Abstract
Free full text
Inducible cAMP Early Repressor (ICER) and Brain Functions
Abstract
The inducible cAMP early repressor (ICER) is an endogenous repressor of cAMP-responsive element (CRE)-mediated gene transcription and belongs to the CRE-binding protein (CREB)/CRE modulator (CREM)/activating transcription factor 1 (ATF-1) gene family. ICER plays an important role in regulating the neuroendocrine system and the circadian rhythm. Other aspects of ICER function have recently attracted heightened attention. Being a natural inducible CREB antagonist, and more broadly, an inducible repressor of CRE-mediated gene transcription, ICER regulates long-lasting plastic changes that occur in the brain in response to incoming stimulation. This review will bring together data on ICER and its functions in the brain, with a special emphasis on recent findings highlighting the involvement of ICER in the regulation of long-term plasticity underlying learning and memory.
Introduction
Regulation of gene transcription through cAMP-responsive element (CRE)-mediated mechanisms is one of the important ways an organism and the brain adapt to ever changing environments. Transcription factors from the CRE-binding protein (CREB)/CRE modulator (CREM)/activating transcription factor 1 (ATF-1) gene family bind to CREs in promoter regions of different genes and mediate the response of the cell to extracellular stimuli [1–5]. CREB was the first CRE-binding factor to be characterized [6, 7]. Numerous studies have established a strong connection between CREB and neuronal plasticity [8–11]. However, the outcome of CRE-mediated gene transcription does not solely depend on CREB binding, but on the competitive binding of several dimerized transcription factors, including activators and repressors of gene transcription. Among the members of the CREB/CREM/ATF-1 gene family, the inducible cAMP early repressor (ICER) is unique in that it is not only a potent endogenous repressor of CRE-mediated gene transcription, but also is highly inducible by a variety of stimuli [12, 13]. In contrast, most other members of the family are non-inducible and are ubiquitously expressed [14–16].
Until recently, the main interest in ICER in the field of neuroscience was connected to its role in regulation of the circadian rhythm and neuroendocrine function [17–19]. However, other roles of ICER have recently come under the spotlight, shifting attention to its potential role in the regulation of neuronal plasticities that underlie higher nervous functions, such as learning and memory in particular [20, 21].
ICER Gene Structure and Regulation
Gene Structure
ICER is a product of the CREM gene, and ICER messenger RNAs (mRNAs) are transcribed through an alternative intronic promoter (P2) [12, 13]. The CREM gene contains multiple exons and gives rise to several alternative splicing variants that encode both transcriptional activator and repressor proteins (Fig. 1a) [22, 23]. One of the several interesting features of the CREM gene is that it possesses two DNA-binding domains (DBD I and DBD II), only one of which is included into the final protein.
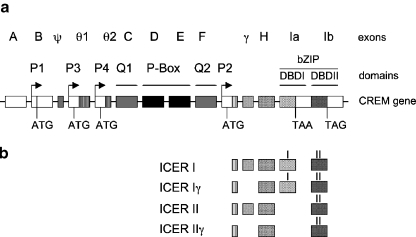
Schematic representation of the gene encoding CREM/ICER. a The intron/exon structure of the CREM/ICER gene. Also shown are CREM promoters (P1, P3, and P4); ICER promoter (P2); glutamine-rich domains (Q1 and Q2); kinase-inducible domain (P-Box); and DNA-binding domains (DBD I and DBD II) of the ICER gene, containing leucine zipper and basic regions (bZIP). ATG, initiation methionine; TAA and TAG, stop codons. b Schematic mRNA structure of four ICER isoforms and corresponding exons. ICER I mRNA contains sequences encoding both DBD I and II, but the stop codon located in the carboxyl terminus of DBD I prevents insertion of DBD II into the protein
Transcription from the constitutively active P1 promoter yields CREMs. CREMs share extensive homology with CREB: a DBD in the carboxyl terminus, and a kinase-inducible domain (P-Box) and a γ-domain (absent in CREMγ) in the amino terminus (Fig. 1a). CREM, τ in addition contains glutamine-rich activation domains (Q1 and Q2).
ICER, on the other hand, is transcribed from the internal P2 promoter (Fig. 1b). ICERs contain a short and conserved N-terminal sequence followed by a γ-domain (absent in γ-isoforms) and one of the DBDs, thus encoding small proteins with the predicted molecular weight of 12 and 13.5 kDa (~15 and ~19 kDa in SDS-PAGE). The two DBDs encoded by the CREM gene are composed of basic and leucine zipper domains, which are involved in both dimerization and DNA binding of CREM and ICER [12, 13, 22].
Isoforms
Alternative splicing of the ICER transcript results in four different isoforms: ICER I, ICER Iγ, ICER II, and ICER IIγ (Fig. 1b). ICER I isoforms contain DBD I, while ICER II isoforms contain DBD II. The two γ isoforms (ICER Iγ and ICER IIγ) lack exon γ. ICER I mRNA contains sequences encoding both DBD I and II, but the stop codon located in the carboxyl terminus of DBD I prevents insertion of DBD II into the protein [23]. All ICER isoforms homodimerize or heterodimerize with other members of the CREB/CREM/ATF-1 family [12, 13, 24, 25].
Repressor Activity
ICERs lack activation domains and the kinase-inducible domain (Fig. 1), but the DBD allows their homo- or heterodimerization and binding to CRE elements. The lack of a transactivation domain makes ICERs potent repressors of cAMP-induced transcription of CRE-containing genes. However, not much is known at the moment about the levels of ICER required to suppress CRE-regulated transcription in vivo. In cultured pinealocytes, co-transfection with ICER completely abolishes cAMP-mediated induction of various CRE-containing reporters, starting at substoichiometric concentrations [13]. Co-transfection of a promoter-luciferase reporter construct of corticotrophin releasing hormone (CRH, also referred as CRF) with ICER I cDNA did not significantly affect basal CRH promoter activity, but dose-dependently inhibited forskolin-stimulated promoter activity [24]. More experiments are required to determine the effectiveness of ICER’s transcription repressor activity in the nervous system.
Regulation
The promoter of ICER (P2) contains a cluster of four CRE-like cAMP autoregulatory elements (CAREs) organized in two tandems, CARE1–CARE2 and CARE3–CARE4. These CAREs are strongly inducible and are recognized by a variety of CRE-binding proteins, including CREB. Phosphorylated CREB binds to CAREs in the P2 promoter and rapidly activates ICER expression [12]. After induction, ICER can compete with CREB for the ICER promoter, thus suppressing its own transcription, which in effect constitutes a negative autoregulatory loop [12]. This negative feedback loop serves as a temporal gene controlling mechanism that allows the cAMP-dependent signaling cascade to prepare for subsequent incoming signals [26, 27].
Different binding affinities of the different CAREs for CREB, CREM, and ICER proteins together with different transcription-driving efficiencies of the CAREs provide additional options for fine gene regulation [12, 28]. Moreover, ICER expression can be activated in a non-CRE-dependent manner [29]. Further characterization of the relationships between different extracellular stimuli, intracellular cascades, and resulting ICER activation will help to advance our understanding of the “fine-tuning” of transcription in response to differential stimulation.
Intracellular levels of ICER protein are controlled by transcription regulation and by protein degradation through the ubiquitin–proteasome system [30]. Different ICER isoforms have different half-lives: ICER I is the most stable followed by ICER II, whereas γ-isoforms are short-lived, with a half-life of ~3 h [30]. The different half-lives of different isoforms may contribute to the temporal regulation of CRE-dependent gene transcription during the course of physiological phenomena.
ICER Distribution and Induction Factors in the Brain
ICER is expressed at uniformly low levels in the central nervous system, with the exception of some neuroendocrine structures, such as the pineal gland and hypothalamic nuclei, and sensory input and relay nuclei including the olfactory bulb and sensory brain stem nuclei [13, 18, 29, 31–33]. However, drastic upregulation of ICER expression has been demonstrated in response to a variety of stimuli [26].
The well-studied signal transduction pathway regulating ICER transcription is the adenylate cyclase–cAMP–cAMP-dependent protein kinase (PKA)–CREB–ICER pathway. Numerous studies have demonstrated ICER induction in a variety of cell cultures after treatments that increase intracellular cAMP and induce PKA activation [12, 13, 24, 28, 34, 35]. ICER can also be induced through PKA-independent pathways such as the Ras-dependent nerve growth factor (NGF) pathway and the Janus kinase/signal transducer and activator of transcription pathway, the protein kinase C (PKC) pathway, as well as other pathways [26, 28, 29, 36]. Interestingly, some cell culture studies showed that histone deacetylase (HDAC) is required for the induction of ICER expression [36, 37]. Application of brain-derived neurotrophic factor (BDNF) and dopamine to neuronal primary cultures also increases ICER levels [29, 35].
In Vivo Induction
Seizure activity strongly induces ICER. Electroconvulsive shock upregulates ICER mRNA in rat cortex and hippocampus [38], as do kainate-induced seizures [39], prolonged pilocarpine-induced seizures [40], and amygdala kindling stimulation [21]. Both agonists and antagonists of glutamate receptors induce ICER mRNA expression [39, 41–43].
Different types of stress, as well as CRH injections, increase ICER expression in a variety of neuroendocrine structures as well as in the striatum [12, 44–46]. Antidepressant and amphetamine treatments also upregulate ICER expression [46, 47], as do lithium chloride and nociceptin injections [48–50].
Purely physiological stimulation, such as exposure of dark-reared rats to light [39] and exploration of enriched environments [31, 32], also have the capacity to substantially upregulate ICER expression. Moreover, ICER mRNA is upregulated not only immediately after fear conditioning training but is also upregulated specifically when mice are presented with the conditioned stimulus (tone or context) 24 h later [21].
The ICER induction observed in response to a variety of physiological and non-physiological stimuli suggests that it may play an important role in restricting/suppressing responses to environmental stimuli. Thus, ICER may serve as a filter ensuring that only important, strong, or persistent incoming stimulation is transduced into plastic changes of the nervous system.
Time Course of Induction
ICER is classified as an early response gene [12]. Studies using different types of stimulation have demonstrated that ICER induction is relatively slow compared to the induction time course of other immediate early genes (IEGs; ICER expression peaks around 2–6 h after stimulation, depending on types of stimulation and cell types). Once induced, however, ICER upregulation lasts longer than the upregulation of other IEGs in the cell (often more than 24 h) [24, 35, 39, 41, 51–54].
Amphetamine injection or restraint stress increases ICER mRNA levels in the striatum, reaching maximum levels around 3–4 h later followed by slow return to control levels within 12 h [25, 46]. Kindling stimulation causes a sharp increase in c-fos mRNA followed by rapid decline [21]. On the other hand, kindling increases ICER mRNA levels 1 h post-stimulation, which remains above basal levels for more than 6 h thereafter [21]. After pilocarpine-induced prolonged seizures, ICER mRNA reaches maximum levels around 6 h, and then gradually declines; ICER protein levels are upregulated longer than 24 h after seizures [29]. During exploration of a novel enriched environment, the increase in c-Fos protein levels reaches maximum levels after 1 h and then declines [32]. By contrast, induction of ICER reaches a maximum around 6 h after exploration and remains upregulated even after 5 days of exploration [32].
ICER as a Transcription Regulator
The delayed and prolonged time course of ICER induction suggests that, after reaching a sufficient concentration in the nucleus, ICER acts to suppress gene transcription of other IEGs and/or their target late-response genes and eventually switches off the CRE-mediated gene transcription initiated by the original stimuli [1, 4, 18, 25, 26, 32]. This is supported by the observations that ICER attenuates c-fos mRNA expression in cultured cells [28, 55]; that increase in ICER expression after nociceptin injection coincides with decreased c-Fos protein expression [48]; and that kindling stimulus-induced c-Fos protein expression is attenuated by ICER overexpression in vivo [21]. Further support is provided by findings that herpes simplex virus (HSV)-ICER injections into the rat nucleus accumbens repress amphetamine-induced expression of BDNF [46] and upregulation of ICER reduces expression of the α1 subunit of gamma-aminobutyric acid (GABA)A receptors in primary neocortical cell cultures [35]. Thus, ICER may serve as an important factor in shutting down cAMP-inducible gene transcription [32], together with the inactivating of phosphorylated CREB by dephosphorylation [5].
Being an inducible and powerful repressor of cAMP-dependent transcription, ICER can potentially affect the expression of numerous CRE-containing genes, including genes whose products are critically involved in neuronal plasticity; genes that encode transcription factors (c-fos, JunD, Krox-20, Krox-24, creb, icer); genes that encode neurotransmission-related proteins (enkephalin, galanin, somatostatin, CRH, tyrosine hydroxylase, GABAA receptor subunits, β-adrenergic receptor subunits, inducible nitric oxide synthase); and genes that encode growth factors, such as BDNF [1, 5, 26]. In addition, ICER can regulate transcription by binding to sequences other than CRE (e.g., activator protein 1, AP1) [26].
As different ICER isoforms utilize different DBD domains, by analogy with other CREM isoforms, they may have different affinity for heterodimerization with other members of the CREB/CREM/ATF-1 gene family [22, 23] and different CRE affinity [35]. For example, ICER I is slightly more potent than ICER IIγ in suppressing endogenous and forskolin-stimulated CRH promoter activity in cultured cells [24]. However, Misund and colleagues did not find any substantial difference in the repression of CRE-driven transcription between the ICER I and IIγ isoforms in human embryonic kidney (HEK) 293 cell variants with controllable overexpression of these isoforms [55]. The authors hypothesized that the putative different biological functions of the ICER isoforms may instead be related to differences in their expression levels in response to different types of stimulation or in different cell types.
In addition to the regulation of CREB-dependent transcription at the level of competition for CRE sites, ICER is proposed to regulate CREB at the level of protein stability, since in cell cultures co-transfected with CREB- and ICER-containing vectors, ICER significantly decreases the intracellular levels of exogenously expressed CREB [56]. Thus, ICER may regulate CRE-mediated transcription in several different ways. Together with the already mentioned potential differential affinity of the four ICER isoforms toward particular dimerization partners and different CRE sites, as well as tissue/cell-type specificity, this simultaneous versatility and variability gives ICER the potential to play very important roles in “fine-tuning” CRE-mediated transcription according to different incoming information. The final outcome may be further affected by interactions occurring between two binding events: ICER binding to CREs and transcription activator binding to other sites [34]. All these aspects warrant careful further investigation, specifically in application to neurons.
Role of ICER in Circadian Regulation
In neuroendocrine tissues, ICER is the most abundant of all CREM isoforms [12, 13, 15, 44]. ICER plays an important role in the neuroendocrine system, regulating melatonin synthesis during the course of the circadian rhythm [19, 57] and coordinating reaction to hypothalamic–pituitary–adrenal (HPA) axis stimulation [44, 45]. The role of ICER in circadian regulation has already received much attention and is summarized in a series of excellent reviews [17–19, 58].
Role of ICER in Apoptosis
ICER plays an important physiological role in apoptosis in the nervous system [26, 39, 59–63]. Stimuli evoking neuronal cell death in the brain upregulate endogenous ICER expression [39]. Endogenous ICER expression is also upregulated in a variety of cell cultures undergoing apoptosis [59–62]. Moreover, both adenoviral vector-driven overexpression of ICER IIγ and cell transfection with any of the four ICER isoforms also result in apoptosis [39, 41, 59, 60, 62]. While all four ICER isoforms are induced after pro-apoptotic treatment, isoforms (Iγ and IIγ) lacking the γ domain show the strongest induction [60, 62]. ICER might promote apoptosis through downregulation of the anti-apoptotic gene bcl-2 [59, 62, 64]. ICER may interfere with the pro-survival action of CREB and contribute to neurodegeneration from neurotoxicity, trauma, and trophic deprivation or disorder [62]. However, studies employing in vivo ICER overexpression did not report any signs of neuronal cell death [20, 21, 46]. Recent findings [62] suggest that in the intact brain the pro-apoptotic action of ICER may be more tightly regulated and less pronounced [21], thus explaining the discrepancy between the in vitro and in vivo findings.
ICER and General Behavior
Data regarding involvement of ICER in the regulation of basic behavioral functions are sometimes contradictory, reflecting differences between ICER manipulating procedures (viral vector transfer or chronic genetic manipulation) and animal species used (mice or rats). Overall, the accumulated results suggest that ICER may play different roles depending on the site of manipulation (brain structure) and type of behavior (Table 1).
Table 1
Behavioral consequences of in vivo manipulations of ICER levels
| Models | |||||
|---|---|---|---|---|---|
| Species | Manipulation | General behaviors | Epileptogenesis | Learning and memory | References |
| Mouse | CREM/ICER knockout | Increased spontaneous locomotor activity | Normal acute pilocarpine-induced status epilepticus | Potential memory enhancement (conditioned suppression of motility test) | [40, 65] |
| Reduced anxiety-like behavior | Increased spontaneous seizures after pilocarpine-induced status epilepticus | Enhanced long-term fear memory | [21] | ||
| ICER knockout | Normal locomotor activity, sensory and motor functions, and emotional reactivity | Accelerated kindling development | Normal odor discrimination memory | ||
| ICER overexpression (forebrain-specific, pCaMKII) | Normal locomotor activity, sensory and motor functions, and emotional reactivity | Normal basal neuronal excitability (electric stimulation of amygdala) | Impaired long-term fear memory | [21] | |
| Rat | ICER overexpression (rAAV vector) dorsal hippocampus | No change in general motor activity | Not tested | Normal odor discrimination memory | |
| 3-month-old rats—normal Barnes maze and passive avoidance performance | [20] | ||||
| Normal basal neuronal excitability (electric stimulation of amygdala). Retarded kindling development | 15-month-old rats—impaired Barnes maze and passive avoidance performance | ||||
| ICER overexpression (HSV vector) nucleus accumbens shell | Decreased spontaneous locomotor activity | Not tested | Not tested | [46] | |
| Potentiated amphetamine-induced locomotor activity | |||||
| Increased responsiveness to natural rewards | |||||
| Antidepressant-like effect in the forced swim test | |||||
| Increased neophobia and anxiety-like behavior | |||||
ICER-specific overexpressing (OE) or knockout (KO) mice display normal locomotor activity in the home cage and open field [21]. Moreover, overexpression of ICER in hippocampus does not lead to changes in rat locomotor activity [20]. On the other hand, overexpression of ICER in the nucleus accumbens decreases the spontaneous activity of rats in the open field but strongly potentiates amphetamine-induced locomotor activity [46]. Characterization of CREM/ICER-KO mice (KO mice lacking all CREM isoforms including ICER) yielded contradictory results that might stem from differences in genetic background and experimental procedures used. For example, Maldonado and colleagues observed significantly increased spontaneous locomotor activity in mutant animals; moreover, mutant animals displayed homogeneously high activity during both light and dark cycle periods [65]. By contrast, Conti and colleagues observed no differences in basal locomotion in the home-cage activity of mutant and wild-type mice [47].
Overexpression of either ICER I or II in the entire forebrain does not affect mouse behavior in the elevated plus-maze test [21], while overexpression of ICER in the nucleus accumbens produces robust anxiogenic-like effects and increases “neophobia” in rats [46]. CREM/ICER-KO mice show decreased anxiety in the elevated plus- and zero-maze tests [65]. ICER-specific KO mice do not show changes in the elevated plus-maze; however, longer latencies to enter the light compartment during the light/dark transition test suggest that these mice have increased anxiety [21].
Overexpression of ICER in the nucleus accumbens increases responsiveness to natural rewards such as familiar sucrose and social interaction, and produces antidepressant-like effects in the forced swim test [46]. On the other hand, CREM/ICER-KO mice show similar levels of immobility compared to wild-type mice in the forced swim and tail suspension tests [47].
ICER, Stress, and Depression-like Behavior
Stress induces ICER expression in different parts of the HPA axis, and ICER regulates transcription of the CRH gene during stress [24, 25, 45]. Under normal conditions, stimulation of the cAMP cascade by stress produces a dual effect: first facilitating CRH gene transcription via phospho-CREB, and later inhibiting it via induction of ICER. Thus, ICER activation, by suppressing CRH transcription, serves as a protective mechanism helping to avoid the consequences of excessive expression of CRH [24, 25, 45].
Decreased depression-like behavior in ICER-overexpressing rats [46] may, in part, result from reduced CRH production, as the overproduction of CRH is believed to contribute to depression [66, 67]. ICER may also be a critical molecular mediator of tricyclic antidepressant action, as suggested by its strong induction in response to desipramine treatment [47]. Although ICER is not essential for the behavioral antidepressant effect of the drug, it is critical for desipramine-mediated reduction of stress-induced plasma corticosterone levels [47].
The results obtained through ICER gene manipulations may provide important clues that will help to reveal the precise molecular mechanisms underlying chronic stress states and will contribute toward development of novel antidepressant drugs.
ICER and Regulation of Epileptogenesis
Seizures alter the activity of the CREB/CREM/ATF-1 gene family and their target genes. Kindling is widely studied as a model of epileptogenesis and as a form of neuroplasticity [68–70]. Furthermore, kindling development requires gene transcription and translation [68, 71]. The term kindling refers to the phenomenon that periodic mild electrical stimulation of one of many brain sites at stimulus intensities initially too low to produce any effects eventually leads to the development and gradual intensification of elicited motor seizures [72]. CRE binding of CREB is transiently upregulated after kindling stimulation [73]. As mentioned previously, ICER mRNA levels are increased in the hippocampus and other brain regions after seizures provoked by kindling stimulation [21], electroconvulsive shock [38], kainate [39], and pilocarpine [40].
The finding that CREM/ICER-KO mice develop more severe epilepsy after pilocarpine-induced status epilepticus (SE) than non-mutant littermates supports a close tie between ICER and the genesis of seizures [40]. Furthermore, using ICER-specific mutant mice (both ICER-OE and ICER-KO mice) we have recently shown that ICER negatively regulates epileptogenesis: Overexpression of ICER leads to the retardation of kindling development, while lack of ICER significantly facilitates kindling [21]. These anti-epileptic effects of ICER fit nicely with the data showing that prolonged hyperactivation of CREB-mediated gene transcription leads to sporadic epileptic seizures [74]. The increased ICER levels may achieve the anti-epileptic effect by restricting and suppressing excessive CRE-dependent transcription. On the other hand, the removal of ICER from the system compromises endogenous suppression of CRE-dependent gene activation and results in aberrant synaptic plasticity and seizure development [21, 40].
Pilocarpine-induced SE upregulates ICER, which binds to the CRE site in the Gabra1 promoter and decreases expression of GABAA receptor α1 subunits [29]. This leads to a decrease in the number of GABAA α1γ2-containing receptors in the dentate gyrus of hippocampus, potentially promoting network disinhibition [29, 35]. A similar process occurs in hippocampal cell cultures [35].
The apparent discrepancy of the above findings might be due to differences between the effects of constitutive changes in ICER levels (in the case of gene manipulations) and the effects of transient ICER upregulation (in the case of intact systems). Another possible explanation is that the effects of whole forebrain (or brain) changes in ICER levels differ from those of region-specific changes in ICER levels. Further studies are needed to better understand the role of ICER in epilepsy, but even in the current state, all these findings clearly show that ICER does play an important role in the regulation of epileptogenesis.
ICER and Memory
The cAMP–PKA–CREB system is evolutionarily well conserved [5, 11]. The indispensable role of CREB and CRE-mediated gene transcription in neuronal plasticity underlying learning and memory has been established in a variety of animal species [75–79]. Manipulation of CREB levels or activity is considered to be a promising way to study and, eventually, improve memory [10, 80]. However, studies to date have yielded mixed results [81–84], highlighting that manipulation of a constitutively expressed transcription factor is complicated by wide non-selective changes and produces compensatory changes in expression of other CREB/CREM/ATF-1 gene family members [85–87]. Being a potent endogenous repressor of CREB, ICER is strategically poised to play an important role in memory formation. Its low basal level of expression and high inducibility in response to various types of stimulation suggest that ICER is an ideal potential target for memory-improving manipulations. However, until recently, little attention has been given to the role of ICER in learning and memory.
Machado and colleagues proposed that ICER belongs to a subgroup of IEGs, called induced preferentially by depolarization-IEGs, that are more likely to play specific roles in synaptic plasticity than IEGs, which are induced by a number of factors in addition to depolarization [88]. Exploration of enriched environments upregulates ICER expression in the barrel-related columns of primary somatosensory cortex, an important site related to familiarization with novel environments and learning about new stimuli [31, 32]. Increased ICER mRNA levels are also detected in the amygdala after fear conditioning training and testing [21].
The overexpression of ICER in dorsal hippocampus through a recombinant adeno-associated virus (rAAV) vector did not cause 3-month-old rats to show changes in memory tests, which included a passive avoidance task and the Barnes spatial maze [20]. When the same animals were re-tested in these tasks at 15 months of age, ICER-overexpressing rats performed significantly worse than the sham-operated control rats of the same age. Although rAAV-mediated ICER overexpression was widespread and robust, it did not cover 100% of hippocampal neurons. Mouravlev et al. proposed that in young adult rats the remaining non-transfected neurons were sufficient to maintain normal function [20]. However, as the CREB system function declined with age, the same level of ICER overexpression became disruptive, as the non-transduced cells were no longer able to compensate.
To investigate the role of ICER in neuronal plasticity and memory in detail, we have generated two types of ICER mutant mice: ICER-overexpressing (ICER-OE) and ICER-specific knockout (ICER-KO) mice [21]. In accordance with the non-constitutive and inducible nature of ICER, specific overexpression or deletion of the gene did not cause any compensatory changes in the expression level of CREB or CREM isoforms. While both ICER-OE and ICER-KO mice were spared of any robust changes in locomotor activity, sensory functions, and emotional responses, they demonstrated specific changes in memory, as assessed using fear conditioning. After conditioning with two tone-foot shock pairs, the mice were sequentially tested for short-term (1 h) and long-term (24 h) tone-dependent fear memory, followed by long-term context-dependent fear memory (48 h). Overexpression of ICER led to impaired long-term tone- and context-dependent fear memory, leaving short-term memory unchanged (Fig. 2a). By contrast, deletion of ICER in ICER-KO mice—although inefficient in the case of the standard fear conditioning protocol (ceiling effect, Fig. 2b)—led to enhancement of long-term memory formation when a weak conditioning protocol (producing weak long-term memory in controls) was employed (Fig. 2c).
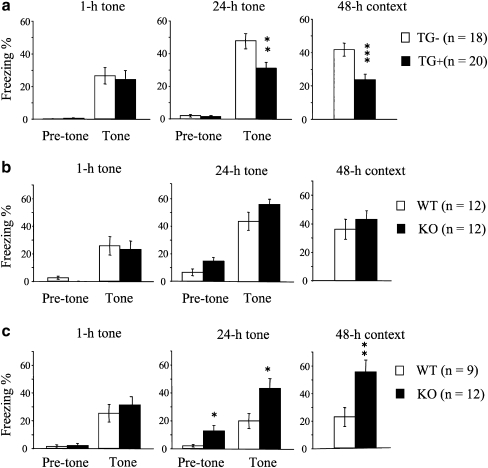
Fear conditioning in ICER mutant mice. Conditioned freezing to tone and context was compared in ICER-overexpressing (OE) transgenic mice (TG+) and their non-TG littermates (TG−; a), in ICER-knockout (KO) mice and their wild-type (WT) littermates after a standard conditioning protocol (b), and in ICER-KO mice and WT littermates after a weak conditioning protocol (c). a No difference was observed between non-TG mice and ICER-OE mice 1 h after conditioning (1 h tone). However, 24 h (24 h tone) and 48 h (48 h context) after conditioning, tone-dependent and context-dependent freezing were significantly attenuated in ICER-OE mice. b After being subjected to a standard conditioning protocol, ICER-KO mice and WT littermates exhibited similar freezing levels during the tone-dependent tests performed 1 h (1 h tone) and 24 h (24 h tone) after conditioning and during the context-dependent test (48-h context). c After being subjected to a weak conditioning protocol, there was no significant difference in conditioned freezing between ICER-KO and WT mice 1 h after conditioning (1 h tone). However, ICER-KO mice showed increased freezing during both pre-tone and tone presentation in the tone-dependent test performed 24 h after conditioning (24 h tone). Context-dependent freezing in ICER-KO mice was also enhanced (48 h context). Data are means ± SEM; number of animals per group are in parentheses. *p <
< 0.05; **p
0.05; **p <
< 0.01; ***p
0.01; ***p <
< 0.001 compared to non-TG or WT littermates (with modifications from [21])
0.001 compared to non-TG or WT littermates (with modifications from [21])
The results showing that overexpression of ICER specifically impairs long-term fear memory resemble those reported previously in mice in the case of CREB downregulation [75, 82, 89] or overexpression of dominant negative mutant CREB [90, 91] and are consistent with the role of ICER as an endogenous CREB antagonist. The disruption of CREB gene results not only in the impairment of long-term memory but also leads to the significant upregulation of several CREM isoforms, ICER in particular [86]. This raises the possibility that behavioral phenotypes of CREB-KO mice may not entirely result from the lack of CREB but, in part, may result from increased ICER levels. On the other hand, ICER-KO mice show enhanced fear memory, though only when a weak conditioning protocol is used. Again, these data are consistent with the results of CREB manipulations: In rats, the overexpression of CREB promotes the formation of long-term memory under training conditions that do not produce strong long-term memory among controls [92–94]. Taken together, the data show that both manipulations, the increase in the amount of transcription activator (CREB) or removal of transcription suppressor (ICER), change behavior in the same direction (i.e., enhance long-term memory).
Neither overexpression nor deletion of ICER affected memory tested in the odor discrimination task [21]. The role of ICER may be critical only for some types of memory; however, extensive data clearly demonstrate that CREB and CRE-dependent transcription play an important role in a wide array of memory tasks [75, 82, 95, 96]. It might also be the case that ICER-induced inhibition of transcription play a crucial role in “single-trial” or “sub-threshold” learning, but more robust repeated training (as in an odor discrimination task) is able to overcome the effects of loss or overexpression of ICER. Further detailed examination of other types of memory is necessary in order to fully delineate memory systems critically dependent on CREB/ICER regulation.
Further Possible Directions: ICER and Drug Addiction
Through the occupation of CRE elements in the promoters of various critical genes and counteraction of CREB-induced activation, ICER has the potential to affect different types of higher brain functions. One of the promising directions to study is ICER’s involvement in the regulation of reward learning and drug addiction. CREB is involved in regulation of drug reward [97–101], and overexpression of dominant-negative CREB increases cocaine reward [102, 103]. Involvement of ICER in the regulation of drug reward is supported by the data obtained using the psychostimulant amphetamine. Amphetamine administration increases ICER mRNA levels in the ventral striatum in a dose-dependent manner; this effect diminishes progressively with repeated drug administration [46]. In addition, overexpression of ICER in the nucleus accumbens strongly potentiates amphetamine-induced locomotor activity [46]. The same authors have found that overexpression of ICER also affects an animal’s responses to natural rewards, leading to increased preference of familiar sucrose solution and increased social reward. Moreover, since both dopamine-β-hydroxylase and tyrosine hydroxylase genes contain CRE elements [104, 105], potentially, ICER may be able to modulate synthesis of dopamine as well as other catecholamines. Application of dopamine to primary rat neocortical cultures induces ICER activation and downregulates expression of the α1 subunit of GABAA receptor, leading to the downregulation of α1-containing GABAA receptors at the cell surface [35]. Hu et al. proposed that the dopamine neurotransmitter system uses the CREB signaling pathway to regulate inhibition in the brain, and ICER may be an important contributor to altered inhibitory processes in different pathological conditions, such as Parkinson’s disease, brain trauma, and drug addiction [35].
Conclusions
Recent findings clearly show that ICER is involved in the regulation of plasticity underlying epileptogenesis and fear memory, as well as reaction to stress. However, further work is needed to assess ICER’s involvement in plasticity underlying other types of learning and memory. The availability of genetically manipulated mice, the expression of ICER using virus vectors, and the conditional manipulation of ICER gene provide rich opportunities to dissect the physiological role of ICER in detail.
ICER acts as a negative regulator of long-term neuronal plasticity most possibly through suppression of plasticity-related gene expression (Fig. 3). As expression of ICER may be induced by the same stimuli and through the same molecular cascades that initiate formation of memory traces, ICER may play an overall positive role in the formation of adaptively meaningful learning and memory. By suppressing cAMP-induced transcription, ICER may serve as a filter that increases the “signal-to-noise ratio,” sifts away weak stimulation, and allows only strong/persistent information to gain access to long-term storage. However, the same filter might become counterproductive if the activating part of the process (e.g., CREB activity) weakens due to some circumstances. Accordingly, manipulations of ICER levels may, depending on the context, play both positive and negative roles in the organism.
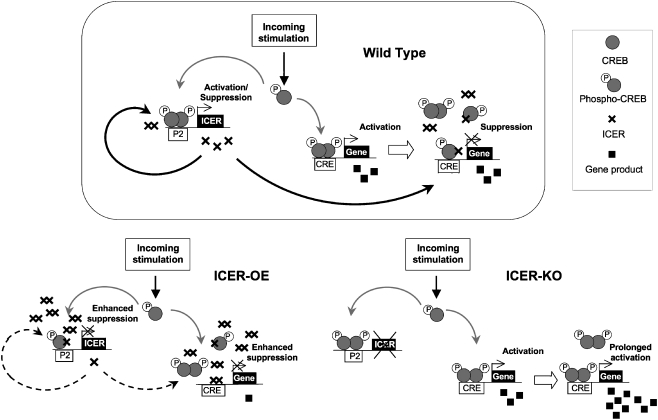
Simplified schematic diagram of ICER acting as a regulator of long-term plasticity. In wild-type mice, incoming stimuli activate protein kinases and activated kinases phosphorylate CREB. Phosphorylated CREB activates CRE-containing gene transcription. Phosphorylated CREB also activates P2 promoter and initiates ICER transcription. ICER is a transcription repressor, and after reaching a certain concentration, starts to suppress CRE-dependent activation of gene expression, including its own expression (through the formation of ICER homodimers or ICER-CREB heterodimers that bind to CREs and block transcription). In ICER-OE mice, suppression of CRE-mediated gene transcription is enhanced by a constitutively available excess amount of ICER. In ICER-KO mice, lack of ICER-mediated suppression results in a prolonged activation of CRE-mediated gene transcription. Accumulated gene products that result from the transcription-translation of the gene are indicated as filled squares; ICER proteins are indicated as crosses; the transcription activator CREB is indicated by gray filled circles. P, phosphate group; P2, ICER promoter in the CREM/ICER gene; CRE, CRE-containing promoters (figure with modifications was adapted from [21])
In the neuroendocrine system, ICER induction by cAMP contributes to a refractory phase during which additional cAMP stimuli fail to elicit a full transcriptional response [106]. This mechanism has been proposed to make the transcriptional response to repetitive or prolonged stress dependent on the frequency or the duration of previous stress episodes [44, 106]. We would like to extend this further and speculate that, by the same mechanism, ICER may contribute to weaker memory formation after massed training procedures. In a similar framework, Won and Silva have proposed that a refractory phase caused by ICER repression may play an important role in memory allocation in neuron networks, promoting dynamic memory allocation to different sets of neurons [107]. All these hypotheses position ICER as an important player in metaplasticity [108].
As we have tried to show, interesting data on ICER function in the brain have emerged recently. Overexpression of ICER, achieved through gene manipulation or viral vectors, is a powerful tool. However, while considering these results, one needs to be aware that the consequences of stable and long-lasting overexpression of ICER may be different from the consequences of the phasic, transient changes in gene expression that takes place under normal conditions in response to different incoming stimuli [46]. But even with this constraint, the data obtained so far provide valuable insight into the role of ICER regulation of CRE-mediated transcription in brain function, firmly positioning it as an important “stimulus-transcription coupling” agent [109, 110]. The data showing that ICER acts as a regulator of long-term memory formation and epileptogenesis suggest that the dynamic balance of CREB/ICER function is a crucial factor that determines which events will be fixed for long-term storage through neural plasticity. Further studies and a better understanding of the role of ICER in the regulation of long-term plasticity may contribute to the development of medications for various pathological conditions, such as post-traumatic stress disorder, drug addiction, epilepsy, and dementias.
Acknowledgments
The authors thank Dr. Nobuhiko Kojima for the critical reading of an early draft and for generating Fig. 3, and everyone who responded to the authors’ inquiry regarding ICER and shared their recent findings.
References
Articles from Molecular Neurobiology are provided here courtesy of Springer
Full text links
Read article at publisher's site: https://doi.org/10.1007/s12035-009-8072-1
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007%2Fs12035-009-8072-1.pdf
Subscription required at HumanaPress
http://www.humanapress.com/ArticleDetail.pasp?issn=0893-7648&acode=MN:40:1:73
Citations & impact
Impact metrics
Citations of article over time
Article citations
Multi-faceted regulation of CREB family transcription factors.
Front Mol Neurosci, 17:1408949, 06 Aug 2024
Cited by: 0 articles | PMID: 39165717 | PMCID: PMC11333461
Review Free full text in Europe PMC
A liver immune rheostat regulates CD8 T cell immunity in chronic HBV infection.
Nature, 631(8022):867-875, 10 Jul 2024
Cited by: 3 articles | PMID: 38987588 | PMCID: PMC11269190
Interleukin 6 (IL-6) Regulates GABAA Receptors in the Dorsomedial Hypothalamus Nucleus (DMH) through Activation of the JAK/STAT Pathway to Affect Heart Rate Variability in Stressed Rats.
Int J Mol Sci, 24(16):12985, 19 Aug 2023
Cited by: 3 articles | PMID: 37629166 | PMCID: PMC10455568
Genetics and Molecular Biology of Memory Suppression.
Neuroscientist, 30(3):315-327, 15 Dec 2022
Cited by: 2 articles | PMID: 36524276 | PMCID: PMC10570929
Review Free full text in Europe PMC
Reversal of spatial memory impairment by phosphodiesterase 3 inhibitor cilostazol is associated with reduced neuroinflammation and increased cerebral glucose uptake in aged male mice.
Front Pharmacol, 13:1031637, 21 Dec 2022
Cited by: 3 articles | PMID: 36618932 | PMCID: PMC9810637
Go to all (43) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Inducible cAMP early repressor (ICER) in the nervous system--a transcriptional regulator of neuronal plasticity and programmed cell death.
J Neurochem, 87(6):1313-1320, 01 Dec 2003
Cited by: 52 articles | PMID: 14713288
Review
Requirement for cAMP-response element (CRE) binding protein/CRE modulator transcription factors in thyrotropin-induced proliferation of dog thyroid cells in primary culture.
Eur J Biochem, 259(1-2):370-378, 01 Jan 1999
Cited by: 16 articles | PMID: 9914516
Inducible cAMP early repressor acts as a negative regulator for kindling epileptogenesis and long-term fear memory.
J Neurosci, 28(25):6459-6472, 01 Jun 2008
Cited by: 31 articles | PMID: 18562617 | PMCID: PMC6670907
Coupling signalling pathways to transcriptional control: nuclear factors responsive to cAMP.
Recent Prog Horm Res, 52:121-39; discussion 139-40, 01 Jan 1997
Cited by: 18 articles | PMID: 9238850
Review





