Abstract
Free full text

HeLa cell-adherent enteropathogenic Escherichia coli in children under 1 year of age in Thailand.
Abstract
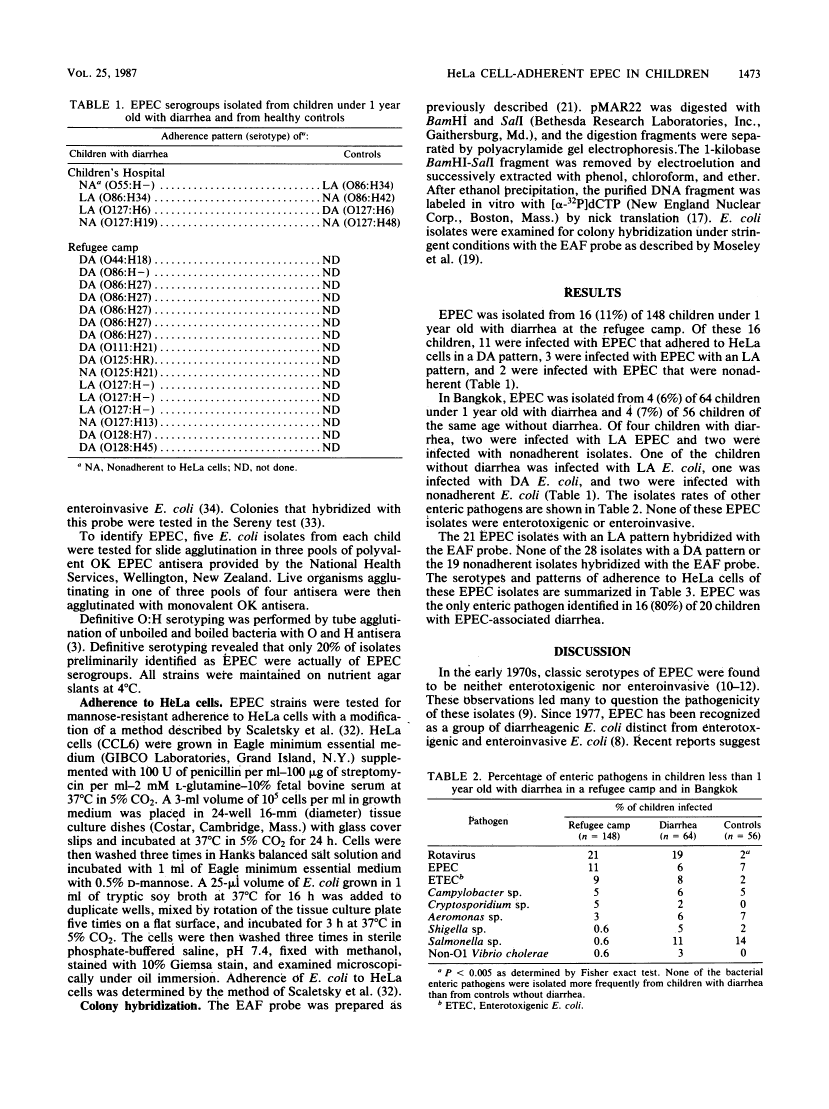
Enteropathogenic Escherichia coli (EPEC) was isolated from 11% of 148 Hmong children under 1 year old with diarrhea at a refugee camp in northern Thailand. Of 16 children with EPEC-associated diarrhea, 11 were infected with EPEC that adhered to HeLa cells in a diffuse pattern, 3 were infected with EPEC that adhered to HeLa cells in a localized adherence (LA) pattern, and 2 were infected with EPEC that were nonadherent. In Bangkok, EPEC was isolated from 6% of 64 children under 1 year old with diarrhea and 7% of 56 children of the same age without diarrhea. Of four children with diarrhea, two were infected with EPEC with an LA pattern, and two were infected with nonadherent EPEC. Of four children without diarrhea, one was infected with EPEC with an LA pattern, one was infected with EPEC that adhered in a diffuse pattern, and two were infected with nonadherent EPEC. The 21 EPEC isolates with an LA pattern hybridized with the EPEC adherence factor DNA probe. EPEC was the only enteric pathogen identified in 16 (80%) of 20 children with EPEC-associated diarrhea. EPEC was as frequently isolated from children under 1 year old as were other bacterial enteric pathogens. The problem of identifying EPEC with pools of polyvalent antisera are described, and the need to identify additional enteropathogenic determinants of EPEC is discussed.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (839K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldini MM, Kaper JB, Levine MM, Candy DC, Moon HW. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2(3):534–538. [Abstract] [Google Scholar]
- Baldini MM, Nataro JP, Kaper JB. Localization of a determinant for HEp-2 adherence by enteropathogenic Escherichia coli. Infect Immun. 1986 Apr;52(1):334–336. [Europe PMC free article] [Abstract] [Google Scholar]
- Bettelheim KA, Thompson CJ. New method of serotyping Escherichia coli: implementation and verification. J Clin Microbiol. 1987 May;25(5):781–786. [Europe PMC free article] [Abstract] [Google Scholar]
- Bronsdon MA. Rapid dimethyl sulfoxide-modified acid-fast stain of Cryptosporidium oocysts in stool specimens. J Clin Microbiol. 1984 Jun;19(6):952–953. [Europe PMC free article] [Abstract] [Google Scholar]
- Clausen CR, Christie DL. Chronic diarrhea in infants caused by adherent enteropathogenic Escherichia coli. J Pediatr. 1982 Mar;100(3):358–361. [Abstract] [Google Scholar]
- Dean AG, Ching YC, Williams RG, Harden LB. Test for Escherichia coli enterotoxin using infant mice: application in a study of diarrhea in children in Honolulu. J Infect Dis. 1972 Apr;125(4):407–411. [Abstract] [Google Scholar]
- Edelman R, Levine MM. From the National Institute of Allergy and Infectious Diseases. Summary of a workshop on enteropathogenic Escherichia coli. J Infect Dis. 1983 Jun;147(6):1108–1118. [Abstract] [Google Scholar]
- Gangarosa EJ, Merson MH. Epidemiologic assessment of the relevance of the so-called enteropathogenic serogroups of Escherichia coli in diarrhea. N Engl J Med. 1977 May 26;296(21):1210–1213. [Abstract] [Google Scholar]
- Goldschmidt MC, DuPont HL. Enteropathogenic Escherichia coli: lack of correlation of serotype with pathogenicity. J Infect Dis. 1976 Feb;133(2):153–156. [Abstract] [Google Scholar]
- Gross RJ, Scotland SM, Rowe B. Enterotoxin testing of Escherichia coli causing epidemic infantile enteritis in the U.K. Lancet. 1976 Mar 20;1(7960):629–631. [Abstract] [Google Scholar]
- Herrmann JE, Blacklow NR, Perron DM, Cukor G, Krause PJ, Hyams JS, Barrett HJ, Ogra PL. Enzyme immunoassay with monoclonal antibodies for the detection of rotavirus in stool specimens. J Infect Dis. 1985 Oct;152(4):830–832. [Abstract] [Google Scholar]
- Leksomboon U, Echeverria P, Suvongse C, Duangmani C. Viruses and bacteria in pediatric diarrhea in Thailand: a study of multiple antibiotic-resistant enteric pathogens. Am J Trop Med Hyg. 1981 Nov;30(6):1281–1290. [Abstract] [Google Scholar]
- Levine MM, Nataro JP, Karch H, Baldini MM, Kaper JB, Black RE, Clements ML, O'Brien AD. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985 Sep;152(3):550–559. [Abstract] [Google Scholar]
- Maniatis T, Jeffrey A, Kleid DG. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. [Europe PMC free article] [Abstract] [Google Scholar]
- Moon HW, Whipp SC, Argenzio RA, Levine MM, Giannella RA. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983 Sep;41(3):1340–1351. [Europe PMC free article] [Abstract] [Google Scholar]
- Moseley SL, Echeverria P, Seriwatana J, Tirapat C, Chaicumpa W, Sakuldaipeara T, Falkow S. Identification of enterotoxigenic Escherichia coli by colony hybridization using three enterotoxin gene probes. J Infect Dis. 1982 Jun;145(6):863–869. [Abstract] [Google Scholar]
- Nataro JP, Baldini MM, Kaper JB, Black RE, Bravo N, Levine MM. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J Infect Dis. 1985 Sep;152(3):560–565. [Abstract] [Google Scholar]
- Nataro JP, Scaletsky IC, Kaper JB, Levine MM, Trabulsi LR. Plasmid-mediated factors conferring diffuse and localized adherence of enteropathogenic Escherichia coli. Infect Immun. 1985 May;48(2):378–383. [Europe PMC free article] [Abstract] [Google Scholar]
- O'Brien AD, LaVeck GD, Thompson MR, Formal SB. Production of Shigella dysenteriae type 1-like cytotoxin by Escherichia coli. J Infect Dis. 1982 Dec;146(6):763–769. [Abstract] [Google Scholar]
- Patel JR, Daniel J, Mathan M, Mathan VI. Isolation and identification of enteroviruses from faecal samples in a differentiated epithelial cell line (HRT-18) derived from human rectal carcinoma. J Med Virol. 1984;14(3):255–261. [Europe PMC free article] [Abstract] [Google Scholar]
- Polotsky YE, Dragunskaya EM, Seliverstova VG, Avdeeva TA, Chakhutinskaya MG, Kétyi I, Vertényl A, Ralovich B, Emödy L, Málovics I, et al. Pathogenic effect of enterotoxigenic Escherichia coli and Escherichia coli causing infantile diarrhoea. Acta Microbiol Acad Sci Hung. 1977;24(3):221–236. [Abstract] [Google Scholar]
- Rajan DP, Mathan VI. Prevalence of Campylobacter fetus subsp. jejuni in healthy populations in southern India. J Clin Microbiol. 1982 May;15(5):749–751. [Europe PMC free article] [Abstract] [Google Scholar]
- Reis MH, Matos DP, de Castro AF, Toledo MR, Trabulsi LR. Relationship among enterotoxigenic phenotypes, serotypes, and sources of strains in enterotoxigenic Escherichia coli. Infect Immun. 1980 Apr;28(1):24–27. [Europe PMC free article] [Abstract] [Google Scholar]
- Robins-Browne RM, Still CS, Miliotis MD, Richardson NJ, Koornhof HJ, Freiman I, Schoub BD, Lecatsas G, Hartman E. Summer diarrhoea in African infants and children. Arch Dis Child. 1980 Dec;55(12):923–928. [Europe PMC free article] [Abstract] [Google Scholar]
- Rothbaum R, McAdams AJ, Giannella R, Partin JC. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology. 1982 Aug;83(2):441–454. [Abstract] [Google Scholar]
- Rothbaum RJ, Partin JC, Saalfield K, McAdams AJ. An ultrastructural study of enteropathogenic Escherichia coli infection in human infants. Ultrastruct Pathol. 1983 Jun;4(4):291–304. [Abstract] [Google Scholar]
- Sack DA, Sack RB. Test for enterotoxigenic Escherichia coli using Y-1 adrenal cells in miniculture. Infect Immun. 1975 Feb;11(2):334–336. [Europe PMC free article] [Abstract] [Google Scholar]
- Scaletsky IC, Silva ML, Toledo MR, Davis BR, Blake PA, Trabulsi LR. Correlation between adherence to HeLa cells and serogroups, serotypes, and bioserotypes of Escherichia coli. Infect Immun. 1985 Sep;49(3):528–532. [Europe PMC free article] [Abstract] [Google Scholar]
- Scaletsky IC, Silva ML, Trabulsi LR. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984 Aug;45(2):534–536. [Europe PMC free article] [Abstract] [Google Scholar]
- Taylor DN, Echeverria P, Pál T, Sethabutr O, Saiborisuth S, Sricharmorn S, Rowe B, Cross J. The role of Shigella spp., enteroinvasive Escherichia coli, and other enteropathogens as causes of childhood dysentery in Thailand. J Infect Dis. 1986 Jun;153(6):1132–1138. [Abstract] [Google Scholar]
- Toledo MR, Alvariza M do C, Murahovschi J, Ramos SR, Trabulsi LR. Enteropathogenic Escherichia coli serotypes and endemic diarrhea in infants. Infect Immun. 1983 Feb;39(2):586–589. [Europe PMC free article] [Abstract] [Google Scholar]
- Toledo MR, Trabulsi LR. Correlation between biochemical and serological characteristics of Escherichia coli and results of the Serény test. J Clin Microbiol. 1983 Mar;17(3):419–421. [Europe PMC free article] [Abstract] [Google Scholar]
- Ulshen MH, Rollo JL. Pathogenesis of escherichia coli gastroenteritis in man--another mechanism. N Engl J Med. 1980 Jan 10;302(2):99–101. [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Clinical Microbiology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jcm.25.8.1472-1475.1987
Read article for free, from open access legal sources, via Unpaywall:
https://jcm.asm.org/content/jcm/25/8/1472.full.pdf
Free to read at intl-jcm.asm.org
http://intl-jcm.asm.org/cgi/content/abstract/25/8/1472
Free after 4 months at intl-jcm.asm.org
http://intl-jcm.asm.org/cgi/reprint/25/8/1472.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
Molecular characterization of Escherichia coli isolated from milk samples with regard to virulence factors and antibiotic resistance.
Vet World, 14(9):2410-2418, 17 Sep 2021
Cited by: 4 articles | PMID: 34840461 | PMCID: PMC8613785
A localized adherence-like pattern as a second pattern of adherence of classic enteropathogenic Escherichia coli to HEp-2 cells that is associated with infantile diarrhea.
Infect Immun, 67(7):3410-3415, 01 Jul 1999
Cited by: 49 articles | PMID: 10377120 | PMCID: PMC116525
Adhesion and its role in the virulence of enteropathogenic Escherichia coli.
Clin Microbiol Rev, 7(2):152-173, 01 Apr 1994
Cited by: 50 articles | PMID: 8055465 | PMCID: PMC358315
Review Free full text in Europe PMC
Adhesive properties of enteropathogenic Escherichia coli isolated from infants with acute diarrhea in Africa.
Eur J Clin Microbiol Infect Dis, 8(11):979-983, 01 Nov 1989
Cited by: 12 articles | PMID: 2513199
Diarrhoeal disease in children less than one year of age at a children's hospital in Guangzhou, People's Republic of China.
Trans R Soc Trop Med Hyg, 85(5):667-669, 01 Sep 1991
Cited by: 14 articles | PMID: 1781004
Go to all (9) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
HeLa cell adherence and cytotoxin production by enteropathogenic Escherichia coli isolated from infants with diarrhea in Thailand.
J Clin Microbiol, 25(8):1519-1523, 01 Aug 1987
Cited by: 12 articles | PMID: 3305567 | PMCID: PMC269261
Tissue culture-adherent Escherichia coli in infantile diarrhea.
J Infect Dis, 165(1):141-143, 01 Jan 1992
Cited by: 35 articles | PMID: 1727883
Nationwide surveillance program to identify diarrhea-causing Escherichia coli in children in Thailand.
J Clin Microbiol, 28(3):469-472, 01 Mar 1990
Cited by: 8 articles | PMID: 2182667 | PMCID: PMC269646
Traditional enteropathogenic Escherichia coli of infantile diarrhea.
Rev Infect Dis, 9(1):28-53, 01 Jan 1987
Cited by: 118 articles | PMID: 3547577
Review