Abstract
Free full text

Epithelial Origin of Myofibroblasts during Fibrosis in the Lung
Abstract
An understanding of the mechanisms underlying pulmonary fibrosis remains elusive. Once believed to result primarily from chronic inflammation, it is now clear that inflammation and chronic fibrosis, especially in diseases such as idiopathic pulmonary fibrosis/usual interstitial pneumonia, are often dissociated, and that inflammation is neither necessary nor sufficient to induce fibrosis. The origin of the primary effector cell of fibrosis in the lung, the myofibroblast, is not clearly established. Three potential sources have been hypothesized. Although conversion of resident fibroblasts and differentiation of circulating bone marrow–derived progenitors likely play a role, the possible contribution of alveolar epithelial cells (AECs), through a process termed “epithelial–mesenchymal transition” (EMT), has only recently received consideration. A process by which epithelial cells lose cell–cell attachment, polarity and epithelial-specific markers, undergo cytoskeletal remodeling, and gain a mesenchymal phenotype, EMT plays a prominent role in fibrogenesis in adult tissues such as the kidney. This review summarizes the evidence supporting a central role for EMT in the pathogenesis of lung fibrosis, the potential for EMT in AECs in vitro and in vivo and role of transforming growth factor-β1 in this process, and the implications of epithelium-driven fibrosis on future research and treatment. Potential pathways involved in EMT are also discussed. It is hoped that a major shift in current paradigms regarding the genesis of pulmonary fibrosis and dissection of the relevant pathways may allow development of targeted interventions that could potentially reverse the process and ameliorate the debilitating effects of abnormal repair and progressive fibrosis.
Changes in paradigms, although slow and usually difficult, are fundamental to most significant advances in science. Such a paradigm change may be underway in the current investigation into mechanisms of chronic injury and fibrosis in the lung. Historically, idiopathic pulmonary fibrosis/usual interstitial pneumonia (IPF/UIP) has been viewed as the result of ongoing inflammation and cellular injury, with subsequent activation and proliferation of resident mesenchymal elements in the lung (1). Observations from both animal models of fibrosis and patients with IPF have led to recent reassessment of the concept that inflammation is the major pathogenic event in IPF. Inflammation is not a prominent feature in IPF biopsies and antiinflammatory therapies have shown little benefit in the treatment of IPF. αvβ6 integrin knockout mice that cannot activate transforming growth factor (TGF)-β1 develop an exaggerated inflammatory response to bleomycin, yet have near-complete attenuation of the fibrotic response, indicating that inflammation and fibrosis can be dissociated (2). These and other observations have contributed to the evolving view that IPF is a disorder that involves abnormal wound healing, and that ongoing epithelial injury and/or activation may lie at the heart of fibrogenesis and mesenchymal cell proliferation, independent of inflammation. This hypothesis has been advanced in a number of recent reviews (3, 4). This article outlines the evidence that supports a central role for the alveolar epithelium in the development of IPF and reviews recent data that suggest that, more than just serving as the pathogenic instigator, alveolar epithelial cells (AECs) themselves may acquire a mesenchymal phenotype and serve as an important source of fibroblasts and myofibroblasts through a process known as epithelial–mesenchymal transition (EMT). Under this new paradigm, the alveolar epithelium should be viewed as one of the key participants in fibrosis, serving as a “multipotent” progenitor with considerable plasticity and the capacity to participate in alternate pathways: re-epithelialization to restore normal alveolar architecture, apoptosis, or fibrogenesis through EMT. Understanding the factors that determine cell fate decisions of AECs along these pathways will be important in further elucidating the pathogenesis of IPF.
MYOFIBROBLASTS ARE KEY MEDIATORS OF FIBROSIS IN THE LUNG
The myofibroblast is believed to play a central role in the pathogenesis of IPF. Increased numbers of fibroblastic foci are associated with disease progression and a worse prognosis in IPF/UIP (5), and the rapid development of fibrotic lesions composed of proliferating myofibroblasts and fibroblasts underlies the pathogenesis of irradiation-induced pulmonary fibrosis (6). These activated fibroblasts are characterized by a spindle or stellate morphology with intracytoplasmic stress fibers, a contractile phenotype, expression of various mesenchymal immunocytochemical markers (including, most reliably, α-smooth muscle actin [α-SMA]), and collagen production (7). They are the key mediators of extracellular matrix deposition, structural remodeling, and destruction of alveocapillary units during and after lung injury (8), and as such, knowledge of their cellular source is critical to the understanding of the pathogenesis of IPF in particular and fibrosis of the lung in general. Three hypotheses have been proposed with regard to the cellular origin of the myofibroblast. The first, and historically most prevalent, hypothesis postulates that resident intrapulmonary fibroblasts respond to a variety of stimuli during fibrogenic responses and differentiate into myofibroblasts (8). TGF-β1, a key regulator of fibrosis, induces transdifferentiation of fibroblasts in vitro through a Smad3-dependent mechanism (9). Although this hypothesis is tempting in its simplicity, an alternate hypothesis has recently been proposed that bone marrow–derived progenitors contribute to myofibroblast induction and proliferation during pulmonary fibrosis. Epperly and colleagues (6) demonstrated using transplantation of green fluorescent protein–positive bone marrow into wild-type mice that marrow-derived cells constitute 20 to 50% of cells in fibrotic areas during irradiation-induced fibrosis. Direkze and colleagues (10) demonstrated multiple organ engraftment by bone marrow–derived fibroblasts and myofibroblasts in mice after radiation injury. Consistent with these results, Hashimoto and colleagues (11) showed that collagen-producing lung fibroblasts in bleomycin-induced pulmonary fibrosis can be derived from bone marrow progenitor cells. However, these marrow-derived fibroblasts did not express α-SMA and were resistant to fibroblast–myofibroblast conversion by TGF-β1. A novel third possible source of fibroblasts and/or myofibroblasts in pulmonary fibrosis has recently been proposed: that AECs, through the process of EMT, also play a significant role. It is important to stress here that these potential sources of myofibroblasts are not mutually exclusive and the relative contribution of each source to the progression of fibrosis remains to be determined.
EMT
EMT is a process by which fully differentiated epithelial cells undergo phenotypic transition to fully differentiated mesenchymal cells, often fibroblasts and myofibroblasts (12). This is a form of metaplasia, but does not always require cell division. For clarity, it is important, especially in the case of the alveolar epithelium, to distinguish this type of transition from epithelial–epithelial transdifferentiation processes, which classically refer to differentiated cells changing into other differentiated cells (13). Although transdifferentiation of one AEC type to another (e.g., type II [AT2] to type I [AT1]) is well described (14, 15), complete phenotypic switching of fully differentiated alveolar epithelium across embryonic lineages has been believed until recently to be unlikely. However, the notion that many adult cell types can exhibit considerable phenotypic plasticity is being increasingly accepted (13).
The process of EMT has long been known to play a pivotal role in cellular transdifferentiation during development and tumor progression. Epiblasts undergo EMT early in development to form primary mesenchyme. Secondary epithelia are created through mesenchymal–epithelial transitions. These secondary epithelia then differentiate to form fully differentiated adult epithelia, or can undergo a second round of EMT to form a variety of mesenchymal and connective tissue cells, such as adipocytes, chondrocytes, osteoblasts, myocytes, and fibroblasts (16). One of the critical aspects of EMT is the ability of epithelial cells to lose polarity, disassemble cell adhesion systems, produce cell-motility machinery, and move from one location to another (12).
Increasingly, it is being recognized that, in the adult, injury can induce epithelial cells to undergo transition to a mesenchymal phenotype, thereby contributing to fibrosis in a number of organs (17, 18). Fibroblasts and myofibroblasts that have differentiated from epithelium are commonly identified in these tissues through morphologic changes (e.g.. a change from a cuboidal cell shape to an elongated or spindle-shaped form), the acquisition of fibroblast- or myofibroblast-specific markers (e.g., fibroblast-specific protein [FSP1] and α-SMA, respectively), and the loss of characteristic epithelial markers (e.g., E-cadherin and zonula occludens-1 [ZO-1]) (19). EMT has been most extensively investigated as a mechanism underlying fibrosis in renal and lens epithelium. Renal tubular epithelial cells express FSP1 (a member of the S100 family of calcium-binding proteins exclusively expressed in fibroblasts) early after injury during kidney fibrosis in transgenic mice, migrate through damaged basement membranes into the interstitium, and fully transdifferentiate into fibroblasts and myofibroblasts (16, 20). In this setting, 36% of new fibroblasts come from EMT of local epithelium (17). In vitro, rat kidney tubular epithelial cells treated with TGF-β1 lose expression of the epithelial marker E-cadherin, acquire an elongated shape, and increase expression of α-SMA (20). Lens epithelial cells have also been shown to undergo EMT in vivo and in response to TGF-β in vitro, likely via a Smad3-dependent pathway (18). Clearly, EMT may play a pivotal role in the normal differentiation processes of adult tissues and in response to injury.
In nearly every case of EMT in adult tissues, a crucial role for the stimulatory input of soluble growth factors and/or extracellular matrix components (usually collagen) has been demonstrated. Most commonly, epidermal growth factor (EGF), hepatocyte growth factor (HGF), fibroblast growth factors (FGF), and especially the TGF-β family of factors are directly involved (16, 21). Under the influence of these factors, epithelial cells lose polarity, express basement membrane–degrading matrix metalloproteinases, undergo cytoskeletal rearrangements, and express machinery necessary for motility, which often leads to migration and complete transition to a mesenchymal phenotype (16, 19). Whether this transition is irreversible and represents true transdifferentiation has been debated, with some suggestion of an epithelial “phenocopy,” termed “reversible scatter,” being an alternative possibility (19). This reversible scatter is defined as epithelial cells undergoing partial dedifferentiation with loss of polarity and down-regulation of epithelial markers, but losing these characteristics once the inciting stimulus is removed. Importantly, cells undergoing “scatter” do not express appreciable amounts of mesenchymal markers. EGF, HGF, FGF, and TGF-β1 alone often induce a reversible scatter phenomenon. However, prolonged exposure to TGF-β1 (> 4–6 d) is a powerful inducer of complete EMT in many cases, often acting in conjunction with a variety of costimuli (including EGF and activation of the small GTPases Ras and RhoA) (22, 23).
ROLE OF TGF-β IN EMT
TGF-β has been implicated as a “master switch” in the induction of fibrosis in many organs, including the lung (24). Targeted expression of TGF-β1 alone in the lungs of newborn and adult rats induces a dramatic fibrotic response with minimal inflammation (25) and, as discussed above, inability to activate TGF-β1 affords significant protection from bleomycin-induced fibrosis in transgenic mice (2). It makes intuitive sense that TGF-β1 would also play a pivotal role in the induction of EMT, given the progressively more apparent role of EMT in fibrotic processes, the key role of TGF-β1 in fibrosis, and the ability of TGF-β1 to promote loss of the epithelial phenotype. Most commonly, TGF-β1 stimulation of epithelial cells leads to the induction of Smad proteins, which serve both as transcription factors themselves and as inducers of other transcription factors, including Slug, Snail, Scatter, lymphoid enhancing factor-1, and β-catenin (16). These transcription factors lead to expression of the “EMT proteome,” including the cellular machinery necessary for junctional disassembly, cytoskeletal rearrangement, and cellular motility (12). The majority of Smad-dependent target gene transcription is controlled by Smad3 (26), which partners with Smad4 on activation by TGF-β receptor serine/threonine kinases and translocates to the nucleus. EMT in many tissues, including retina, lens, and kidney, is dependent on Smad3 (18, 26).
TGF-β1 can also activate non–Smad-mediated cellular signaling pathways, most importantly involving Rho kinase, which directly activates the cellular machinery necessary for cytoskeletal rearrangement, basement membrane detachment, and E-cadherin down-regulation (27). In most cases, stimulation of these cooperative signaling pathways provides the important physiologic context that allows for induction and specification of EMT within particular tissues. Cross-talk between classical TGF-β pathways and Rho, as well as a host of other modulatory signaling molecules, including Ras, extracellular signal-related kinase (ERK), p38 mitogen-activated proteins kinase (MAPK), Notch, Wnt proteins, nuclear factor-κB, and PI3K, have all been demonstrated to affect the extent and reversibility of EMT (12).
ROLE OF THE ALVEOLAR EPITHELIUM IN FIBROGENESIS
Classically, the alveolar epithelium has been thought of as a passive bystander in the process of pulmonary fibrosis. Recently, there has been a return to the notion proposed by Adamson and colleagues (28) that ongoing AEC injury and retarded wound repair may be central to the pathogenesis of pulmonary fibrosis (3, 4). These authors demonstrated that epithelial injury and blunted epithelial repair is sufficient to promote pulmonary fibrotic processes (28). The extent of hyperoxia-induced fibrosis in cultured murine lung explants correlated directly with the degree of epithelial injury, and inflammatory mechanisms involving alveolar macrophages or polymorphonuclear cells were unnecessary. Consistent with this, AEC apoptosis is detected adjacent to myofibroblast-containing fibroblastic foci, the presumed primary sites of epithelial injury in IPF/UIP. Ongoing apoptosis is believed to be a key component in the progression of IPF/UIP (28, 29) and appears to be essential for the development of TGF-β1–induced lung fibrosis (30).
It has long been recognized that the epithelial cells overlying fibroblastic foci are hyper- and dysplastic, with abnormal morphology and gene expression patterns (1, 31). These cells secrete a variety of profibrotic cytokines, participating in a bidirectional communication network with neighboring fibroblasts whereby each cell type influences the proliferation/survival of the other. The alveolar epithelium serves as a major source of TGF-β1 and many other cytokines, including endothelin-1 and tumor necrosis factor-α, during lung injury and fibrosis (32–34), independent of proinflammatory mediators (35). Instead, changes in and activation of epithelial cells appear to be critical inciting factors in fibrosis initiation. The alveolar epithelium also regulates an intrinsic capacity to respond to TGF-β1 stimulation through differential expression of TGF-β1 receptor subtypes (36). Taken together, these data suggest that the alveolar epithelium plays a major role in the pathogenesis of lung fibrosis, with the capacity to both produce and respond to TGF-β1, regulate the function and differentiation of fibroblasts, and modify cell morphology and gene expression in response to injury, all independent of the degree of inflammation.
The exact nature of the epithelial injury in IPF/UIP is unknown, although it has been speculated that viral infection may play a role. As discussed below, for re-epithelialization to occur, AT2 cells must proliferate and differentiate into AT1 cells. In IPF, this process appears to be impaired, with detection of abnormal, hyperplastic AT2 cells with an intermediate phenotype overlying fibroblastic foci (31). Thus, depending perhaps on the degree and nature of the injury, extent of disruption of underlying basement membrane, and the exact cytokine milieu, injured AECs may face one of several choices: apoptosis; proliferation and differentiation into AT1 cells to effect re-epithelialization; or, as has been recently suggested, EMT, thereby contributing directly to fibrosis.
EMT IN LUNG
The critical importance of the alveolar epithelium and epithelial injury to the process of fibrosis, together with the crucial role for EMT in fibrogenesis in other tissues, naturally raise the question of whether EMT contributes to the pathogenesis of fibrosis in the lung. This possibility seems especially inviting when considering the role of the “multipotent” AT2 cell after injury and recent demonstrations of AEC plasticity. AT2 cells are believed to serve as the progenitors for repair of the alveolar epithelium after injury, being capable of both self-renewal and of giving rise to AT1 cells through a process of transdifferentiation (37). Similar to observations in vivo, AT2 cells in primary culture lose their phenotypic hallmarks and gradually acquire the morphologic features of AT1 cells (38). The cells increasingly express all available AT1 cell phenotypic markers, suggesting that AT2 cells in vitro transdifferentiate toward an AT1 cell phenotype (AT1-like cells) (15, 39). Experimental conditions have also been developed that induce cells that have acquired AT1 cell characteristics to revert to an AT2 cell phenotype (39), suggesting far greater plasticity in expression of the differentiated AEC phenotypes than previously believed. This remarkable phenotypic plasticity suggests that, under certain conditions in which transition to an AT1 cell phenotype is inhibited (e.g., injury), AT2 cells may also have the capacity to undergo transition to fibroblasts and myofibroblasts through the process of EMT.
There have been hints that this may in fact be the case. AECs overlying fibroblastic foci in IPF/UIP appear histologically somewhat similar to fibroblasts (4). Iyonaga and colleagues (40) detailed marked changes in cytokeratin expression patterns in AECs from patients with IPF/UIP, and noted that epithelial cells in IPF lung tissues may be fundamentally different in function and nature. AECs exposed to TGF-β1 down-regulate surfactant protein C production and express extracellular matrix components, such as fibronectin (41). One important study demonstrating Wnt pathway activation in IPF/UIP postulated that some fraction of the abnormal fibroblasts in IPF/UIP could directly derive from epithelial cell precursors at sites of ongoing injury and repair (42). These findings suggest a significant alteration in AEC differentiation state and function during fibrosis and in response to fibrogenic stimuli.
Recently, our group directly addressed the hypothesis that AECs undergo EMT. We demonstrated that pure cultures of primary AECs, as well as an AEC line (RLE-6TN), undergo EMT in response to extended exposure to TGF-β1 in culture (43). TGF-β1 causes loss of epithelial cell markers such as aquaporin-5, cytokeratins, and ZO-1, while dramatically up-regulating mesenchymal cell markers, including α-SMA, vimentin, desmin, and type I collagen, concurrent with transition to a fibroblast-like morphology. Analysis of the transition over time reveals a gradual increase in α-SMA expression concomitant with a progressive increase in longitudinal stress fiber formation (Figure 1). This gradual change parallels a decrease in nuclear expression of thyroid transcription factor (TTF)-1, an epithelial-specific transcription factor. Complete transition in primary cells requires prolonged exposure to TGF-β1 of nearly 2 wk, although coexpression of epithelial and mesenchymal markers can be seen as early as Day 4 after addition of TGF-β1. We also examined tissue from patients with advanced IPF/UIP. Over 80% of the hyperplastic epithelial cells overlying fibroblastic foci were noted to coexpress epithelial (TTF-1 and pro–surfactant protein B) and mesenchymal (α-SMA) markers (Figure 2), suggesting that they may have been undergoing EMT at the time of tissue sampling. Altogether, these findings conclusively demonstrate the occurrence of EMT in AECs in vivo, and suggest that EMT may play an important part in the pathogenesis of IPF/UIP and other fibrotic lung disorders.
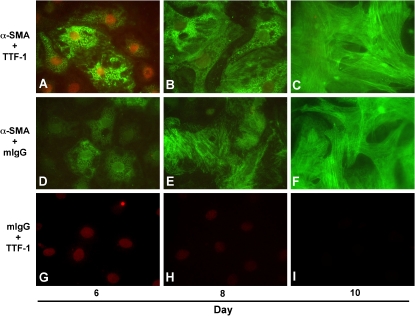
Alveolar epithelial–mesenchymal transition (EMT) in vitro. Colocalization of mesenchymal (α-smooth muscle actin [α-SMA]) and epithelial (thyroid transcription factor [TTF]-1) markers in primary alveolar epithelial cells (AECs) during EMT. Immunoreactivity for α-SMA (green) and TTF-1 (red) was assessed on Days 6, 8, and 10 of primary culture of AECs in minimal defined serum-free medium (MDSF) + TGF-β1. On Day 6, individual AECs are identified that coexpress both nuclear TTF-1 and cytoplasmic α-SMA. Expression of α-SMA increased gradually over time in culture (A–C, D–F), and paralleled a concomitant decrease in expression of TTF-1 (A–C, G–I), along with transition from epithelial- to fibroblast-like morphology. Reprinted by permission from Reference 43.
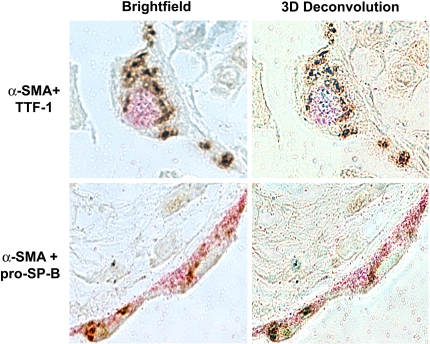
Evidence for alveolar epithelial EMT in vivo. Myofibroblast and AEC markers colocalized in approximately 80% of AECs overlying fibroblastic foci in lung tissue from humans with idiopathic pulmonary fibrosis using three-dimensional deconvolution microscopy. Both pro–surfactant protein B (pro–SP-B) and TTF-1 (pink) colocalized with α-SMA (brown) to the same optical section in all cells analyzed. Reprinted by permission from Reference 43.
These findings have recently been corroborated by the work of a number of other investigators. Similar changes in epithelial morphology were observed after transduction of rat lung explants with a retroviral vector encoding TGF-β1 (34). Using isolated rat type II cells in primary culture, Yao and colleagues (44) demonstrated that treatment with TGF-β1 results in cytoskeletal rearrangements, assumption of a fibroblast-like morphology, down-regulation of E-cadherin, increased collagen I production, and induction of α-SMA, indicative of EMT. A549 cells, a human lung epithelial cell line, also undergo an EMT-like process in response to TGF-β1, as evidenced by increased expression of fibronectin and connective tissue growth factor (CTGF) and loss of E-cadherin, but not in response to tumor necrosis factor-α or interleukin-1β (45). Other recent preliminary work demonstrated that the hyperplastic epithelial cells in areas of fibrosing lung have transcriptional profiles indicative of an EMT, and that the fibroblasts underlying areas of epithelial hyperplasia express significant numbers of epithelial markers (46). Treatment of mouse AT2 cells in primary culture with a combination of TGF-β1 and EGF induced expression of FSP1 with loss of E-cadherin and acquisition of a fibroblast-like morphology (47). Evaluation of biopsy samples from stable lung transplant patients demonstrated markers of EMT in epithelial cells, suggesting that fibroblasts may also originate from airway epithelial cells (48). These recent findings definitively demonstrate the occurrence of EMT in alveolar and possibly airway epithelial cells both in vitro and in vivo, that it is mediated by TGF-β1, and that this process represents a potentially important mechanism of fibroblast and/or myofibroblast production during pulmonary fibrosis and other disorders characterized by epithelial injury and remodeling.
Little is known regarding the downstream cellular mechanisms of EMT in alveolar epithelium. As stated earlier, most TGF-β–mediated EMT responses require Smad-mediated signaling in cooperation with a number of costimuli. Interestingly, Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis (49), but the possible role of Smad3 in the induction of EMT in lung has not been explored. EMT in A549 cells was dependent on Smad2 (45), but its importance in primary cells or in vivo remains to be determined. A growing body of evidence implicates endothelin signaling in fibrogenesis in lung and other tissues (32, 50), but its relationship to EMT in lung is completely unknown. A deeper and more mechanistic understanding of the process of EMT in lung will require elucidation of the importance of these Smad-mediated and non–Smad-mediated signaling pathways in AECs.
CONCLUSIONS
At this point, one can only speculate on the relative contribution of alveolar epithelial EMT to the production of intrapulmonary fibroblasts and/or myofibroblasts during pulmonary fibrotic processes in vivo. However, given that EMT contributes at least one-third of the fibroblast population during fibrosis in other organs, that alveolar epithelial–fibroblastic cross-talk and interactions are involved in fibrosis in the lung, that AECs undergo EMT in vitro in response to TGF-β1 (Figure 2), and that TGF-β1 is expressed at sites of epithelial injury and adjacent fibrosis in vivo, it is likely that conversion of resident AECs to fibroblasts and activated fibroblasts (myofibroblasts) contributes significantly to lung fibrogenesis in vivo (Figure 3). It should be noted, however, that it is unclear at this point whether AT1, AT2, or both AT1 and AT2 pneumocytes can undergo EMT. It is possible that, depending on the degree of injury, AT1 cells may be more likely to undergo apoptosis whereas AT2 cells preferentially undergo EMT. It is also interesting to speculate that certain injurious stimuli may induce EMT rather than apoptosis. In this regard, whether and to what extent EMT is a feature of all pulmonary fibrotic processes also remains to be determined. It will be interesting to establish whether EMT only occurs in diseases such as IPF/UIP in which fibrosis appears to be the result of repetitive injury reflected by patchy fibrosis on biopsy. This could be compared with scleroderma, which has few fibroblastic foci and in which the pathologic changes are relatively homogeneous and appear to be the result of a single “hit.” Elucidation of these issues and the mechanisms underlying EMT in lung await further investigation. The potential for prevention or reversibility of this process is unknown; however, the identification of specific pathways involved in EMT during fibrosis in the lung may allow targeted interventions to be developed that could ameliorate the devastating effects of abnormal repair and fibrosis.
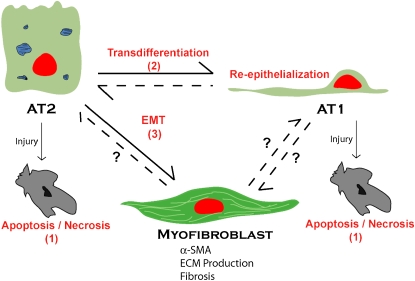
Alveolar epithelial transdifferentiation pathways. AECs demonstrate a previously unappreciated pluripotency. Under normal conditions, alveolar type II (AT2) cells transdifferentiate into alveolar type I (AT1) cells. In vitro, AT1 cells can also transdifferentiate into AT2 cells. Depending on the cellular environment and stimuli, AECs respond to injury by traveling down one of a number of pathways: apoptosis/necrosis (1); proliferation, transdifferentiation, and re-epithelialization (2); or EMT (3) to a myofibroblast phenotype, resulting in extracellular matrix (ECM) deposition, destruction of lung architecture, and fibrosis.
Acknowledgments
The authors thank E.D. Crandall and P.W. Shaul for critically reviewing the manuscript and helpful comments.
Notes
Supported by the Hastings Foundation and by the following grants from the National Institutes of Health: K12 HD47349 (Pediatric Critical Care Scientist Development Program) to B.C.W., and R01 HL38578 and HL62569 to Z.B.
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
Articles from Proceedings of the American Thoracic Society are provided here courtesy of American Thoracic Society
Full text links
Read article at publisher's site: https://doi.org/10.1513/pats.200601-004tk
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2658689?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/110223346
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1513/pats.200601-004tk
Article citations
Understanding myofibroblast origin in the fibrotic lung.
Chin Med J Pulm Crit Care Med, 2(3):142-150, 17 Sep 2024
Cited by: 0 articles | PMID: 39403408 | PMCID: PMC11471099
Review Free full text in Europe PMC
PTGES is involved in myofibroblast differentiation via HIF-1α-dependent glycolysis pathway.
J Cell Mol Med, 28(20):e70157, 01 Oct 2024
Cited by: 0 articles | PMID: 39417702 | PMCID: PMC11484478
Periostin in Bronchiolitis Obliterans Syndrome after Lung Transplant.
Int J Mol Sci, 25(19):10423, 27 Sep 2024
Cited by: 0 articles | PMID: 39408746 | PMCID: PMC11477235
Deficiency of Secreted Phosphoprotein 1 Alleviates Hyperoxia-induced Bronchopulmonary Dysplasia in Neonatal Mice.
Inflammation, 29 Jun 2024
Cited by: 0 articles | PMID: 38951356
Mechanisms and therapeutic research progress in intestinal fibrosis.
Front Med (Lausanne), 11:1368977, 14 Jun 2024
Cited by: 0 articles | PMID: 38947241
Go to all (322) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis.
Am J Pathol, 166(5):1321-1332, 01 May 2005
Cited by: 628 articles | PMID: 15855634 | PMCID: PMC1606388
Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix.
Proc Natl Acad Sci U S A, 103(35):13180-13185, 21 Aug 2006
Cited by: 846 articles | PMID: 16924102 | PMCID: PMC1551904
Detection of epithelial to mesenchymal transition in airways of a bleomycin induced pulmonary fibrosis model derived from an alpha-smooth muscle actin-Cre transgenic mouse.
Respir Res, 8:1, 07 Jan 2007
Cited by: 119 articles | PMID: 17207287 | PMCID: PMC1781437
Recent advances in molecular targets and treatment of idiopathic pulmonary fibrosis: focus on TGFbeta signaling and the myofibroblast.
Curr Med Chem, 16(11):1400-1417, 01 Jan 2009
Cited by: 88 articles | PMID: 19355895
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: R01 HL38578
Grant ID: HL62569
NICHD NIH HHS (1)
Grant ID: K12 HD47349




