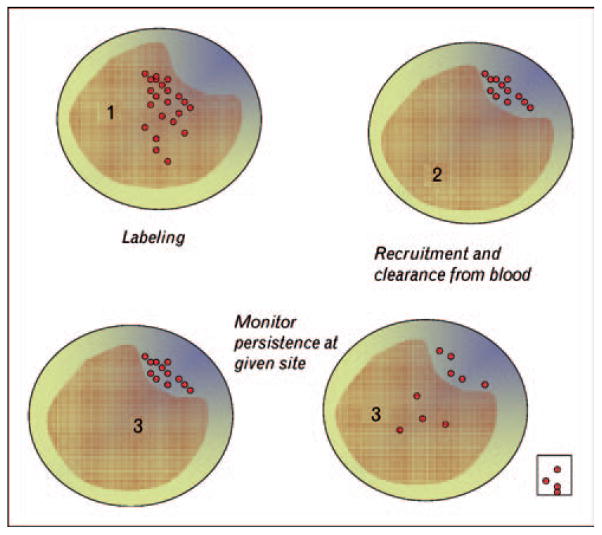
Cartoon depicts cross-sections of an artery containing a raised area corresponding with an atherosclerotic plaque. Nonbiodegradable latex beads are used to label circulating monocyte subsets in mice (labeled monocytes depicted as red spheres, time point 1). These monocytes carry the label out of the bloodstream as they enter tissues, such as inflamed atherosclerotic plaques. Labeled monocytes are largely cleared from the circulation during a 5-day time period (time point 2), as they are recruited to tissues and undergo normal turnover. Because the label cannot be lost from plaques if monocyte-derived cells die, any subsequent loss of the label within plaques after an initial plateau during recruitment would indicate egress of phagocytes from the plaque (time point 3, right). This egress may occur through the bloodstream or involve migration to distal sites like lymph nodes (time point 3, boxed area). By contrast, persistence of the label to the same level as the initial plateau following recruitment would indicate little to no emigration out of plaques (time point 3, left).
Possible mechanisms of trapping within lesions
The mechanisms that limit exodus of monocyte-derived cells from lesions remain largely to be explored in vivo. As discussed above, one cause of limited egress may stem from the unusual anatomy of the intima that is separated from afferent lymphatic vessels by a significant barrier for migration [26], thus cutting off would be emigrants from easy access to the typical route of mobilization from tissues. However, anatomical limitations are not likely the entire problem, given that egress can be restored to some extent when the conditions that drive progressive disease are lifted. Lipid-derived signals are strong candidates for impairing migration, and oxidized phospholipids along with LPA are disease-relevant mediators that may shift the fate of monocyte-derived cells to a more sessile phenotype [24,36] and promote retention in plaques. Cholesterol loading, especially when free cholesterol accumulates in the plasma membrane, suppresses macrophage chemotaxis in vitro by preventing appropriate activation of RhoA [37]. Beyond lipids, other features of the plaque, such as the expression of CX3CL1 by plaque smooth muscle cells may retain CX3CR1+ macrophages [38].
Who’s who: macrophages versus dendritic cells in plaques
Because cells with a dendritic cell character rather than classical macrophages readily traffic out of tissues to lymph nodes through lymphatics, the migratory fate of the monocyte-derived cell that enter plaques would be expected to be influenced by their differentiation to dendritic cells versus macrophages. So, then the question becomes one of how these two patterns of differentiation can be distinguished in tissues. In mice, one of the classical markers for dendritic cells is CD11c [39]. High expression of CD11c is a reliable way to distinguish macrophages from dendritic cells in lymphoid organs [39,40], and it is an appealing marker for dendritic cells because its expression level is not regulated by dendritic cell maturation; both immature, or resting dendritic cells, and mature, or activated dendritic cells, express it. However, it is important to be aware that much of the work on dendritic cells in vivo has focused on dendritic cells in lymphoid organs: spleen, lymph nodes, and thymus. Although it is tempting to assume that the features of the dendritic cells in these organs might be readily extrapolated to peripheral organs and sites of inflammation, like atherosclerotic plaques, this assumption may be false. Indeed, the developmental origins of dendritic cells in lymphoid organs versus peripheral organs may be distinct [41], with monocytes participating only in the latter. It is already well established that CD11c is not a useful marker for distinguishing macrophages and dendritic cells in the lung, as both cell types express high levels [42–45]. Our recent experience in comparing potential macrophages and dendritic cells in a variety of peripheral organs suggests that each organ expresses a pattern of markers distinct from other organs and different from spleen and lymph nodes, making it very difficult to assign ‘universal’ markers of dendritic cells versus macrophages in tissues beyond the well characterized primary and secondary lymphoid organs. Thus, caution should be raised in applying CD11c, or any other single marker, as a dendritic cell marker in atherosclerotic plaques. The CD11c+ cells observed to accumulate in the intima of plaque-prone regions may be dendritic cells [16], but they also resemble a subset of CD11c+ monocytes that expresses low levels of CCR2 and Gr1/Ly6C [35•,46]. That dendritic cells are superior to macrophages in emigrating from tissues [9,10,47], especially through lymphatics, suggests to us that the mere capacity to egress from a lesion is a ‘dendritic cell-like’ characteristic, but beyond that, it remains uncertain just how closely plaque monocyte-derived cells resemble what immunologists and inflammation biologists would consider to be typical dendritic cells or macrophages. It will be an exciting challenge in the future to determine whether development of a particular phenotype within a plaque closely correlates with a phagocyte that will possess the capacity to leave it.
Conclusion
Monocyte-derived cells are cleared from inflammatory reactions that resolve by migrating out of the tissue, typically by converting to cells resembling dendritic cells and accessing lymph nodes through lymphatic vessels. In atherosclerosis, this egress from plaques is blocked, highlighting the failure of resolution mechanisms to operate in the disease. Failed egress likely stems from multiple contributing factors ranging from the specialized anatomical features of the artery to the role of lipid mediators in impairing motility. Impaired egress would be expected to compound macrophage accumulation within plaques, contribute to build-up of necrotic pools, and explain in part the reticence of many plaques to regress, or resolve. Restoration of the capacity of monocyte-derived cells to leave plaques would, by contrast, be expected to facilitate regression. However, it is also possible that at least in some cases, egress of monocyte-derived dendritic cell-like cells from plaques with a fragile morphology, especially egress at the arterial lumen, may also contribute to plaque disruption or rupture [48,49] rather than lead to a protective benefit.
Acknowledgments
I am grateful to my colleagues Edward Fisher, Claudia Jakubzick, Jaime Llodra, Veronique Angeli, Frank Tacke, and Ted Kaplan for their important contributions to work discussed in this review. Past and ongoing work carried out in the author’s laboratory discussed in this review has been supported by NIH grants HL069446, AI049653, AI061741, HL084312, and an Established Investigator Award from the American Heart Association (0740052).
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 527).
Full text links
Read article at publisher's site: https://doi.org/10.1097/mol.0b013e32830d5f09
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2652166?pdf=render
Subscription required at www.co-lipidology.com
http://content.wkhealth.com/linkback/openurl?issn=0957-9672&volume=19&issue=5&spage=462
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1097/mol.0b013e32830d5f09
Article citations
Exploring the Association Between Systemic Lupus Erythematosus and High-Density Lipoproteins: A Systematic Review and Meta-Analysis.
ACR Open Rheumatol, 6(10):648-661, 19 Jul 2024
Cited by: 1 article | PMID: 39030864 | PMCID: PMC11471950
Review Free full text in Europe PMC
Hypoxia inducible factor-1α is an important regulator of macrophage biology.
Heliyon, 9(6):e17167, 09 Jun 2023
Cited by: 6 articles | PMID: 37484306 | PMCID: PMC10361316
Review Free full text in Europe PMC
Macrophage Involvement in Aging-Associated Skeletal Muscle Regeneration.
Cells, 12(9):1214, 22 Apr 2023
Cited by: 10 articles | PMID: 37174614 | PMCID: PMC10177543
Review Free full text in Europe PMC
Macrophage Anti-inflammatory Behaviour in a Multiphase Model of Atherosclerotic Plaque Development.
Bull Math Biol, 85(5):37, 29 Mar 2023
Cited by: 3 articles | PMID: 36991234 | PMCID: PMC10060284
Response by Owens and Deaton to Letter Regarding Article, "Dichotomous Roles of Smooth Muscle Cell-Derived MCP1 (Monocyte Chemoattractant Protein 1) in Development of Atherosclerosis".
Arterioscler Thromb Vasc Biol, 43(1):e64, 21 Dec 2022
Cited by: 0 articles | PMID: 36542726 | PMCID: PMC9976800
Go to all (87) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Divergent JAM-C Expression Accelerates Monocyte-Derived Cell Exit from Atherosclerotic Plaques.
PLoS One, 11(7):e0159679, 21 Jul 2016
Cited by: 14 articles | PMID: 27442505 | PMCID: PMC4956249
Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes.
J Exp Med, 200(10):1231-1241, 08 Nov 2004
Cited by: 199 articles | PMID: 15534368 | PMCID: PMC2211916
Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques.
Proc Natl Acad Sci U S A, 101(32):11779-11784, 27 Jul 2004
Cited by: 368 articles | PMID: 15280540 | PMCID: PMC511052
New Classification of Macrophages in Plaques: a Revolution.
Curr Atheroscler Rep, 22(8):31, 18 Jun 2020
Cited by: 7 articles | PMID: 32556603
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (4)
Grant ID: R01 HL069446
Grant ID: HL084312
Grant ID: R01 HL084312
Grant ID: HL069446
NIAID NIH HHS (6)
Grant ID: AI061741
Grant ID: R01 AI061741
Grant ID: AI049653
Grant ID: R01 AI049653
Grant ID: R01 AI049653-07
Grant ID: R01 AI061741-01A2





