Abstract
Free full text

Identification and characterization of IL-10/IFN-γ–producing effector-like T cells with regulatory function in human blood
Abstract
Two subsets of natural and adaptive regulatory T (T reg) cells have been described, but the identity of adaptive type 1 regulatory (Tr1)–like cells in humans is unclear. We analyzed a subset of human blood CD4+ T cells—CD45RA−CD25−interleukin (IL)-7 receptor (R)− cells—that rapidly secreted high levels of IL-10 together with interferon γ, but produced little IL-2. These IL-7R− T cells were rare, anergic, and largely Foxp3−. They expressed low levels of Bcl-2 but high levels of Ki-67 and ICOS, suggesting that they have been recently activated in vivo. Consistently, they responded selectively to persistent foreign and self-antigens under steady-state conditions. Unlike natural CD25+ T reg cells, IL-7R− cells suppressed naive and memory T cell proliferation in an IL-10–dependent fashion, and they required strong T cell receptor stimulation for suppression. To our knowledge, this is the first report that identifies Tr1-like cells in human blood. These IL-10–secreting cells have characteristics of chronically activated Th1 effector cells and are distinct from CD25+ T reg cells.
T reg cells are important for the control of autoimmune diseases and for limiting immune responses (1). “Natural” T reg cells can be identified by CD25 surface expression in both mice and humans, and express high levels of CTLA-4 (2). They mature in the thymus (1) or in the periphery after tolerogenic priming (3), and are anergic in vitro because they lack an IL-2–producing capacity. Nevertheless, T reg cells proliferate in vivo (4, 5) and are highly susceptible to apoptosis (6). They inhibit T cell priming in vitro by a not fully understood cell contact–dependent mechanism (2). It is believed that these cells represent an independent T cell lineage that is determined by the transcription factor Foxp3 (1). The TCR repertoire of natural T reg cells is biased toward autoreactivity (7), and they have been shown to prevent autoimmune diseases in several different models (1). On the other hand, “adaptive” type 1 regulatory (Tr1) cells have also been shown to be important for control of autoimmune diseases (8). These cells do not belong to the natural T reg cell lineage (9, 10) and are characterized by their capacity to produce IL-10 and to inhibit immune responses in an IL-10–dependent manner (8, 10, 11). Tr1-like populations can be generated in vitro in various ways, including repetitive priming with immature DCs, IL-10 plus IFN-α, or immune-suppressive drugs (8, 12–14). Interestingly, IL-10–producing Th1 cells have been recently shown to prevent immunopathology in chronic parasite infections in vivo (15, 16), but their relationship to Tr1 cells is currently unclear because not all described Tr1 populations also produce IFN-γ.
Although various Tr1-like populations can be generated, their identity and phenotype in human blood is currently unknown. Recently, it was shown that human CD25+ T reg cells express low levels of CD127 (17, 18), the IL-7Rα chain that is important for survival and homeostatic maintenance of CD4+ memory T cells (19). Based on this observation, it was speculated that adaptive T reg cells might be present among CD25−IL-7Rlo cells (20). Consistently, adaptive mouse T reg cells that down-regulate inflammatory responses to malaria parasites had a CD127−CD25− phenotype (21). In this report, we show that human blood CD4+CD25−IL-7Rα− cells were activated, effector-like cells that coproduced IL-10 and IFN-γ but not IL-2. These cells were largely Foxp3− but inhibited T cell proliferation in an IL-10–dependent manner, and might thus represent the human counterpart of mouse Tr1-like cells.
RESULTS AND DISCUSSION
Circulating CD4+IL-7R− T cells coproduce IL-10 and IFN-γ but secrete little IL-2
We purified human antigen–experienced (CD45RA−) CD4+ T cells according to CD25 and IL-7Rα expression, and analyzed Foxp3 expression to assess the presence of natural T reg cells in the resulting three populations (Fig. 1, A and B). The majority of the cells had a CD25−/loIL-7Rhi memory phenotype (IL-7R+) and did not express Foxp3 (1 ± 1%). Consistent with two previous reports (17, 18), the CD25+IL-7Rlo phenotype identified natural T reg cells (CD25+ T reg cells; 5 ± 2%) that were mostly Foxp3+ (82 ± 16%). Finally, the smallest fraction of cells (IL-7R−; 1 ± 1%) expressed neither CD25 nor IL-7R, and only a small fraction of these cells expressed Foxp3 (11 ± 6%).

Tr1-like cytokine profile of CD25−IL-7R− T cells. (A) CD25 and IL-7Rα expression on antigen-experienced human blood CD4+CD45RA− T cells ex vivo. (B) Foxp3 expression in CD4+ T cell subsets purified according to CD25 and IL-7Rα expression assessed once in at least 11 different donors. (C) Purified CD25−IL-7R− and IL-7R+ subsets were stimulated with anti-CD3 and anti-CD28 antibodies or with phorbol 12,13-dibutyrate (PdBu) plus ionomycin, and IL-10 and IL-2 secretion were measured (percentages are shown). One representative experiment out of seven with seven different donors is shown. (D) Total CD4+CD45RA− cells were compared with subsets defined by CD25 and IL-7R expression for the secretion of IL-10 and IFN-γ (top), IL-4 (middle), and IL-17 (bottom). Percentages are shown. One representative experiment out of three performed with four different donors is shown. (E) Percentages of total IFN-γ+ cells (left), total IL-10+ cells (middle), and IFN-γ–producing cells among IL-10+ cells (right) in IL-7R−, IL-7R+, and CD25+ subsets in 13 different donors. Horizontal bars represent means. *, P < 0.05; and **, P < 0.005.
We analyzed the cytokine profile of purified CD4+CD45RA− T cell subsets after polyclonal activation with phorbole ester and calcium ionophore or immobilized anti-CD3 and anti-CD28 antibodies. As shown in Fig. 1 C, IL-7R− cells expressed high levels of IL-10 under both conditions but only low levels of IL-2, in particular upon more physiological stimulation with anti-CD3 and anti-CD28 antibodies. Conversely, the majority of IL-7R+ cells produced IL-2, but only very few cells produced IL-10 under both conditions. In a group of 13 donors, a significantly higher portion of IL-10 producers was found in the IL-7R− (9 ± 7%) compared with the IL-7R+ (1 ± 1%) compartment when stimulated with phorbol ester and calcium ionophore (Fig. 1 E). IL-7R+ cells also secreted higher levels of TNF-α than IL-7R− cells (unpublished data). We then assessed if IL-7R− T cells that secreted IL-10 coexpressed cytokines that are characteristic for different Th cell lineages (Fig. 1 D). The cytokine expression after polyclonal restimulation was compared between total CD4+CD45RA− T cells and subsets purified according to CD25 and IL-7Rα expression. Total antigen-experienced CD4+ T cells expressed low levels of IL-10 and a substantial fraction of these cells coproduced IFN-γ, whereas only very few IL-10+ cells also secreted IL-4 or IL-17. The IL-10+ cells that coproduced IFN-γ or IL-4 were strongly diminished among IL-7R+ cells and highly enriched among IL-7R− cells. Notably, nearly all IL-7R− cells that produced IL-10 also secreted IFN-γ (Fig. 1, D and E). Surprisingly, there were also some IL-10+ cells in the T reg cell fraction that coproduced IFN-γ or IL-4, and CD25+ T reg cells contained the highest fraction of cells that coproduced IL-17 and IL-10. Although the IFN-γ–producing CD25+ T cells are most likely contaminating Foxp3− effector cells (Fig. 1 B), the IL-17–producing cells could also represent Foxp3/RORγt-coexpressing cells that were previously described in the mouse (22). In conclusion, the cytokine profile of circulating IL-7R− cells (i.e., cosecretion of IFN-γ and IL-10 combined with low IL-2 production) is consistent with that reported originally for adaptive regulatory Tr1 cells generated in vitro (8, 14). Coproduction of IFN-γ and IL-10 is also characteristic for Th1 cells that prevent immunopathology upon chronic mouse Toxoplasma or Leishmania infections (15, 16). The production of IFN-γ distinguishes human IL-7R− cells, however, from Tr1-like cells generated upon mouse malaria infection (21) and from some Tr1-like populations generated in vitro (12, 13).
CD4+IL-7R− T cells have a low expansion potential
IL-7R expression is characteristic for mouse CD4+ memory T cells because they require IL-7 for their antigen-independent maintenance (19). Memory T cells are characterized by a high secondary expansion potential, whereas effector cells die upon restimulation and T reg cells are anergic in vitro. To analyze the secondary expansion potentials of the purified CD4+ T cell subsets, cells were labeled with CFSE and stimulated with immobilized anti-CD3 antibodies in the absence or presence of anti-CD28 antibodies or exogenous IL-2 (Fig. 2 A). IL-7R+ memory phenotype cells proliferated efficiently upon stimulation with anti-CD3, and expansion was further enhanced by the addition of IL-2 or anti-CD28 antibodies. Conversely, CD25+ T reg cells and IL-7R− cells failed to proliferate upon anti-CD3 stimulation alone, but IL-7R− cells proliferated when exogenous IL-2 was added. Both subsets proliferated upon addition of anti-CD28, but low numbers of IL-7R− cells were recovered after culture (Fig. 2 C), suggesting that they died in culture. In summary, IL-7R+ cells have a high expansion potential, as is characteristic for memory cells, whereas IL-7R− cells are anergic but proliferate in the presence of IL-2.

Low expansion potential of IL-7R− T cells in response to TCR stimulation and homeostatic cytokines. (A) Proliferative response of subsets purified according to CD25 and IL-7Rα expression in response to anti-CD3 antibodies in the absence or presence of exogenous IL-2 or co-stimulatory anti-CD28 antibodies. CFSE dilution of viable cells was assessed on day 4. One representative donor out of three is shown. (B) Proliferative response of subsets purified according to CCR7 and IL-7Rα expression in response to 25 ng/ml IL-7 or IL-15 on day 7. One representative donor out of four is shown. (C) The recovered number of cells after stimulation with IL-7, IL-15, or anti-CD3/28. Horizontal bars represent means. *, P < 0.05.
It was proposed that the lymph node homing receptors CCR7 or CD62L in combination with IL-7R expression defines subsets of central memory, effector memory, and effector cells (23, 24). Memory but not effector CD4+ T cells maintain their number by slow proliferation with homeostatic cytokines (19, 25). In addition, CCR7− effector memory T cells are more responsive to homeostatic cytokines than CCR7+ central memory T cells (26). We therefore compared the proliferative response of CD4+CD25− T cell subsets purified according to CCR7 and IL-7Rα expression to IL-7 or IL-15 (Fig. 2 B). IL-7R+ subsets proliferated with IL-7 and IL-15, showing a higher rate of proliferation in the CCR7− subset, as expected. In contrast, IL-7R− cells that were also heterogeneous for CCR7 expression failed to proliferate with IL-7, as expected. Notably, although CCR7−IL-7R− proliferated in response to IL-15, only few cells were recovered from IL-15–driven cultures (Fig. 2 C), indicating that they failed to survive.
In summary, IL-7R− cells have characteristics of effector cells because they produce high levels of effector cytokines and little IL-2, expand poorly upon TCR stimulation, and die in the presence of homeostatic cytokines.
CD4+IL-7R− T cells respond to persistent antigens
The IL-7R is down-regulated upon TCR stimulation (24), and human CD8 T cells that have down-regulated IL-7R expression are specific for persistent but not for cleared viruses (27). We therefore investigated the proliferative responses of CD4+IL-7R+ and IL-7R− T cell subsets to persistent and vaccination antigens (Fig. 3, A and B) in the presence of low amounts of IL-2 to allow for TCR-driven proliferation of IL-7R− cells (Fig. 2 A). Proliferative responses to the persistent pathogens Candida albicans and human CMV (HCMV), as well as to the self-antigen MelanA, could be readily detected in both IL-7R+ and IL-7R− subsets. IL-7R+ cells also responded to a variety of vaccination antigens, including tetanus toxoid (TT), influenza hemagglutinin, and purified protein derivate (PPD) in vaccinated donors. Conversely, IL-7R− cells responded poorly or not at all to these antigens in the same donors. However, in two donors that had been recently vaccinated against influenza and in one with recent tuberculosis infection, IL-7R− cells did proliferate with hemagglutinin and PPD, respectively (unpublished data), consistent with the view that down-regulation of IL-7R in vivo reflects recent antigenic activation. To assess if antigen-specific IL-7R− cells coproduced IFN-γ and IL-10, proliferating cells were restimulated with autologous monocyte-derived DCs in the absence or presence of the relevant antigens. As shown in Fig. 3 C, a substantial fraction of IL-7R− cells that had proliferated with HCMV or C. albicans coproduced IFN-γ and IL-10 upon antigen-specific restimulation. Conversely, IL-7R+ cells from the same donor produced only IFN-γ under the same conditions. Cells specific for C. albicans but not for HCMV also produced IL-17 upon restimulation (unpublished data). Moreover, in three donors we were able to detect low levels of IL-10 in IL-7R− but not in IL-7R+ subsets in response to HCMV antigens after 24 h ex vivo by ELISA, whereas IFN-γ was detected in the supernatants of both subsets (unpublished data). Collectively our results indicate that IL-7R− cells can produce IFN-γ and IL-10 in response to persistent antigens under steady-state conditions.
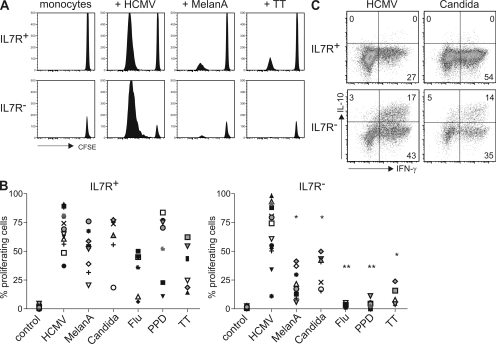
Antigen specificities of IL-7R− T cells. (A) CFSE profiles of CD25−IL-7R− and IL-7R+ subsets in one donor after stimulation in the absence or presence of protein antigens derived from HCMV, MelanA, and TT on day 7. (B) Percentages of purified IL-7R− and IL-7R+ cells that have diluted CFSE on day 7 in at least 20 different donors in response to HCMV (14 donors), C. albicans (Candida; 7 donors), MelanA (8 donors), influenza hemagglutinin (Flu; 6 donors), M. tuberculosis (PPD; 7 donors), and TT (6 donors). Significantly lower proliferation among IL-7R− cells is indicated (*, P < 0.05; and **, P < 0.005). (C) IFN-γ versus IL-10 production of cells that had divided in response to HCMV or Candida in IL-7R+ and IL-7R− subsets after restimulation with the relevant antigen. One representative experiment with different donors out of three (Candida) or five (HCMV) is shown.
The IL-7R− subset is enriched for cells that have been recently activated in vivo
The results so far suggested that IL-7R− cells had been recently activated by persistent antigens. To assess the recent proliferation history of CD4+ T cell subsets, we performed ex vivo Ki-67 staining because it identifies circulating T cells that have proliferated in the last few days (28). As shown in Fig. 4 A, very few IL-7R+ cells were Ki-67+ (1 ± 1%), whereas substantial fractions not only of CD25+ T reg cells, as expected (4, 5), but in particular of IL-7R− cells expressed this proliferation marker (15 ± 8% and 21 ± 10%, respectively). The different proliferation histories of the IL-7R+ and IL-7R− subsets were also reflected by their phenotypes, because the activation markers ICOS and HLA-DR (Fig. 4, B and C) were expressed on comparable fractions of CD25+ T reg cells and IL-7R− cells but not on IL-7R+ cells. We also analyzed the ex vivo expression levels of the antiapoptotic molecule Bcl-2 (Fig. 4 D), which is down-regulated by antigenic stimulation but induced by homeostatic cytokines. Bcl-2 expression levels were high in IL-7R+ cells, lower in CD25+ T reg cells, as reported previously (6), and lowest in IL-7R− cells, and therefore correlated with cell recovery in TCR- or cytokine-driven cultures (Fig. 2). Finally, IL-7R− cells expressed higher levels of the T reg cell marker CTLA-4 than IL-7R+ cells after in vitro activation, and CD25+ T reg cells expressed the highest levels, as expected (Fig. 4 E). Collectively these results are consistent with the view that the IL-7R− subset contains cells that have been recently activated by persistent antigens in vivo. Because IL-7R− cells express low levels of Bcl-2 (Fig. 4 D), responded poorly to homeostatic cytokines in vitro (Fig. 2 B), are specific for persistent antigens (Fig. 3), and have a high turnover rate in vivo (Fig. 4 A), it is tempting to speculate that the persistence of these cells depends on chronic TCR stimulation. Consistent with this hypothesis, a subset of mouse CD4+ T cells was recently identified that rapidly turned over in response to TCR ligands in vivo, and that was distinct from memory-like cells that performed slow homeostatic proliferation with IL-7 or IL-15 (25).
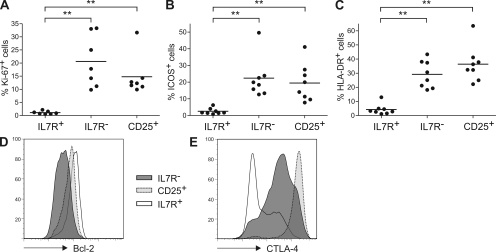
Activation marker expression in IL-7R− T cell subsets. CD4+CD45RA− T cell subsets identified by CD25 and IL-7Rα expression were analyzed once in at least seven healthy donors for the expression of (A) Ki-67, (B) ICOS, or (C) HLA-DR. Significant differences are indicated (**, P < 0.005). Horizontal bars represent means. (D and E) Intracellular expression of (D) Bcl-2 ex vivo and (E) CTLA-4 after stimulation of purified subsets. Histogram plots are representative of single experiments in at least three different donors.
CD4+IL-7R− T cells suppress upon strong TCR activation in an IL-10–dependent manner
The high IL-10–producing capacities of IL-7R− T cells suggested that they possessed suppressive potential. Optimal activation of IL-7R− T cells required strong stimulation (Fig. 2 A and not depicted), whereas in vitro suppression of T cell priming is most efficient after weak stimulation of responder cells (29). In conventional in vitro suppression assays responder and suppressor T cell populations are stimulated by anti-CD3, PHA, or allogenic APCs that activate both populations simultaneously. We designed a novel in vitro suppression assay in which responder and suppressor T cell populations could be selectively stimulated with the superantigens toxic shock syndrome toxin (TSST) and staphylococcal enterotoxin B (SEB), respectively. For this aim, CFSE-labeled responder cells were rendered hyporesponsive to SEB by depleting TCR-Vβ3–, 12–, 14–, and 17–expressing cells, whereas unlabeled putative suppressor populations were depleted of TSST-responsive TCR-Vβ2+ cells. T cell priming by TSST-loaded DCs was then analyzed by CFSE dilution of TCR-Vβ2+ responder cells in the presence of TCR-Vβ2− populations of CD25+ T reg cells, IL-7R− cells, or naive control cells (Fig. 5 A) that were stimulated with different concentrations of SEB. IL-7R− T cells suppressed poorly or not at all in the absence of SEB or at low SEB concentrations, but efficiently suppressed at high SEB concentrations, indicating that they required strong TCR stimulation for suppression. Conversely, CD25+ T reg cells strongly inhibited T cell priming even in the absence of SEB. Thus, CD25+ T reg cells either suppress efficiently upon weak stimulation of TCR-Vβ2− cells by TSST, or alternatively, strong stimulation of highly autoreactive CD25+ T reg cells by autologous DC could be sufficient for suppression. The suppression assay was done with a mixture of myeloid DCs (mDCs) and plasmacytoid DCs (pDCs), the two principal DC populations in human blood, because the former prime naive T cells more potently and the latter induce IL-10 expression in T cells more efficiently. Nevertheless, IL-10–dependent suppression by IL-7R− cells was also observed when mDCs were used alone, but naive T cell proliferation was higher when pDCs were present (unpublished data). To exclude that the suppression induced by IL-7R− cells was mediated by contaminating Foxp3+ cells (Fig. 1 B), we depleted CCR4+ cells from the IL-7R− subset, because CCR4 is expressed on CD25+ T reg cells (30) but not on Th1 cells in human blood (26). CCR4−IL-7R− cells contained only very few Foxp3+ cells (4 ± 2%) but efficiently suppressed naive T cell proliferation when stimulated with SEB (Fig. 5 B). These results suggested that IL-7R− T cells contain suppressor cells that are distinct from natural T reg cells. Consistently, suppression by IL-7R− cells but not by CD25+ T reg cells was inhibited upon IL-10 neutralization (Fig. 5, B and C). Importantly, both CD25+ T reg cells and IL-7R− T cells also suppressed proliferation of IL-7R+ memory T cells (Fig. 5 B). CD25+ T reg cells inhibited naive T cell proliferation with similar efficiency in the absence and presence of IL-10 neutralization (80 ± 18% and 75 ± 19%, respectively; Fig. 5 C, left). Conversely, the suppression induced by IL-7R− cells was 30 ± 20% and was significantly (P < 0.005) reduced to 10 ± 17% upon IL-10 neutralization (Fig. 5 C, right). The different suppressive efficiencies of the two subsets might be explained by the fact that CD25+ T reg cells are a nearly homogenous population of 82 ± 16% Foxp3-expressing cells (Fig. 1 B), whereas a smaller fraction of IL-7R− cells produce IL-10 (Fig. 1 E). Consistent with a higher suppressive potential, CD25+ T reg cells were still suppressive at a 1:4 dilution (Fig. 5 D), whereas IL-7R− cells were already poorly suppressive at a 1:2 dilution (not depicted). However, neutralization of IL-10 did not significantly revert the suppression induced by CD25+ T reg cells at different cellular dilutions, consistent with the view that the CD25+ T reg cells and IL-7R− cells suppress via different mechanisms.
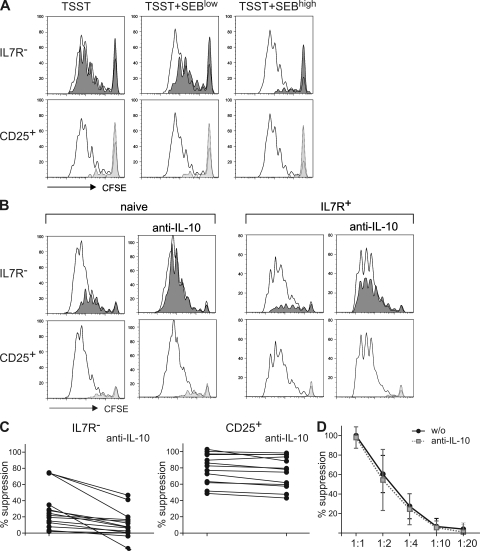
Different requirements for suppression of CD25+ T reg cells and IL-7R− cells. (A and B) CFSE-labeled SEB-hyporesponsive naive T cells were stimulated with DCs pulsed with TSST and different amounts of SEB in the presence of different TSST hyporesponsive T cell populations. Open histograms show CFSE profiles of TCR-Vβ2+ responder cells when naive control cells were present, whereas shaded histograms show the effects of the presence of IL-7R− cells (top) or of CD25+ T reg cells (bottom). (A) CFSE profiles in the absence or presence of 50 pg/ml (low) or 1 ng/ml (high) SEB are representative of four independent experiments with different donors. (B) Suppression of naive cells (representative of 14 donors; left) or of IL-7R+ memory cells (representative of 6 donors; right) in the absence or presence of IL-10 neutralization induced by CCR4−IL-7R− cells (top) or by CD25+ T reg cells (bottom). (C) Suppression induced by CCR4−IL-7R− cells (left) and by CD25+ T reg cells (right) in 14 different donors in the absence or presence of IL-10 neutralization. (D) Effects of T reg cell dilution on suppression in the absence or presence of anti–IL-10. The means of six independent experiments are shown.
Human Tr1-like cells could so far be generated only in vitro, and this is to our knowledge the first report that identifies circulating human cells with a Tr1-like cytokine profile and function in vivo. We show that these cells have a CD4+CD25−IL-7R− phenotype, are chronically activated by persistent antigens, and are effector-like cells that coproduce IL-10 and IFN-γ but not IL-2. They are distinct from natural CD25+ T reg cells and can inhibit naive and antigen-experienced T cell activation in an IL-10–dependent manner. We therefore suggest that they might represent the human counterpart of the mouse Tr1 cells or the IL-10–producing Th1 cells that limit immunopathology in parasitic infections (15, 16). The identification of circulating Tr1-like cells should facilitate their further characterization in human clinical studies.
MATERIALS AND METHODS
Media and reagents.
Recombinant cytokines (IL-2, IL-4, IL-7, IL-15, and IL-10) were purchased from R&D Systems. For FACS sorting (FACSAria and FACSDiva; BD) and analysis (LSRII and FACSCalibur; BD), the following antibodies were used: CD4–Alexa Fluor 405 (TT1), CD19-Cy5 (BU12), CD45RA–Alexa Fluor 700/-Cy5/-FITC (4G11), and HLA-DR–Cy5 (L243; in-house conjugates); ICOS-PE, IL-7R–PE/–Bio, TCR-Vβ2–FITC/-Bio, TCR-Vβ3–, 12–, 14–, and 17–FITC, and CD25-FITC (Beckman Coulter); CD25-PE (Miltenyi Biotec); CD25- allophycocyanin and IL-17A–Alexa Fluor 647 (eBioscience); CD4–Alexa Fluor 700, CD14-Cy5, CCR4–Pe-Cy7, IFN-γ–FITC, IL-10-PE/allophycocyanin, IL-2–allophycocyanin, and IL-4–PE (BD); streptavidin-allophycocyanin/–Pacific blue (Invitrogen); CCR7 (R&D Systems); and goat anti–mouse IgG2a–Bio (SouthernBiotech).
Cell culture.
PBMCs were obtained from buffy coats of healthy donors (DRK) by Ficoll-Hypaque gradient (Sigma-Aldrich). All experiments with patient material were approved by the local ethics committee (Charite Ethikkommision). DCs were labeled with CD1c-FITC and BDCA-4–PE antibodies and were positively selected with anti-FITC and anti-PE microbeads (Miltenyi Biotec), followed by sorting into pDC and mDC subsets by the exclusion of contaminating B cells (CD19+) and monocytes (CD14+). Monocytes were purified with anti-CD14 magnetic beads (Miltenyi Biotec). Naive and memory CD4+ T cell subsets were enriched with magnetic beads, and IL-7R/CD25–expressing subsets were purified by cell sorting. Cells were cultured as described previously (26). T cell stimulation was performed with 0.1 µM phorbol 12,13-dibutyrate and 1 µg/ml ionomycin (Sigma-Aldrich), or with optimal concentrations of either immobilized anti-CD3 antibodies alone (2 µg/ml) or at 0.1 µg/ml in the presence of 6 µg/ml anti-CD28 antibodies (BD).
Intracellular stainings.
Foxp3 staining was performed with the anti–human Foxp3 staining set (eBioscience) according to the manufacturer's instructions. Cytokine-producing capacity and CTLA-4 expression were assessed after stimulation for 6 and 4 h, respectively, and addition of 10 µg/ml Brefeldin A (Sigma-Aldrich) after 2 h. After fixation with 2% paraformaldehyde (PFA; Merck), cells were permeabilized with PBS/0.5% saponin (Sigma-Aldrich) and stained for cytokines. CTLA-4 was stained with anti–CTLA-4 (BNI-3; BD) antibody followed by an FITC-coupled anti–mouse IgG2a antibody (SouthernBiotech). To examine Ki-67 and Bcl-2 expression, PBMCs were first labeled with antibodies against CD127, CD25, CD4, and CD45RA, fixed with 2% PFA, and stained with anti–Ki-67–FITC (BD) in PBS/0.5% saponin or with anti-Bcl2–FITC (Dako) in PBS/1% Tween (Sigma-Aldrich).
Analysis of antigen specificities.
Antigen specificities were analyzed as previously described (26). 5 × 104 monocytes were incubated with antigens and CFSE-labeled autologous T cell subsets at a 1:1 ratio in medium with human serum. CFSE dilution was analyzed after 7 d of culture. The antigens used were HCMV lysate (1:1,000), 0.5 particles/ml of C. albicans extract (provided by G. Gerna and D. Lilleri, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy), 1 µg/ml rhMelanA, 1 µg/ml rh-hemagglutinin–influenza A virus (both from Prospec-Tany TechnoGene), 2 µg/ml TT (Novartis), and 1 µg/ml PPD of Mycobacterium tuberculosis (provided by M. Jacobsen, MPI Immunology, Berlin, Germany). 1 ng/ml IL-2 corresponding to 2.4 IU/ml was added where indicated in the figures. In some experiments, antigen specificity was confirmed by restimulation with autologous monocytes or monocyte-derived DCs and the relevant antigens (Fig. 3 C), including an overlapping peptide pool of MelanA (JPT Peptide Technologies; not depicted), followed by intracellular cytokine staining of CFSE− T cells. In some experiments, cytokine production of antigen-specific cells was analyzed by ELISA, as described previously (26). In brief, irradiated monocytes preincubated with antigens were cultured 1:1 with autologous T cell subsets, and the release of cytokines was analyzed after 24 h of stimulation.
Suppression assay.
Responder T cells were rendered hyporesponsive to SEB by depleting TCR-Vβ3, 12, 14, and 17+ cells by cell sorting and labeled with CFSE. They were co-cultured at a 1:1 ratio with unlabeled regulatory subsets or naive control cells that were rendered hyporesponsive to TSST by depleting TCR-Vβ2+ cells. 2–4 × 103 DCs (mDCs and pDCs at a 1:1 ratio) that had been pulsed with different concentrations of SEB and/or TSST (Sigma-Aldrich) for 30 min at 37°C were added as stimulators. After 5 d, cells were stained and TCR-Vβ2+ cells analyzed for CFSE dilution by FACS. Where indicated in the figures, IL-10 was neutralized by the addition of anti–IL-10 and anti–IL-10R antibodies at 10 µg/ml (BD). To calculate the percent suppression, we set the CFSE mean fluorescence intensity (MFI) of undivided cells as 100% suppression and the MFI of TCR-Vβ2+ cells in the presence of unlabeled naive TCR-Vβ2− control cells as 0% suppression.
Statistics.
Statistical significance was calculated using the two-tailed Student's t test. P < 0.05 (*) and P < 0.005 (**) were regarded as statistically significant.
Acknowledgments
We thank C. Romagnani, A. Hauser, A. Scheffold, and A. Thiel for critical reading and comments. A special thanks to Dr. Knels from the Red Cross Blood Bank in Dresden, and to Prof. G. Gerna and Dr. D. Lilleri of the Virology Department of Pavia Hospital for providing us with the HCMV and C. albicans preparations. Furthermore, we would also like to thank the laboratory managers, and in particular T. Kaiser and K. Raba from the flow cytometry facility of the German Rheumatism Research Center for their invaluable support.
This work was supported by the Charité Medical School and the Deutsche Forschungsgemeinschaft (junior group grant SFB650 TPN01).
The authors have no conflicting financial interests.
References
- Sakaguchi S. 2004. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses.Annu. Rev. Immunol. 22:531–562 [Abstract] [Google Scholar]
- Shevach E.M. 2001. Certified professionals: CD4+CD25+ suppressor T cells.J. Exp. Med. 193:F41–F46 [Europe PMC free article] [Abstract] [Google Scholar]
- Seddon B., Mason D. 1999. Peripheral autoantigen induces regulatory T cells that prevent autoimmunity.J. Exp. Med. 189:877–882 [Europe PMC free article] [Abstract] [Google Scholar]
- Vukmanovic-Stejic M., Zhang Y., Cook J.E., Fletcher J.M., McQuaid A., Masters J.E., Rustin M.H., Taams L.S., Beverley P.C., Macallan D.C., Akbar A.N. 2006. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo.J. Clin. Invest. 116:2423–2433 [Europe PMC free article] [Abstract] [Google Scholar]
- Fisson S., Darrasse-Jeze G., Litvinova E., Septier F., Klatzmann D., Liblau R., Salomon B.L. 2003. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state.J. Exp. Med. 198:737–746 [Europe PMC free article] [Abstract] [Google Scholar]
- Taams L.S., Smith J., Rustin M.H., Salmon M., Poulter L.W., Akbar A.N. 2001. Human anergic/suppressive CD4(+)CD25(+) T cells: a highly differentiated and apoptosis-prone population.Eur. J. Immunol. 31:1122–1131 [Abstract] [Google Scholar]
- Hsieh C.S., Liang Y., Tyznik A.J., Self S.G., Liggitt D., Rudensky A.Y. 2004. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors.Immunity. 21:267–277 [Abstract] [Google Scholar]
- Groux H., O'Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J.E., Roncarolo M.G. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis.Nature. 389:737–742 [Abstract] [Google Scholar]
- Bluestone J.A., Abbas A.K. 2003. Natural versus adaptive regulatory T cells.Nat. Rev. Immunol. 3:253–257 [Abstract] [Google Scholar]
- Vieira P.L., Christensen J.R., Minaee S., O'Neill E.J., Barrat F.J., Boonstra A., Barthlott T., Stockinger B., Wraith D.C., O'Garra A. 2004. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells.J. Immunol. 172:5986–5993 [Abstract] [Google Scholar]
- Asseman C., Mauze S., Leach M.W., Coffman R.L., Powrie F. 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation.J. Exp. Med. 190:995–1004 [Europe PMC free article] [Abstract] [Google Scholar]
- Jonuleit H., Schmitt E., Schuler G., Knop J., Enk A.H. 2000. Induction of interleukin 10–producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells.J. Exp. Med. 192:1213–1222 [Europe PMC free article] [Abstract] [Google Scholar]
- Barrat F.J., Cua D.J., Boonstra A., Richards D.F., Crain C., Savelkoul H.F., de Waal-Malefyt R., Coffman R.L., Hawrylowicz C.M., O'Garra A. 2002. In vitro generation of interleukin 10–producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)– and Th2-inducing cytokines.J. Exp. Med. 195:603–616 [Europe PMC free article] [Abstract] [Google Scholar]
- Levings M.K., Sangregorio R., Galbiati F., Squadrone S., de Waal Malefyt R., Roncarolo M.G. 2001. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells.J. Immunol. 166:5530–5539 [Abstract] [Google Scholar]
- Jankovic D., Kullberg M.C., Feng C.G., Goldszmid R.S., Collazo C.M., Wilson M., Wynn T.A., Kamanaka M., Flavell R.A., Sher A. 2007. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection.J. Exp. Med. 204:273–283 [Europe PMC free article] [Abstract] [Google Scholar]
- Anderson C.F., Oukka M., Kuchroo V.J., Sacks D. 2007. CD4+CD25−Foxp3− Th1 cells are the source of IL-10–mediated immune suppression in chronic cutaneous leishmaniasis.J. Exp. Med. 204:285–297 [Europe PMC free article] [Abstract] [Google Scholar]
- Liu W., Putnam A.L., Xu-Yu Z., Szot G.L., Lee M.R., Zhu S., Gottlieb P.A., Kapranov P., Gingeras T.R., Fazekas de St Groth B., et al. 2006. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells.J. Exp. Med. 203:1701–1711 [Europe PMC free article] [Abstract] [Google Scholar]
- Seddiki N., Santner-Nanan B., Martinson J., Zaunders J., Sasson S., Landay A., Solomon M., Selby W., Alexander S.I., Nanan R., et al. 2006. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells.J. Exp. Med. 203:1693–1700 [Europe PMC free article] [Abstract] [Google Scholar]
- Bradley L.M., Haynes L., Swain S.L. 2005. IL-7: maintaining T-cell memory and achieving homeostasis.Trends Immunol. 26:172–176 [Abstract] [Google Scholar]
- Banham A.H. 2006. Cell-surface IL-7 receptor expression facilitates the purification of FOXP3(+) regulatory T cells.Trends Immunol. 27:541–544 [Abstract] [Google Scholar]
- Couper K.N., Blount D.G., Wilson M.S., Hafalla J.C., Belkaid Y., Kamanaka M., Flavell R.A., de Souza J.B., Riley E.M. 2008. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection.PLoS Pathog. 4:e1000004. [Europe PMC free article] [Abstract] [Google Scholar]
- Lochner M., Peduto L., Cherrier M., Sawa S., Langa F., Varona R., Riethmacher D., Si-Tahar M., Di Santo J.P., Eberl G. 2008. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORγt+ T cells.J. Exp. Med. 205:1381–1393 [Europe PMC free article] [Abstract] [Google Scholar]
- Huster K.M., Busch V., Schiemann M., Linkemann K., Kerksiek K.M., Wagner H., Busch D.H. 2004. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets.Proc. Natl. Acad. Sci. USA. 101:5610–5615 [Europe PMC free article] [Abstract] [Google Scholar]
- Lozza L., Rivino L., Guarda G., Jarrossay D., Rinaldi A., Bertoni F., Sallusto F., Lanzavecchia A., Geginat J. 2008. The strength of T cell stimulation determines IL-7 responsiveness, secondary expansion, and lineage commitment of primed human CD4(+)IL-7R(hi) T cells.Eur. J. Immunol. 38:30–39 [Abstract] [Google Scholar]
- Purton J.F., Tan J.T., Rubinstein M.P., Kim D.M., Sprent J., Surh C.D. 2007. Antiviral CD4+ memory T cells are IL-15 dependent.J. Exp. Med. 204:951–961 [Europe PMC free article] [Abstract] [Google Scholar]
- Rivino L., Messi M., Jarrossay D., Lanzavecchia A., Sallusto F., Geginat J. 2004. Chemokine receptor expression identifies pre–T helper (Th)1, pre–Th2, and nonpolarized cells among human CD4+ central memory T cells.J. Exp. Med. 200:725–735 [Europe PMC free article] [Abstract] [Google Scholar]
- van Leeuwen E.M., de Bree G.J., Remmerswaal E.B., Yong S.L., Tesselaar K., ten Berge I.J., van Lier R.A. 2005. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells.Blood. 106:2091–2098 [Abstract] [Google Scholar]
- Pitcher C.J., Hagen S.I., Walker J.M., Lum R., Mitchell B.L., Maino V.C., Axthelm M.K., Picker L.J. 2002. Development and homeostasis of T cell memory in rhesus macaque.J. Immunol. 168:29–43 [Abstract] [Google Scholar]
- George T.C., Bilsborough J., Viney J.L., Norment A.M. 2003. High antigen dose and activated dendritic cells enable Th cells to escape regulatory T cell-mediated suppression in vitro.Eur. J. Immunol. 33:502–511 [Abstract] [Google Scholar]
- Iellem A., Mariani M., Lang R., Recalde H., Panina-Bordignon P., Sinigaglia F., D'Ambrosio D. 2001. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+CD25+ regulatory T cells.J. Exp. Med. 194:847–853 [Europe PMC free article] [Abstract] [Google Scholar]
Articles from The Journal of Experimental Medicine are provided here courtesy of The Rockefeller University Press
Full text links
Read article at publisher's site: https://doi.org/10.1084/jem.20082238
Read article for free, from open access legal sources, via Unpaywall:
https://rupress.org/jem/article-pdf/206/5/1009/1200051/jem_20082238.pdf
Free after 6 months at intl.jem.org
http://intl.jem.org/cgi/reprint/206/5/1009.pdf
Free after 6 months at intl.jem.org
http://intl.jem.org/cgi/content/full/206/5/1009
Free to read at intl.jem.org
http://intl.jem.org/cgi/content/abstract/206/5/1009
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1084/jem.20082238
Article citations
Beyond Immune Balance: The Pivotal Role of Decidual Regulatory T Cells in Unexplained Recurrent Spontaneous Abortion.
J Inflamm Res, 17:2697-2710, 01 May 2024
Cited by: 1 article | PMID: 38707955 | PMCID: PMC11070170
Review Free full text in Europe PMC
Erratum: Type 1 regulatory T cell-mediated tolerance in health and disease.
Front Immunol, 13:1125497, 24 Jan 2023
Cited by: 0 articles | PMID: 36761160 | PMCID: PMC9903213
Type 1 regulatory T cell-mediated tolerance in health and disease.
Front Immunol, 13:1032575, 28 Oct 2022
Cited by: 12 articles | PMID: 36389662 | PMCID: PMC9650496
Review Free full text in Europe PMC
How the Immune System Responds to Allergy Immunotherapy.
Biomedicines, 10(11):2825, 05 Nov 2022
Cited by: 1 article | PMID: 36359345 | PMCID: PMC9688006
Review Free full text in Europe PMC
Roles of type 1 regulatory T (Tr1) cells in allergen-specific immunotherapy.
Front Allergy, 3:981126, 03 Aug 2022
Cited by: 8 articles | PMID: 35991310 | PMCID: PMC9381954
Review Free full text in Europe PMC
Go to all (107) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cells with regulatory function of the innate and adaptive immune system in primary Sjögren's syndrome.
Clin Exp Immunol, 157(3):343-349, 01 Sep 2009
Cited by: 53 articles | PMID: 19664141
Responses of CD4(+) CD25(+) Foxp3(+) and IL-10-secreting type I T regulatory cells to cluster-specific immunotherapy for allergic rhinitis in children.
Pediatr Allergy Immunol, 23(2):140-149, 23 Dec 2011
Cited by: 30 articles | PMID: 22192331
CD55 costimulation induces differentiation of a discrete T regulatory type 1 cell population with a stable phenotype.
J Immunol, 191(12):5895-5903, 06 Nov 2013
Cited by: 26 articles | PMID: 24198281
IL-2- and CD25-dependent immunoregulatory mechanisms in the homeostasis of T-cell subsets.
J Allergy Clin Immunol, 123(4):758-762, 01 Apr 2009
Cited by: 159 articles | PMID: 19348914
Review






