Abstract
Free full text

Risk of Cataract after Exposure to Low Doses of Ionizing Radiation: A 20-Year Prospective Cohort Study among US Radiologic Technologists
Abstract
The study aim was to determine the risk of cataract among radiologic technologists with respect to occupational and nonoccupational exposures to ionizing radiation and to personal characteristics. A prospective cohort of 35,705 cataract-free US radiologic technologists aged 24–44 years was followed for nearly 20 years (1983–2004) by using two follow-up questionnaires. During the study period, 2,382 cataracts and 647 cataract extractions were reported. Cigarette smoking for ≥5 pack-years; body mass index of ≥25 kg/m2; and history of diabetes, hypertension, hypercholesterolemia, or arthritis at baseline were significantly (p ≤ 0.05) associated with increased risk of cataract. In multivariate models, self-report of ≥3 x-rays to the face/neck was associated with a hazard ratio of cataract of 1.25 (95% confidence interval: 1.06, 1.47). For workers in the highest category (mean, 60 mGy) versus lowest category (mean, 5 mGy) of occupational dose to the lens of the eye, the adjusted hazard ratio of cataract was 1.18 (95% confidence interval: 0.99, 1.40). Findings challenge the National Council on Radiation Protection and International Commission on Radiological Protection assumptions that the lowest cumulative ionizing radiation dose to the lens of the eye that can produce a progressive cataract is approximately 2 Gy, and they support the hypothesis that the lowest cataractogenic dose in humans is substantially less than previously thought.
A cataract is an ocular lens opacity associated with visual impairment and may be classified according to anatomic location into nuclear, cortical, posterior subcapsular, or mixed types. Estimates indicate that nearly 13 million people in the United States suffer from cataracts, and this number and the associated surgical procedures are expected to increase dramatically in the coming decades (1). The only effective treatment for cataract is cataract extraction, which accounts for some 60 percent of the estimated $6 billion annual Medicare expenditure on vision-related services (2).
The lens is relatively radiosensitive, and cataract formation has long been documented as a major ocular complication associated with exposure to ionizing radiation (3). However, the lowest cataractogenic dose and the dose-response relation of cataracts in humans are not well established. For radiation protection purposes, the National Council on Radiation Protection and the International Commission on Radiological Protection assume that the minimum dose required to produce a detectable cataract is about 2 Gy in a single exposure and 5 Gy for fractionated or protracted exposure (4, 5). This assumption was supported by studies of cataract data 19 years after the atomic bombings in Hiroshima, Japan, which suggested that a point estimate for the threshold dose for radiation-induced cataract was approximately 1.5 Gy (6–8). However, a more recent reanalysis of cataract prevalence among the atomic bomb survivors that used the 2002 Dosimetry System (DS02) provided no significant evidence for a dose-response threshold (9).
The latency period, the time between irradiation and the appearance of lens opacities, is also uncertain. Early studies on radiation-induced cataract among the atomic bomb survivors exposed to 1 Gy or more showed an approximate average latency period for development of lens opacities of 2–3 years, depending upon the dose to the eye (8, 10). However, a more recent study on infants treated with protracted radium irradiation for skin hemangioma suggested that the latency period for cataract formation is probably longer for smaller doses and may reach 30–45 years (11). Long-term follow-up studies are needed to analyze the risk of cataract formation over extended time periods following low-dose ionizing radiation. The aim of the current study was to examine the incidence of cataract among US radiologic technologists over a long period of time, with attention given to individual characteristics, especially occupational and nonoccupational ionizing radiation exposure.
MATERIALS AND METHODS
The US Radiologic Technologists Study cohort and study methods have been described in detail previously (12, 13). Briefly, beginning in 1983, baseline questionnaires were mailed to all 132,454 radiologic technologists who had been certified for at least 2 years by the American Registry of Radiologic Technologists between 1926 and 1980 and were located alive. A total of 90,305 (68.2 percent) subjects responded to the baseline questionnaire that elicited information about medical outcomes, sociodemographic and lifestyle factors such as smoking and alcohol consumption, and personal diagnostic and therapeutic radiation for medical reasons. Study participants were asked to indicate the number of diagnostic x-rays they had received and the part of the body x-rayed, including head (categorized as skull, cervical spine, or other face/neck) and trunk (categorized as chest, clavicle, shoulders, ribs, abdomen, thoracic spine, lumbar spine, lumbarsacral, pelvis, or other). There were no questions about cataract or cataract extractions in the baseline questionnaire.
Also elicited from the baseline questionnaires was lifetime work history as a radiologic technologist, including facility type (hospital, physician's office) for each job, procedures performed, other practices, and protective measures used. This occupational history was used in addition to film badge readings to develop individual ionizing radiation dose for each technologist, as described previously (14). In brief, the current occupational dosimetry system provides individual annual probability density functions that represent a distribution of possible true values of the dose to the lens of the eye. The data used in the current dosimetry version included individual film-badge measurements from a commercial dosimetry provider or military dose registries, dose records provided by employers, individual work history, and protection practices from three cohort surveys. For early years (particularly before 1960), when individual measurements were unavailable, dose estimates were derived from the literature, being mindful of the national radiation protection standards at a time when most large institutions attempted conformity. Monte Carlo methods were used to simulate 100 air-kerma dose realizations from each annual dose density, and the mean of the 100 annual doses was taken to be the annual mean dose. These air-kerma dose estimates were used to calculate the dose to the lens for each year of employment by using a kerma-to-eye lens dose factor relevant to energies of diagnostic radiography. The calculated dose was adjusted upward, using the apron attenuation factor, if the badge was reported to have been worn under the apron.
During 1994–1998 and 2003–2005, second and third questionnaires were mailed to all technologists located alive at that time. In addition to general information such as hair color, eye color, and skin complexion, these questionnaires also inquired about a personal history of cataract, cataract extraction, and other medical conditions; medication use; vitamin supplement intake; and lifetime residential history.
To address ultraviolet effects from sunlight, we estimated the potential cumulative residential exposure to sunlight at baseline by using the reported solar ultraviolet radiation from the Robertson-Berger (RB) meter network located in many states (15). We merged a study participant's residential history with measurements of solar radiation between 280 nm and 330 nm (middle ultraviolet radiation spectrum or ultraviolet B) obtained from RB measurements, which were made for each state as a function of altitude, latitude, and cloud cover. One RB unit corresponds to approximately 0.35 Jm−2. By summing the levels in yearly intervals as the participant moved from residence to residence, we derived an estimate of the average annual residential ultraviolet exposure between birth and age 13 years and over the lifespan up to the calendar year of the baseline questionnaire.
The research protocol for the cohort study of US radiologic technologists was approved annually by the US National Cancer Institute's Special Studies Institutional Review Board and the University of Minnesota's Institutional Review Board.
Recent studies on the risk of age-related cataract associated with ionizing radiation exposure showed a stronger association for posterior subcapsular cataracts than for other cataract types (9, 16). Since posterior subcapsular cataracts occur at younger ages (17, 18), and information on the anatomic location of the cataract was unavailable, we restricted our analysis to subjects aged 24–44 years at baseline, that is, at the time of the first questionnaire. Of all individuals eligible at baseline (n = 66,379), 54 percent responded to both the second and third questionnaires (n = 35,870), and we included only these subjects. Compared with nonrespondents, questionnaire respondents were more likely to be younger (mean age, 34.3 years vs. 34.0 years), to be female (82.6 percent vs. 75.8 percent), to never smoke (54.1 percent vs. 49.6 percent), and to use multivitamins (38.0 percent vs. 33.5 percent). Both populations were similar in terms of other cataract risk factors.
We excluded subjects who reported that they never worked as a radiologic technologist (n = 390) or had improbably high (>80 mGy) occupational doses (n = 1,885). We also excluded all subjects who reported in either the second (n = 96) or third (n = 37) questionnaire having a cataract or cataract extraction prior to 1985 (figure 1). Thirty-two subjects who reported a cataract but whose year of diagnosis was unknown were also excluded. The total analytic cohort size was 35,705. Participants were followed from the return date of the baseline questionnaire until the return date of the third questionnaire or diagnosis of first cataract, whichever occurred first.
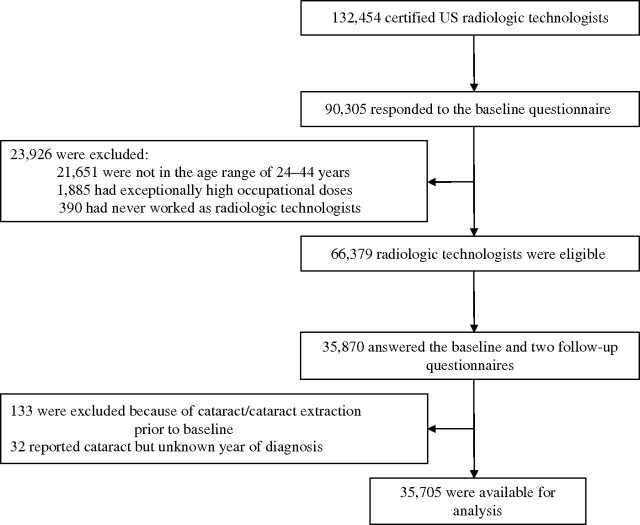
Inclusions and exclusions in the analytic cohort for risk of cataracts, US Radiologic Technologists Study, 1983–2004.
Cox regression with years of follow-up as the time scale was used to estimate hazard ratios and 95 percent confidence intervals (19) of cataract in univariate analyses. We then adjusted for sex and age at baseline. The full multivariate model included the following baseline values of factors primarily related to cataract (20–22): age at baseline (in 1-year categories); sex; body mass index categorized as underweight (<20 kg/m2), normal weight (20–<25 kg/m2), overweight (25–<30 kg/m2), and obese (≥30 kg/m2) (23); education; marital status; skin complexion; hair and iris color; mean annual residential exposure to ultraviolet radiation (categorized as <105 RB, 105–<115 RB, 115–130 RB, and >130 RB units ×10−4); alcohol consumption (categorized as never, <1, 1–2, 3–6, 7–10, and >10 drinks per week); cigarette smoking (categorized as never smoker, <5, 5–<15, 15–<25, and ≥25 pack-years of smoking); intake of multivitamin, vitamin C, or vitamin E supplements; aspirin use (categorized as never, <1, 1–14, and >14 days of use per month); and chronic diseases including diabetes, hypertension, cardiovascular diseases, and arthritis. Tests for trend of ordinal variables were based on the slope of the category median values. If values for categorical variables were missing for more than 2 percent of subjects, they were modeled as a separate unknown category. Otherwise, they were included in the reference category.
Variables significantly related to cataract were included in Cox regression models that used data on each individual's history of diagnostic and therapeutic radiation exposure. To examine the dose-response trends for personal diagnostic radiation exposure, we summed the overall and specific types of diagnostic x-rays reported by the technologists. Potential confounders were also included when calculating the linear excess relative risk (ERR) per Gy (ERR/Gy) and likelihood-ratio-based 95 percent confidence interval for occupational ionizing radiation dose to the lens of the eyes, modeled as a continuous variable, using EPICURE software (24). Calculating ERR/Gy has the advantage that the results can be more easily compared with those of other studies. By adding 1.0 to the ERR, one obtains the relative risk at 1 Gy of radiation to the lens of the eye.
The analysis was repeated for radiation dose as a categorical variable with seven equal groups, using cutpoints of 0.95, 16.1, 22.0, 28.9, 37.2, and 49.1 mGy. To address cataract risk by age at onset, multivariate analyses were repeated for significant exposure variables, with cataract reported before age 50 years as the outcome variable. In this analysis, participants were followed from the return date of the baseline questionnaire to the earliest of diagnosis of first cataract, age 49 years, or date of the third questionnaire. The multivariate analyses were similarly performed for cataract extraction, where participants were followed from the return date of the baseline questionnaire to the earliest of year of surgery or third questionnaire.
RESULTS
Subjects were followed an average of 19.2 years (standard deviation, 1.8), with a total of 685,341 person-years of observation. As shown in table 1, the cohort was predominantly female (83 percent), and 66 percent of the study members began working as radiologic technologists at age 20 years or younger. Nearly 80 percent of the study population had worked for at least 6 years at baseline.
TABLE 1.
Characteristics of the study population at the time of the baseline questionnaire (n = 35,705), US Radiologic Technologists Study, 1983–2004
| No. | % | |
| Age (years) | ||
    23–29 23–29 | 7,735 | 21.7 |
    30–34 30–34 | 12,018 | 33.7 |
    35–39 35–39 | 9,865 | 27.6 |
    40–44 40–44 | 6,087 | 17.1 |
| Sex | ||
    Men Men | 6,199 | 17.4 |
    Women Women | 29,506 | 82.6 |
| Education | ||
    Radiation technology program Radiation technology program | 19,898 | 55.7 |
    Any college or higher Any college or higher | 14,135 | 39.6 |
    Other/unknown Other/unknown | 1,672 | 4.7 |
| Skin complexion | ||
    Fair Fair | 17,404 | 48.7 |
    Medium Medium | 17,206 | 48.2 |
    Dark Dark | 893 | 2.5 |
    Unknown Unknown | 202 | 0.6 |
| Marital status | ||
    Married Married | 27,825 | 77.9 |
    Other/unknown Other/unknown | 3,675 | 10.3 |
    Never married Never married | 4,205 | 11.8 |
| Body mass index (kg/m2) | ||
    <20 <20 | 6,413 | 18.0 |
    20–<25 20–<25 | 19,779 | 55.4 |
    25–<30 25–<30 | 6,517 | 18.3 |
    ≥30 ≥30 | 2,211 | 6.2 |
    Unknown Unknown | 785 | 2.2 |
| No. of years of work as a radiologic technologist | ||
    <6 <6 | 6,962 | 19.5 |
    6–<10 6–<10 | 11,939 | 33.4 |
    10–<13 10–<13 | 7,218 | 20.2 |
    ≥13 ≥13 | 9,009 | 25.2 |
    Unknown Unknown | 577 | 1.6 |
| Age first worked as a radiologic technologist (years) | ||
    14–18 14–18 | 10,726 | 30.0 |
    19–20 19–20 | 12,831 | 35.9 |
    21–25 21–25 | 9,753 | 27.3 |
    26–43 26–43 | 1,818 | 5.1 |
    Unknown Unknown | 577 | 1.6 |
During the study period, 2,382 cataracts (of which 591 occurred before age 50 years) and 647 cataract extractions (of which 183 occurred before age 50 years) were reported. The risk of cataract increased with age by 15 percent per year. Being female; being nonmarried; smoking 15 or more pack-years of cigarettes; having a body mass index of 25 kg/m2 or higher; and having a history of diabetes, hypertension, hypercholesterolemia, or arthritis at baseline were also significantly (p ≤ 0.05) associated with increased risk of cataract (table 2). Consuming 1–10 drinks of alcohol per week was related to reduced risk of cataract compared with consuming less than 1 alcoholic drink per week. Subjects with the highest mean annual residential ultraviolet exposure at age 13 years had a higher risk of cataract, but this comparison did not reach statistical significance after adjustment for other variables.
TABLE 2.
Hazard ratios and 95% confidence intervals for cataract, according to selected personal exposures and medical conditions at baseline, among subjects in the US Radiologic Technologists Study, 1983–2004
| Covariate | Cases (N = 2,382) | Age and sex adjusted * | Multivariate analysis† | ||
| HR | 95% CI‡ | HR | 95% CI | ||
| Age at baseline (years)§ | |||||
    <30 <30 | 165 | 1¶ | 1¶ | ||
    30–34 30–34 | 463 | 1.98 | 1.66, 2.37 | 1.90 | 1.59, 2.27 |
    35–39 35–39 | 762 | 4.13 | 3.49, 4.89 | 3.67 | 3.09, 4.36 |
    40–44 40–44 | 992 | 9.25 | 7.84, 10.91 | 7.59 | 6.39, 9.03 |
| Sex | |||||
    Men Men | 425 | 1 | 1 | ||
    Women Women | 1,957 | 1.08 | 0.97, 1.20 | 1.26 | 1.12, 1.42 |
| Marital status | |||||
    Currently married Currently married | 1,814 | 1 | 1 | ||
    Never married Never married | 240 | 1.30 | 1.14, 1.49 | 1.20 | 1.04, 1.38 |
    Other# Other# | 328 | 1.21 | 1.08, 1.36 | 1.18 | 1.05, 1.34 |
| Education | |||||
    High school/other# High school/other# | 98 | 1 | 1 | ||
    Technologic training Technologic training | 1,350 | 1.06 | 0.86, 1.30 | 1.08 | 0.88, 1.33 |
    College/graduate College/graduate | 934 | 1.16 | 0.94, 1.42 | 1.17 | 0.95, 1.44 |
| Iris color | |||||
    Dark Dark | 812 | 1 | 1 | ||
    Grey (hazel/green) Grey (hazel/green) | 843 | 1.02 | 0.92, 1.12 | 1.02 | 0.92, 1.13 |
    Blue Blue | 696 | 0.93 | 0.84, 1.03 | 0.95 | 0.85, 1.06 |
    Other# Other# | 31 | 1.04 | 0.73, 1.49 | 1.06 | 0.71, 1.59 |
| Skin complexion | |||||
    Fair Fair | 1,164 | 1 | 1 | ||
    Medium Medium | 1,133 | 0.98 | 0.90, 1.06 | 0.96 | 0.88, 1.05 |
    Dark Dark | 68 | 1.00 | 0.78, 1.28 | 0.90 | 0.70, 1.16 |
    Other# Other# | 17 | 1.08 | 0.67, 1.75 | 1.08 | 0.60, 1.91 |
| Hair color | |||||
    Dark Dark | 1,146 | 1 | 1 | ||
    Light Light | 1,104 | 0.95 | 0.87, 1.03 | 0.97 | 0.88, 1.06 |
    Red/auburn Red/auburn | 108 | 0.91 | 0.75, 1.11 | 0.86 | 0.71, 1.06 |
    Other# Other# | 24 | 0.97 | 0.65, 1.45 | 0.94 | 0.59, 1.51 |
| Body mass index (kg/m2) | |||||
    <20 <20 | 298 | 1¶ | 1¶ | ||
    20–<25 20–<25 | 1,189 | 1.10 | 0.97, 1.25 | 1.10 | 0.97, 1.25 |
    25–<30 25–<30 | 583 | 1.50 | 1.30, 1.74 | 1.43 | 1.24, 1.66 |
    ≥30 ≥30 | 245 | 1.74 | 1.46, 2.06 | 1.44 | 1.21, 1.72 |
    Unknown# Unknown# | 67 | 1.46 | 1.12, 1.91 | 1.38 | 1.05, 1.80 |
| Lifetime mean annual residential ultraviolet exposure (RB‡ units × 10−4) | |||||
    <105 <105 | 336 | 1¶ | 1 | ||
    105–<115 105–<115 | 554 | 0.92 | 0.80, 1.06 | 0.85 | 0.70, 1.04 |
    115–<130 115–<130 | 507 | 0.98 | 0.86, 1.13 | 0.96 | 0.78, 1.19 |
    ≥130 ≥130 | 818 | 1.08 | 0.95, 1.23 | 0.95 | 0.77, 1.17 |
    Unknown# Unknown# | 167 | 0.97 | 0.81, 1.17 | 0.87 | 0.66, 1.15 |
| Lifetime mean annual residential ultraviolet exposure at age 13 years (RB units × 10−4) | |||||
    <105 <105 | 439 | 1¶ | 1 | ||
    105–<115 105–<115 | 647 | 0.99 | 0.88, 1.12 | 1.10 | 0.93, 1.33 |
    115–<130 115–<130 | 513 | 0.99 | 0.88, 1.13 | 1.02 | 0.84, 1.24 |
    ≥130 ≥130 | 695 | 1.16 | 1.03, 1.31 | 1.19 | 0.98, 1.45 |
    Unknown# Unknown# | 88 | 1.04 | 0.83, 1.31 | 1.13 | 0.81, 1.57 |
| Cigarette smoking (pack-years) | |||||
    Never# Never# | 1,116 | 1¶ | 1¶ | ||
    <5 <5 | 333 | 0.95 | 0.84, 1.07 | 0.98 | 0.87, 1.11 |
    5–<15 5–<15 | 390 | 1.09 | 0.97, 1.22 | 1.11 | 0.99, 1.25 |
    15–<25 15–<25 | 288 | 1.22 | 1.07, 1.39 | 1.25 | 1.09, 1.43 |
    ≥25 ≥25 | 255 | 1.33 | 1.15, 1.53 | 1.33 | 1.15, 1.53 |
| Alcohol consumption (drinks/week) | |||||
    <1 <1 | 1,061 | 1 | 1 | ||
    Never# Never# | 452 | 1.05 | 0.94, 1.17 | 1.05 | 0.94, 1.17 |
    1–2 1–2 | 320 | 0.81 | 0.71, 0.92 | 0.84 | 0.74, 0.96 |
    3–6 3–6 | 318 | 0.86 | 0.76, 0.97 | 0.88 | 0.78, 1.00 |
    7–10 7–10 | 130 | 0.83 | 0.69, 0.99 | 0.84 | 0.70, 1.01 |
    >10 >10 | 101 | 0.95 | 0.77, 1.17 | 0.92 | 0.75, 1.13 |
| Hypercholesterolemia (>240 mg/dl) | |||||
    Never# Never# | 2,333 | 1 | 1 | ||
    Yes Yes | 49 | 1.73 | 1.30, 2.30 | 1.49 | 1.12, 1.99 |
| Hypertension | |||||
    Never# Never# | 2,220 | 1 | 1 | ||
    Yes Yes | 162 | 1.55 | 1.32, 1.82 | 1.24 | 1.05, 1.46 |
| Myocardial infarction or cerebrovascular accident | |||||
    Never# Never# | 2,368 | 1 | 1 | ||
    Yes Yes | 14 | 1.30 | 0.77, 2.20 | 0.98 | 0.58, 1.67 |
| Diabetes mellitus | |||||
    Never# Never# | 2,307 | 1 | 1 | ||
    Yes Yes | 75 | 4.96 | 3.94, 6.25 | 4.10 | 3.24, 5.20 |
| Arthritis | |||||
    Never# Never# | 2,169 | 1 | 1 | ||
    Rheumatoid Rheumatoid | 45 | 1.53 | 1.14, 2.05 | 1.33 | 0.99, 1.79 |
    Other Other | 168 | 1.68 | 1.44, 1.97 | 1.51 | 1.29, 1.78 |
| Vitamin C supplement | |||||
    Never# Never# | 2,090 | 1 | 1 | ||
    Yes Yes | 292 | 0.96 | 0.85, 1.08 | 0.91 | 0.78, 1.05 |
| Vitamin E supplement | |||||
    Never# Never# | 2,193 | 1 | 1 | ||
    Yes Yes | 189 | 1.06 | 0.91, 1.23 | 1.08 | 0.90, 1.29 |
| Multivitamin intake | |||||
    Never# Never# | 1,477 | 1 | 1 | ||
    Yes Yes | 905 | 0.97 | 0.89, 1.06 | 0.98 | 0.90, 1.08 |
| Aspirin use (days/month) | |||||
    Never# Never# | 1,254 | 1¶ | 1¶ | ||
    <1 <1 | 306 | 1.02 | 0.90, 1.16 | 1.05 | 0.93, 1.19 |
    1–14 1–14 | 553 | 0.90 | 0.82, 1.00 | 0.91 | 0.82, 1.00 |
    >14 >14 | 269 | 1.25 | 1.09, 1.42 | 1.16 | 1.02, 1.33 |
A trend of increased cataract risk was found with increasing number of personal diagnostic x-rays (ptrend < 0.001). Compared with those with five or fewer x-rays, subjects with 25 or more x-rays at baseline had adjusted hazard ratios of 1.41 (95 percent confidence interval (CI): 1.19, 1.68) (table 3) and 2.40 (95 percent CI: 1.38, 4.19) for cataract and cataract before age 50 years, respectively (data not shown). For cataract at any age, subjects who reported, at baseline, having three or more diagnostic x-rays to the face or neck showed an adjusted hazard ratio of 1.25 (95 percent CI: 1.06,1.47; ptrend < 0.01) compared with those who had not undergone x-ray procedures to this part of the body. No association was observed for x-rays to body regions remote from the eyes, such as the lumbosacral spine. Although the fully adjusted increased risk of cataract associated with history of any radiotherapy did not reach statistical significance, history of radiotherapy to the head at age 15 years or younger was related to an adjusted hazard ratio of 1.41 (95 percent CI: 1.00, 1.99). The corresponding risk for radiotherapy to the head after age 15 years was 1.27 (95 percent CI: 0.86, 1.87).
TABLE 3.
Hazard ratios for cataract, according to diagnostic x-rays and radiotherapy history at baseline, among subjects in the US Radiologic Technologists Study, 1983–2004
| No. of cases | Age and sex adjusted | Multivariate analysis* | |||
| HR† | 95% CI† | HR | 95% CI | ||
| Total no. of diagnostic x-rays | |||||
    <5 <5 | 259 | 1‡ | 1‡ | ||
    5–9 5–9 | 637 | 1.06 | 0.92, 1.23 | 1.03 | 0.89, 1.19 |
    10–14 10–14 | 533 | 1.18 | 1.02, 1.37 | 1.11 | 0.95, 1.29 |
    15–24 15–24 | 573 | 1.43 | 1.23, 1.67 | 1.28 | 1.10, 1.49 |
    ≥25 ≥25 | 380 | 1.72 | 1.46, 2.03 | 1.41 | 1.19, 1.68 |
| No. of x-rays to the | |||||
    Skull Skull | |||||
        Never Never | 1,562 | 1‡ | 1 | ||
        1 1 | 524 | 1.20 | 1.09, 1.32 | 1.10 | 1.00, 1.22 |
        2 2 | 163 | 1.25 | 1.06, 1.46 | 1.03 | 0.88, 1.22 |
        ≥3 ≥3 | 133 | 1.37 | 1.15, 1.64 | 1.11 | 0.92, 1.33 |
    Face/neck§ Face/neck§ | |||||
        Never Never | 1,859 | 1‡ | 1‡ | ||
        1 1 | 236 | 1.18 | 1.03, 1.36 | 1.09 | 0.95, 1.25 |
        2 2 | 121 | 1.23 | 1.02, 1.48 | 1.11 | 0.92, 1.33 |
        ≥3 ≥3 | 166 | 1.51 | 1.29, 1.77 | 1.25 | 1.06, 1.47 |
    Clavicle Clavicle | |||||
        Never Never | 2,256 | 1 | 1 | ||
        1 1 | 69 | 0.96 | 0.76, 1.22 | 0.90 | 0.71, 1.15 |
        2 2 | 35 | 1.18 | 0.84, 1.65 | 1.07 | 0.77, 1.50 |
        ≥3 ≥3 | 22 | 0.74 | 0.49, 1.13 | 0.68 | 0.45, 1.04 |
    Chest Chest | |||||
        ≤2 ≤2 | 205 | 1‡ | 1‡ | ||
        3–5 3–5 | 654 | 1.05 | 0.90, 1.23 | 1.01 | 0.86, 1.18 |
        6–10 6–10 | 907 | 1.22 | 1.04, 1.42 | 1.09 | 0.94, 1.28 |
        ≥11 ≥11 | 616 | 1.45 | 1.23, 1.70 | 1.16 | 0.98, 1.38 |
    Lumbosacral Lumbosacral | |||||
        Never Never | 1,662 | 1‡ | 1 | ||
        1 1 | 349 | 1.13 | 1.01, 1.27 | 1.04 | 0.93, 1.17 |
        2 2 | 176 | 1.36 | 1.16, 1.59 | 1.14 | 0.97, 1.34 |
        ≥3 ≥3 | 195 | 1.34 | 1.15, 1.55 | 1.03 | 0.88, 1.21 |
| Ever had a computed tomography scan | |||||
    Never¶ Never¶ | 2,278 | 1‡ | 1 | ||
    1 1 | 79 | 1.28 | 1.03, 1.61 | 1.14 | 0.91, 1.43 |
    ≥2 ≥2 | 25 | 1.43 | 0.96, 2.12 | 1.16 | 0.78, 1.72 |
| Any radiotherapy | |||||
    Never¶ Never¶ | 2,252 | 1‡ | 1‡ | ||
    1 1 | 106 | 1.19 | 0.98, 1.45 | 1.18 | 0.97, 1.43 |
    ≥2 ≥2 | 24 | 1.43 | 0.96, 2.14 | 1.38 | 0.92, 2.07 |
| Ever had radiotherapy to the head/neck | |||||
    Never¶ Never¶ | 2,323 | 1 | 1 | ||
    Yes Yes | 59 | 1.39 | 1.08, 1.80 | 1.34 | 1.04, 1.74 |
| Age at radiotherapy to the head/neck (years) | |||||
    Never¶ Never¶ | 2,323 | 1 | 1 | ||
    ≤15 ≤15 | 33 | 1.43 | 1.01, 2.02 | 1.41 | 1.00, 1.99 |
    >15 >15 | 26 | 1.35 | 0.92, 1.99 | 1.27 | 0.86, 1.87 |
Total number of x-rays was associated with increased risk of cataract extraction (table 4). Compared with subjects with fewer than five x-rays, those with a history of more than 25 x-rays had an adjusted hazard ratio of 1.50 (95 percent CI: 1.09, 2.06) for cataract extraction. Radiotherapy to the head and neck was associated with an adjusted hazard ratio of 1.71 (95 percent CI: 1.09, 2.68) for cataract extraction.
TABLE 4.
Hazard ratios for cataract extraction, according to diagnostic x-rays and radiotherapy history at baseline, among subjects in the US Radiologic Technologists Study, 1983–2004
| No. of cases | Age and sex adjusted | Multivariate analysis* | |||
| HR† | 95% CI† | HR | 95% CI | ||
| Total no. of diagnostic x-rays | |||||
    <5 <5 | 68 | 1‡ | 1‡ | ||
    5–9 5–9 | 192 | 1.20 | 0.91, 1.58 | 1.16 | 0.88, 1.53 |
    10–14 10–14 | 148 | 1.21 | 0.91, 1.62 | 1.12 | 0.83, 1.50 |
    15–24 15–24 | 123 | 1.11 | 0.82, 1.50 | 0.98 | 0.72, 1.34 |
    ≥25 ≥25 | 116 | 1.81 | 1.33, 2.47 | 1.50 | 1.09, 2.06 |
| No. of x-rays to the | |||||
    Skull Skull | |||||
        Never Never | 439 | 1 | 1 | ||
        1 1 | 135 | 1.09 | 0.90, 1.32 | 1.03 | 0.85, 1.26 |
        2 2 | 36 | 0.94 | 0.67, 1.32 | 0.79 | 0.56, 1.12 |
        ≥3 ≥3 | 37 | 1.29 | 0.92, 1.80 | 1.04 | 0.73, 1.48 |
    Face/neck§ Face/neck§ | |||||
        Never Never | 507 | 1‡ | 1 | ||
        1 1 | 75 | 1.41 | 1.11, 1.80 | 1.30 | 1.02, 1.67 |
        2 2 | 23 | 0.85 | 0.56, 1.29 | 0.78 | 0.51, 1.19 |
        ≥3 ≥3 | 42 | 1.38 | 1.00, 1.89 | 1.17 | 0.84, 1.62 |
    Clavicle Clavicle | |||||
        Never Never | 605 | 1 | 1 | ||
        1 1 | 20 | 1.00 | 0.64, 1.56 | 0.95 | 0.61, 1.49 |
        2 2 | 17 | 1.99 | 1.23, 3.22 | 1.86 | 1.14, 3.03 |
        ≥3 ≥3 | 5 | 0.60 | 0.25, 1.44 | 0.56 | 0.23, 1.36 |
    Chest Chest | |||||
        ≤2 ≤2 | 55 | 1‡ | 1 | ||
        3–5 3–5 | 180 | 1.07 | 0.79, 1.45 | 1.05 | 0.77, 1.42 |
        6–10 6–10 | 239 | 1.16 | 0.86, 1.56 | 1.07 | 0.79, 1.45 |
        ≥11 ≥11 | 173 | 1.42 | 1.04, 1.94 | 1.19 | 0.86, 1.66 |
    Lumbosacral Lumbosacral | |||||
        Never Never | 467 | 1 | 1 | ||
        1 1 | 82 | 0.94 | 0.75, 1.19 | 0.89 | 0.70, 1.13 |
        2 2 | 41 | 1.10 | 0.80, 1.51 | 0.94 | 0.68, 1.30 |
        ≥3 ≥3 | 57 | 1.33 | 1.01, 1.75 | 1.06 | 0.78, 1.43 |
    Ever had a computed tomography scan Ever had a computed tomography scan | |||||
        Never¶ Never¶ | 627 | 1 | 1 | ||
        1 1 | 14 | 0.80 | 0.47, 1.36 | 0.72 | 0.42, 1.23 |
        ≥2 ≥2 | 6 | 1.19 | 0.53, 2.66 | 1.00 | 0.45, 2.25 |
| Any radiotherapy | |||||
    Never¶ Never¶ | 605 | 1‡ | 1‡ | ||
    1 1 | 35 | 1.47 | 1.04, 2.07 | 1.43 | 1.02, 2.02 |
    ≥2 ≥2 | 7 | 1.54 | 0.73, 3.25 | 1.53 | 0.72, 3.23 |
| Ever had radiotherapy to the head/neck | |||||
    Never¶ Never¶ | 627 | 1 | 1 | ||
    Yes Yes | 20 | 1.76 | 1.13, 2.76 | 1.71 | 1.09, 2.68 |
| Age at radiotherapy to the head/neck (years) | |||||
    Never¶ Never¶ | 627 | 1 | 1 | ||
    ≤15 ≤15 | 11 | 1.80 | 0.99, 3.27 | 1.74 | 0.96, 3.17 |
    >15 >15 | 9 | 1.72 | 0.89, 3.33 | 1.67 | 0.86, 3.24 |
The median occupational ionizing radiation dose to the lens was estimated to be 28.1 mGy in the entire cohort. In the dose-response analysis, the ERR/Gy for occupational exposure to ionizing radiation for cataract was 1.98 (95 percent CI: −0.69, 4.65; p = 0.15) after adjusting for sex, year of birth, and baseline data on marital status, body mass index, diabetes, smoking, hypercholesterolemia, hypertension, alcohol consumption, arthritis, diagnostic x-rays, and radiotherapy to the head. Workers in the highest category of occupational dose to the lens of the eye (mean dose, 60.1 mGy) had an adjusted hazard ratio of 1.18 (95 percent CI: 0.99, 1.40; p = 0.06) compared with individuals in the lowest category of occupational dose (mean dose, 5.1 mGy). The plot of categorical risk estimates (figure 2) did not suggest curvilinearity. When multivariate analyses were repeated for cataracts that occurred before age 50 years, the ERR/Gy was 3.29 (95 percent CI: −3.23, 9.80). Multivariate analyses carried out for occupational lens dose and cataract extractions showed an ERR/Gy of 1.50 (95 percent CI: −3.43, 6.43) (data not shown).
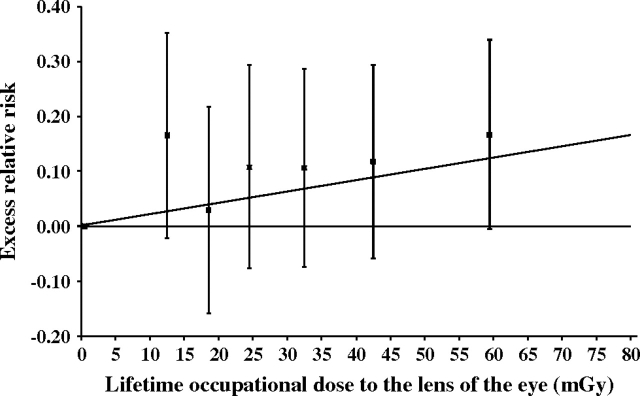
Dose response for occupational radiation to the lens of the eye and cataract risk, US Radiologic Technologists Study, 1983–2004. The excess relative risk (ERR) estimate is equivalent to the relative risk minus 1 and was adjusted for baseline values of age, year of birth, sex, body mass index, marital status, cigarette smoking, diabetes, hypertension, hypercholesterolemia, alcohol consumption, arthritis, and radiation-associated variables (number of diagnostic x-rays, age at radiotherapy to the head) in the log-linear term of the model. The ERR is usually expressed per unit of radiation dose, and a significant relation is indicated when the confidence interval does not include 0. The diagonal line represents the best-fitting linear model with an ERR/Gy of 2.0 (95% confidence interval: −0.7, 4.7). Boxes represent ERR estimates for six dose categories. The upper and lower T-shaped lines represent upper and lower adjacent values, respectively.
DISCUSSION
More than 30 years ago, the ionizing radiation dose-response relation for progressive cataract in humans was extensively examined in 233 radiotherapy patients for whom dose estimates were available (25). The authors concluded that the lowest cataractogenic dose for patients with protracted exposure was 5.5 Gy. Our study among radiologic technologists suggests increased risk at doses roughly a magnitude lower, namely, centigrays and not grays. The relation between occupational radiation dose to the lens of the eye and risk of cataract was stronger among radiologic technologists diagnosed before age 50 years, when posterior subcapsular cataracts are the most frequent type of lenticular opacity, suggesting that radiation exposure may have played a role in onset of this type of cataract. Similarly, we found a significant association between history of three or more diagnostic x-rays to the face or neck and increased risk of cataract.
Our results accord well with findings from smaller studies, including the reanalysis of atomic bomb survivors (9), a cohort of patients with chronic exposure to low-dose-rate radiation from 60Cobalt-contaminated steel in their residences (26), studies of children exposed to low doses from the Chernobyl (Ukraine) accident (27), commercial airline pilots (28), and space astronauts (29). All of these studies found a significant association between exposure to low-dose radiation and increased risk of cortical and posterior subcapsular cataract formation or other lenticular changes. Recently published data on 8,607 Chernobyl cleanup workers who were younger than 55 years of age at first eye examination have shown a significant increase in cataract rates with increasing radiation doses, which were, for the most part, less than 500 mGy (16). The authors concluded that any threshold for cataract is several times lower than that upon which current permissible exposure limits are based.
Results of previous studies on cataract and exposure to radiation from personal diagnostic radiographic procedures have been less consistent. Whereas the Blue Mountains Eye Study (30), for example, suggested no association between history of computed tomography scans of the head and cataract, findings from other case-control (31) and follow-up (32) studies of the Beaver Dam Eye Study indicate that computed tomography scans are significantly associated with posterior subcapsular cataract. The possible causal nature of this relation was supported by the lack of an association for computed tomography scans of the head and age-related maculopathy.
Our study was one of the largest undertaken to date on cataract risk with respect to number of cases and size of the baseline cohort. Another study strength includes the comprehensive and prediagnosis data collection, which reduces the possibility of bias due to disease outcome. Nevertheless, some limitations of the study should be considered. Incident cataract cases were identified from the follow-up questionnaires without clinical confirmation. Because we had no information on validity of the reports or on type of lens opacity, we cannot rule out the possibility that some misclassification of outcome occurred. However, data from the Salisbury Eye Study suggested that the positive predictive values of self-reported cataracts and cataract extractions were 76 percent and 95 percent, respectively (33). The positive predictive value in our study could be even higher since Salisbury Eye Study participants were substantially older (mean age, 77 years) and possibly less knowledgeable about medical procedures compared with our cohort of radiologic technologists. Using age- and type-specific proportions of cataract from the Beaver Dam Eye Study (34), we estimate that approximately 75 percent of all cataracts reported in the present study were posterior subcapsular or cortical.
In our analysis, we adjusted for several factors that have been associated with cataract, including diabetes, smoking, and alcohol consumption. There was an attenuation with increasing degree of adjustment for most radiation-related variables, underlying the importance of controlling for these potential confounders to yield unbiased risk estimates. Nevertheless, we did not have information on other important factors, such as ocular trauma. Trauma to the eyes is a well-known risk factor for cataract formation (35) that frequently requires diagnostic radiographs, raising the possibility of confounding by indication. However, ocular trauma is a relatively rare event, especially among women. In one study, fewer than 5 percent of all cataract patients had a history of ocular trauma (28). Using self-reported history of diagnostic radiographic procedures is another limitation of our study. A previous study on the validity of self-reported x-rays found a significant underreporting of approximately 10 percent on number of diagnostic x-rays when compared with medical records (36). Thus, the true number of x-rays that conferred an increased risk of cataract may be somewhat higher than indicated by the current study. The significant association between number of chest radiographs and cataract is intriguing given their very low radiation dose to the lens of the eye. In addition to a possible causal relation, this association can also be explained if individuals with frequent chest x-rays are more likely to undergo routine eye examinations and thus more likely to be diagnosed with cataract. Before being abandoned in the 1970s, mass radiographic screening for tuberculosis was recommended for hospital personnel and other persons in occupations at increased tuberculosis risk (37). Therefore, chest x-rays may have been taken not only for diagnostic purposes but also to comply with periodic screening examinations.
Similar to several previous epidemiologic studies of ultraviolet radiation and cataract (38–40), we did not measure individual ultraviolet exposure but used ambient levels as a proxy for amount of exposure. This approach may not be valid for our population, where all their work was performed indoors, mostly during daytime, and may partially explain the lack of significant association found between lifetime ultraviolet exposure and cataract in our study. An additional explanation for this negative finding was the potentially low levels of ultraviolet exposure experienced by our cohort of indoor workers compared with the ultraviolet doses previously shown to increase the risk of cortical cataracts (41). We excluded workers who were exposed to improbably high occupational doses (5 percent of the cohort), which, in many cases, was accumulated within 1 or 2 years and may reflect measurement errors (e.g., when the badge was inadvertently left in the examination room, errors in badge placement relative to the filters). The 5 percent with extremely high doses had a calculated ERR/Gy (0.55, p > 0.5) that was markedly lower than the risk calculated for the entire cohort.
The present study confirmed an increased risk of previously described risk factors for cataract, including diabetes, obesity, and alcohol intake. Our analyses agree with data from previous population-based studies (42–44), which found that individuals with diabetes have a threefold to fourfold increased risk of cataract before age 65 years, and with the Blue Mountains Eye Study (45), which showed that a body mass index of >30 kg/m2 was significantly associated with increased risk of cortical cataract. Several previous studies have examined the risk of cataract in alcohol consumers, with inconsistent risk patterns. The results of our analysis suggested that moderate alcohol intake reduced the risk of cataract. This conclusion was supported by the findings from the Blue Mountains Eye Study (46) and a case-control study in the United Kingdom (47). Other risk factors for cardiovascular diseases, such as hypertension and hypercholesterolemia, were also associated with more frequent occurrence of cataract, which agrees with many (45), but not all (48), previous studies. The presumably low frequency of nuclear-type cataract in our cohort may explain the modest risk associated with cigarette smoking compared with several studies that demonstrated stronger associations between smoking and cataract, mostly of the nuclear type (20). The increased risk for women agrees with previous follow-up studies (18, 39, 49) that have shown that women have a greater risk of developing cortical cataract.
In conclusion, our study provides evidence that exposure to relatively low doses of ionizing radiation may be harmful to the lens of the eye and increases the long-term risk of cataract formation. Our findings and the results of recent studies suggest that likelihood of cataract formation increases with increasing exposure to ionizing radiation with no apparent threshold level, a finding that challenges the National Council on Radiation Protection and International Commission on Radiological Protection assumptions that a radiation dose of at least 2 Gy is associated with increased cataract risk.
Acknowledgments
This study was supported in part by contracts N01-CP-31018 and N02-CP-31013 from the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, the National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
The authors are grateful to Jerry Reid of the American Registry of Radiologic Technologists for continued support of this study, Diane Kampa and Allison Iwan of the University of Minnesota for data collection and study coordination, Sheila West of The Johns Hopkins University for her helpful comments, and Dale Preston of Hirosoft International Corporation (Seattle, Washington) for statistical assistance.
Conflict of interest: none declared.
References
Articles from American Journal of Epidemiology are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/aje/kwn171
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/aje/article-pdf/168/6/620/201598/kwn171.pdf
Free to read at aje.oxfordjournals.org
http://aje.oxfordjournals.org/cgi/content/abstract/168/6/620
Free after 12 months at aje.oxfordjournals.org
http://aje.oxfordjournals.org/cgi/content/full/168/6/620
Free after 12 months at aje.oxfordjournals.org
http://aje.oxfordjournals.org/cgi/reprint/168/6/620.pdf
Citations & impact
Impact metrics
Article citations
Exploring Angiotensin II and Oxidative Stress in Radiation-Induced Cataract Formation: Potential for Therapeutic Intervention.
Antioxidants (Basel), 13(10):1207, 08 Oct 2024
Cited by: 0 articles | PMID: 39456460 | PMCID: PMC11504979
Review Free full text in Europe PMC
AVATAR 2.0: next level communication systems for radiotherapy through face-to-face video, biofeedback, translation, and audiovisual immersion.
Front Oncol, 14:1405433, 08 Oct 2024
Cited by: 0 articles | PMID: 39439954 | PMCID: PMC11493730
The natural and artificial intraocular lens in spaceflight.
Eye (Lond), 38(16):3035-3036, 10 Jul 2024
Cited by: 0 articles | PMID: 38987644
Is Ocular Safety in Orthopaedics Overlooked? A Systematic Review of Annual Ocular Radiation Exposure and Protective Measures.
Clin Orthop Relat Res, 482(11):1954-1967, 02 Aug 2024
Cited by: 1 article | PMID: 39115540
Review
Deciphering the molecular landscape of ionising radiation-induced eye damage with the help of genomic data mining.
Arh Hig Rada Toksikol, 75(2):91-101, 29 Jun 2024
Cited by: 0 articles | PMID: 38963141 | PMCID: PMC11223508
Go to all (172) article citations
Other citations
Wikipedia
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Occupational radiation exposure and risk of cataract incidence in a cohort of US radiologic technologists.
Eur J Epidemiol, 33(12):1179-1191, 27 Aug 2018
Cited by: 36 articles | PMID: 30151727 | PMCID: PMC10645574
[Risk of deterministic effects after exposure to low doses of ionizing radiation: retrospective study among health workers in view of a new publication of International Commission on Radiological Protection].
G Ital Med Lav Ergon, 38(2):83-88, 01 Apr 2016
Cited by: 1 article | PMID: 27459840
Occupational radiation exposure and excess additive risk of cataract incidence in a cohort of US radiologic technologists.
Occup Environ Med, 77(1):1-8, 02 Dec 2019
Cited by: 15 articles | PMID: 31792080 | PMCID: PMC10673645
Low- and moderate-dose non-cancer effects of ionizing radiation in directly exposed individuals, especially circulatory and ocular diseases: a review of the epidemiology.
Int J Radiat Biol, 97(6):782-803, 26 Feb 2021
Cited by: 30 articles | PMID: 33471563 | PMCID: PMC10656152
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Intramural NIH HHS
NCI NIH HHS (2)
Grant ID: N02CP31013
Grant ID: N01CP31018
 1
1 




