Abstract
Free full text

Altered nuclear retention of mRNAs containing inverted repeats in human embryonic stem cells: Functional role of a nuclear noncoding RNA
Abstract
In many cells, mRNAs containing inverted repeats (Alu repeats in humans) in their 3′-untranslated regions (3′-UTRs) are inefficiently exported to the cytoplasm. Nuclear retention correlates with adenosine-to-inosine editing and is in paraspeckle-associated complexes containing the proteins p54nrb, PSF and PSP1α. We report that robust editing activity in human embryonic stem cells (hESCs), does not lead to nuclear retention. p54nrb, PSF and PSP1α are all expressed in hESCs, but paraspeckles are absent and only appear upon differentiation. Paraspeckle assembly and function depends on expression of a long nuclear-retained noncoding RNA, hNEAT1. This RNA is not expressed in hESCs, but is induced upon differentiation. Knockdown of hNEAT1 in HeLa cells results both in loss of paraspeckles and enhanced nucleocytoplasmic export of mRNAs containing inverted Alu repeats. Taken together, these results assign a biological function to a large noncoding nuclear RNA in the regulation of mRNA export.
Introduction
Cellular responses to double stranded RNAs (dsRNAs) differ markedly depending on whether these molecules are found in the cytoplasm or in the nucleus. Mammalian cells rarely express dsRNA within the cytoplasm, most likely because of the ensuing dramatic effects on RNA levels, inhibition of protein synthesis, and, if prolonged, cell death (Kumar and Carmichael, 1998; Wang and Carmichael, 2004, and references therein). The primary cytoplasmic response to dsRNAs involves interferons (IFNs), PKR and the 2′,5′-adenylate synthesis (AS)/RNaseL pathway. In lower eukaryotes, or where these pathways are lacking or inactive, the RNA interference (RNAi) pathway provides the primary mechanism to eliminate cytoplasmic dsRNAs.
In the nucleus, dsRNAs are frequently edited by dsRNA dependent adenosine deaminases (ADARs). ADARs are ubiquitously expressed in higher eukaryotes and catalyze the hydrolytic deamination of adenosines (A) to inosines (I) (Bass, 2002; Bass and Weintraub, 1988; Nishikura, 1992; Polson et al., 1991). While some editing directed by short dsRNA structures is site-specific and leads to coding changes in mRNAs, long dsRNA regions (hairpins or sense-antisense hybrids) are edited promiscuously, with up to half of their adenosines being changed to inosines (Bass, 2002). ADAR editing is surprisingly abundant in human cells, and more than 90% of this occurs within inverted repeated Alu elements (IRAlus) (Athanasiadis et al., 2004; Blow et al., 2004; Kim et al., 2004; Levanon et al., 2004). Alu elements are unique to primates and account for almost all of the human short interspersed nuclear elements (SINEs), more than 10% of the genome. Their abundance leads to the frequent occurrence of inverted repeat structures in gene regions. While most IRAlus lie within introns, we have identified 333 human genes with IRAlus in their 3′-UTR regions (Chen et al., 2008).
What is the consequence of the presence of IRAlus in mRNAs? mRNAs with structured or edited 3′-UTRs can be bound by a nuclear complex containing the protein p54nrb, which prevents their export to the cytoplasm (Chen et al., 2008; Prasanth et al., 2005; Zhang and Carmichael, 2001). Such nuclear retention provides a quality control mechanism that prevents inappropriate translation of promiscuously edited RNAs and might be used in other ways to regulate gene expression (Chen and Carmichael, 2008; Chen et al., 2008; Prasanth et al., 2005). p54nrb is concentrated in nuclear structures called paraspeckles, which also contain two related proteins, PSF and PSP1α (Fox et al., 2005; Fox et al., 2002). Paraspeckles are associated not only with nuclear-retained RNAs (Chen et al., 2008; Prasanth et al., 2005), but also with an abundant nuclear-retained noncoding RNA, hNEAT1, which is 3.7 Kb in length (Hutchinson et al., 2007). hNEAT1 has no obvious inverted repeats that could direct editing, and no edited bases within this transcript have been reported. In agreemnt with recent reports (Clemson et al., 2009; Sasaki et al., 2009; Sunwoo et al., 2009) we find that hNEAT1 RNA is required for paraspeckle integrity. We further demonstrate that it influences the nuclear retention of structured or edited mRNAs.
LIN28 is a key regulatory factor in the maintenance of pluripotency (Newman et al., 2008; Rybak et al., 2008; Viswanathan et al., 2008; Xu et al., 2009). Since this factor is abundantly expressed in hESCs (Richards et al., 2004), we suspected that the IRAlus in the 3′-UTR of the Lin28 mRNA may not promote nuclear retention as is seen in other cells (Chen et al., 2008). This is indeed the case. ADAR1 activity is high in hESCs, yet the editing-associated nuclear retention pathway is impaired. Importantly, paraspeckles are not formed and hNEAT1 RNA is not expressed. When hESCs are induced to differentiate into trophoblasts, hNEAT1 is induced and paraspeckles appear. Finally, when hNEAT1 expression is reduced in HeLa cells, not only do paraspeckles disappear, but a number of mRNAs with IRAlus in their 3′-UTRs are more efficiently exported to the cytoplasm.
Results
ADAR1 is active in human ES cells
ADAR1 is responsible for most promiscuous RNA editing (Bass, 2002) and is expressed in hES H1 and H9 cells at a level comparable to that seen in HEK293 and HeLa cells (Figs. 1D and S1). Further, ADAR1 expression is not significantly different when H9 cells are cultured on feeder cells, or grown in feeder-free medium (Fig. S1). Consistent with its expression level, ADAR1 editing activity is robust in hESCs. The LIN28 protein is an important regulator of pluripotency, and is highly expressed in hESCs (Balzer and Moss, 2007; Yu et al., 2007; Viswanathan et al., 2008; Rybak, et al., 2008; Newman, et al. 2008, RNA; Piskounova, et al. 2008). Human Lin28 mRNA contains a single pair of inverted Alu repeats in its 3′-UTR (Fig. 1A, upper panel). We used RT-PCR to amplify the region spanning the AluSc element in the 3′-UTR of Lin28 from total RNA isolated from H9 cells. DNA sequencing of individual clones indicated frequent A-to-I editing in this region (Fig. 1A, lower panel and Fig. S2). In addition, the editing pattern is similar to sequences published in the UCSC genome browser (e.g., CN349329; Fig. 1A) and to Alu editing levels seen in other cells (Chen et al., 2008). Since we examined only one of the Alu elements in this 3′-UTR, it is likely that our results are an underestimate of the true extent of Lin28 transcript diversity generated by editing.

Altered nuclear retention in human ES cells. A. ADAR1 activity in hESCs. The upper panel shows the distribution of Alu elements in the Lin28 transcript. The lower panel shows the sequence of several cDNA clones of Lin28 showing A-to-G changes diagnostic of A-to-I editing. Red arrows indicate the primers used for amplification. B. Lin28 mRNA escapes nuclear retention in H9 cells. Nuclear and cytoplasmic RNAs were isolated from H9 cells and HeLa cells and resolved on a denaturing agarose gel. The probe for the northern blot (red bar) is located upstream of the IRAlus from the 3′-UTR of Lin28. tRNAlys and U6 were used as makers for nuclear and cytoplasmic RNA fractionation. Equivalent fractions of total (T), cytoplasmic (C) and nuclear (N) RNAs were loaded. C. Paics mRNA escapes nuclear retention in H9 cells, but is mostly retained in the nucleus in HeLa cells. The same samples from panel B were used. Two Paics isoforms are shown. The probe for the northern blot (red bar) is located upstream of the IRAlus from the 3′-UTR of Paics. Bands were quantitated using ImageJ software. D. ADAR1 and paraspeckle-related proteins are expressed in H9 cells. Total proteins were collected from HEK293 cells (Lane 1), HeLa cells (Lane 2), H9 cells (Lane 3) and H9 cells differentiated into trophoblasts using BMP4 (Lane 4). Actin was used as loading control, Sox2 was used as a marker for human ES cells and Troma-1 was used as a marker for trophoblasts. E. mRNAs containing IRAlus are associated with p54nrbin both H9 cells and HeLa cells. Immunoprecipitations (IP) were performed from H9 cells and HeLa cells using anti-p54nrb antibody or mock antibody.
Altered nuclear retention pathway in human ES cells
What is the fate of IRAlus in human ES cells? In differentiated cells, the fate of promiscuously edited RNA frequently involves nuclear retention in nuclear complexes containing p54nrb (Zhang and Carmichael, 2001; Prasanth et al., 2005; Chen et al., 2008). We recently published that edited IRAlus in 3′-UTRs of genes can repress gene expression by sequestering mRNAs in the nucleus (Chen et al., 2008). We therefore asked whether the Lin28 transcript is retained in the nucleus in hESCs. Northern blotting was carried out with cytoplasmic and nuclear RNAs isolated from H9 and HeLa cells. Surprisingly, we observed efficient export of Lin28 in H9 cells (Fig. 1B). In contrast, when the Lin28 IRAlus were inserted into a reporter construct, strong retention was seen in HEK293 cells (Chen et al., 2008). This raised the possibility that hESCs export mRNAs with IRAlus in their 3′-UTRs more efficiently than other cells.
However, Lin28 is a stem cell-specific transcript and might have a unique regulation in hESCs. The mRNA for Nicolin 1 contains one pair of inverted Alu repeats in its 3′-UTR and shows strong nuclear retention in both HEK293 cells (Chen et al., 2008) and HeLa cells (data not shown). Since this mRNA is not transcribed in hESCs (Fig. 1E), we sought genes that are transcribed in both hESCs and differentiated cells. From the Gladstone Microarray Data Including Stem Cell Tissue available through the UCSC genome browser we identified several candidate genes containing IRAlus in their 3′-UTRs. Paics (phosphoribosylaminoimidazole carboxylase) is one of them (Fig. 1C, upper panel; Fig. S3) and some available EST sequences show promiscuous editing. Recent biochemical studies showed that PAICS is an important bifunctional enzyme in de novo purine biosynthesis and is especially crucial for rapidly dividing cancer cells which rely heavily on the purine de novo pathway for synthesis of adenine and guanine, while normal cells favor the salvage pathway (Li et al., 2007).
Northern blotting using a probe lying upstream of the inverted Alu repeats in its 3′-UTR showed similar transcription levels of Paics in both H9 cells and HeLa cells (Fig. 1C, lanes 1 and 4). Using equivalent amounts of RNAs from the cytoplasm and the nucleus, we observed distinct nuclear retention of the full-length Paics (3295 nt) in HeLa cells (Fig 1C, lanes 5 and 6, N/C ratio of 3.3), but not in H9 cells (Fig. 1C, lanes 2 and 3). Notably, the Paics probe also detected a shorter isoform of Paics (1562 nt) which corresponds to the polyadenylated cDNA BC019255. This shorter isoform is mostly cytoplasmic in both cell lines, suggesting that nuclear retention in HeLa cells is mediated by the inverted Alu repeats, as we have seen in other cases in differentiated cells (Chen et al., 2008). Consistent with these results, we have observed that multiple mRNAs with IRAlus in their 3′-UTRs (Lin28, Paics and Pccb) are efficiently exported to the cytoplasm in another hESC line, H14 (Fig. S4). Finally, in agreement with what we have generally observed for mRNAs containing IRAlus (Chen et al., 2008), Paics, Nicn1, and Lin28 mRNAs are associated with p54nrb in vivo (Fig. 1E).
Human ES cells lack paraspeckles
In mammalian cells, p54nrb and its partners, such as PSF and PSP1α, likely participate in the regulation of the nuclear retention of inosine containing RNAs (Zhang and Carmichael, 2001; Prasanth et al., 2005; Chen et al., 2008). p54nrb is a multifunctional protein and has been implicated in a variety of nuclear processes (Basu et al., 1997; Emili et al., 2002; Ishitani et al., 2003; Kameoka et al., 2004; Kaneko et al., 2007; Straub et al., 1998; Yang et al., 1993; Yang et al., 1997; Zhang et al., 1993; Zhang and Carmichael, 2001). It was the first RNA-binding protein described which shows high affinity for edited transcripts (Zhang and Carmichael, 2001). p54nrb contains two tandem RNA recognition motif (RRM)-type RNA binding domains and a putative helix-turn-helix motif followed by a highly charged region with a predicted coiled-coil structure. These four regions together comprise the DBHS (Drosophila behavior and human splicing) domain, which is highly conserved among p54nrb, PSF, and PSP1α (Fox et al., 2002; Yang et al., 1993). PSF and PSP1α can each form heterodimers with p54nrb (Akhmedov and Lopez, 2000; Fox et al., 2005; Myojin et al., 2004; Zhang et al., 1993). PSF is also a multifunctional protein. It binds both RNA and DNA (Zhang et al., 1993) and can act in splicing (Gozani et al., 1994; Lindsey et al., 1995; Patton et al., 1993) and transcription (Mathur et al., 2001; Urban et al., 2000). Like p54nrb, PSF also binds tightly to edited substrates (Zhang and Carmichael, 2001). PSP1α is somewhat less abundant, but shares about 50% sequence similarity with p54nrb and PSF in the conserved DBHS domain, and has been recently found to be a marker for nuclear paraspeckles (Fox et al., 2002). Paraspeckles are cell cycle-regulated subnuclear domains of currently unknown function that are dependent on RNA for their structural integrity (Fox et al., 2005). The edited mouse CTN-RNA was shown to at least partialy localize to paraspeckles, suggesting paraspeckles could be sites of nuclear retention of at least a subset of edited dsRNAs (Prasanth et al., 2005). Our recent results on the nuclear retention of IRAlus-containing mRNAs are also consistent with paraspeckle association (Chen et al., 2008).
As shown in Fig. 1D, p54nrb, PSF and PSP1α are expressed at a level in H9 cells similar to that seen in HEK293 cells and HeLa cells. The same result was seen in hESC H1 cells (Fig. S1B) and H14 cells (data not shown). Indeed, in HeLa cells p54nrb, PSF and PSP1α all colocalize in paraspeckles (Fig. 2, panels a–h), although a fraction of all of them are found outside of these structures. Importantly, even though hESCs abundantly express these proteins, typical paraspeckles are not seen (H9 cells, Fig. 2, panels i–l; H14 cells, Fig. 2, panels q–t and Figs. S5 and S9). PSP1α and p54nrb show an almost uniform distribution and colocalization throughout the nucleoplasm, but are excluded from nucleoli. In contrast, hESCs show no apparent defect in nuclear SC35 splicing speckles (Fig. 2, panels u–x). Consistent with what has been observed in other cells (Akhmedov and Lopez, 2000; Fox et al., 2005; Myojin et al., 2004; Zhang et al., 1993), p54nrb, PSF and PSP1α interact with one another in H9 cells, even in the absence of RNA (Fig. 3A and B ).
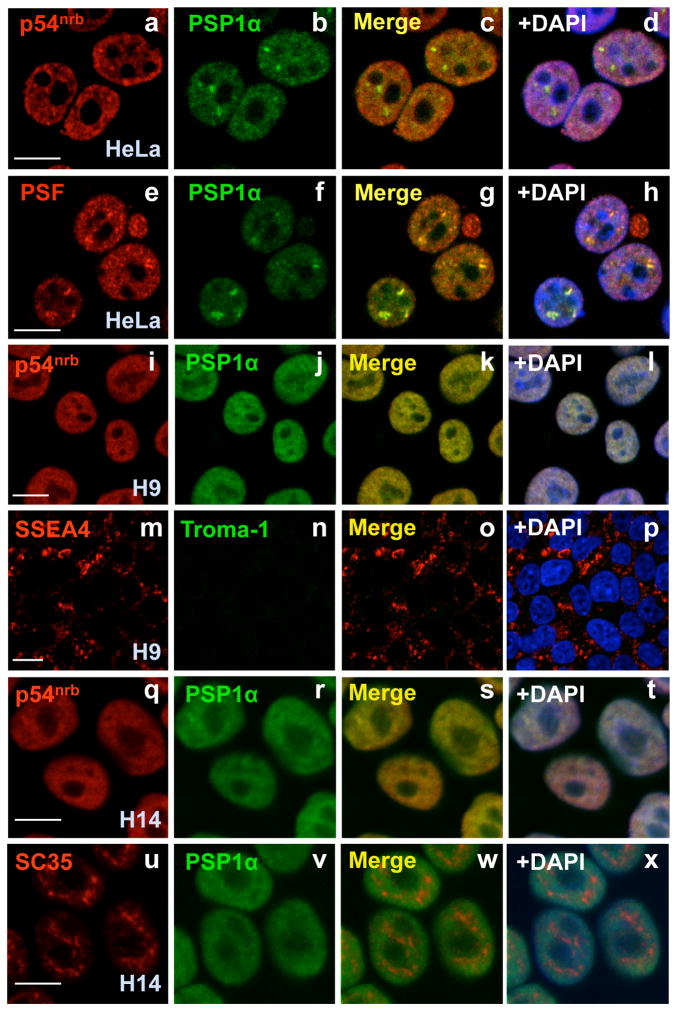
Human ES cells lack paraspeckles. HeLa cells were stained with anti-p54nrb and anti-PSP1α antibodies (a–d) or anti-PSFand anti-PSP1α antibodies (e–h). DAPI was used to indicate DNA. Note that p54nrb, PSF, and PSP1α colocalize with each other and indicate paraspeckles. p54nrb and PSP1α colocalize throughout the nucleoplasm in H9 cells, but show no apparent nuclear paraspeckles (i–l). The same batch of H9 cells was also stained with anti-SSEA4 (stem cell marker) and anti-Troma-1 (trophoblast differentiation marker) (m–p). p54nrb and PSP1α also colocalize throughout the nucleoplasm in H14 cells (q–t), but show no apparent nuclear paraspeckles. H14 cells were also stained with anti-SC35 and anti-PSP1α (u–x). The white scale bar in all images denotes 10 μm.
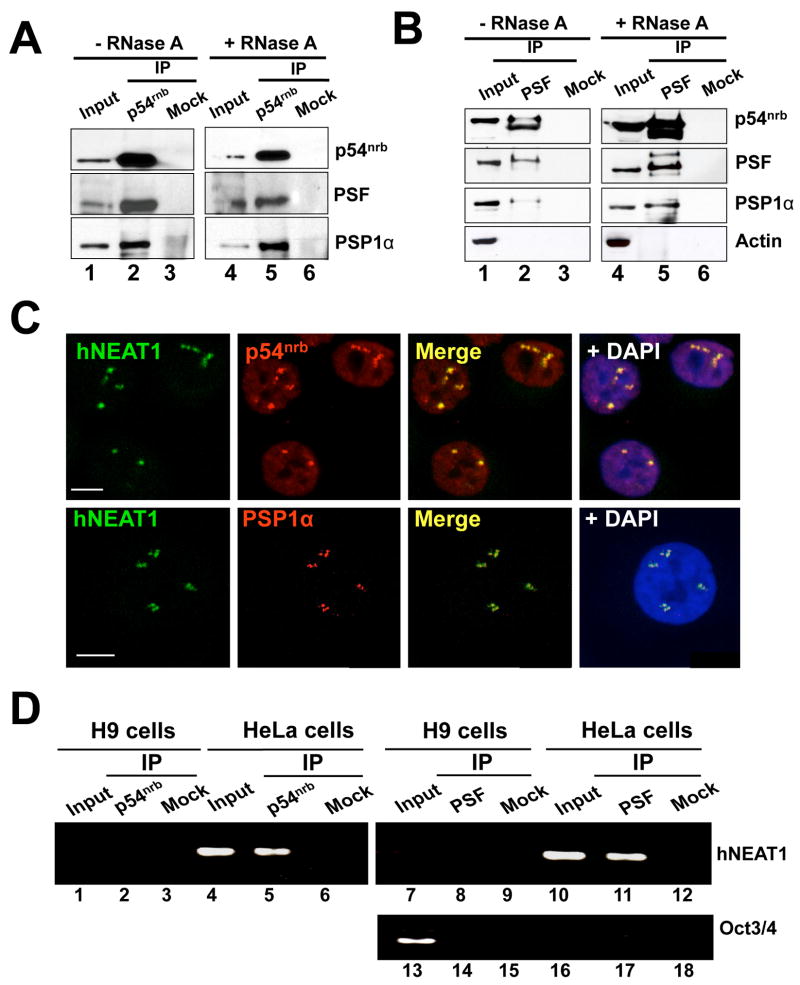
A. RNA-independent interaction of p54nrb with PSF and PSP1α in H9 cells. Total extracts of H9 cells with or without RNaseA treatment were immunoprecipitated with p54nrb antibody or mock antibody, and then immunoblotted with anti-p54nrb anti-PSF and anti-PSP1α antibodies. B. The same immunoprecipitation experiments were performed with anti-PSF antibody in H9 cells. Anti-actin was used as a negative control. C. RNA in situ hybridization was performed with green-dUTP-labeled antisense hNEAT1 probe in HeLa cells, and representative images are shown. p54nrband PSP1α are in red. hNEAT1 colocalizes with p54nrb (upper panel) and PSP1α (lower panel). D. hNEAT1 associates with p54nrband PSF in HeLa cells. IP with anti-p54nrb, anti-PSF, or mock antibody was carried out in extracts from H9 cells or HeLa cells. RT-PCR of hNEAT1 from the IP showed amplification in HeLa cells by anti-p54nrb IP and anti-PSF IP, but not by IP with mock antibody. Oct3/4 is a marker of H9 cells.
Noncoding hNEAT1 RNA localizes to paraspeckles and is not expressed in hESCs
RNA immunoprecipitation experiments revealed that mRNAs for Paics and Lin28 associate with p54nrb in H9 cells (Fig. 1E, lane 2), as in differentiated cells (lane 5). However, these mRNAs are efficiently exported to the cytoplasm in H9 cells (Fig. 1B and 1C) and H14 cells (Fig. S4), indicating that binding to p54nrb is not sufficient for nuclear retention. Rather, retention correlates with the presence of paraspeckles, and these are missing in hESCs (Fig. 2 panels i–t, Fig. S5).
What underlies the failure of paraspeckles to assemble in hESCs? While it is known that RNA plays a role in paraspeckle formation (Fox et al., 2005), structured mRNAs such as those with IRAlus can not themselves play a crucial role because they are expressed in hESCs. Hutchinson et al. (2007) identified NEAT1 (nuclear enriched abundant transcript 1) as a large (3.7 kb) polyadenylated RNA transcript displaying striking nuclear enrichment in both human and mouse cells. In rats, NEAT1 expression can be further induced by virus infection (Saha et al., 2006).
Using in situ hybridization we demonstrated that hNEAT1 localizes to paraspeckles in HeLa cells. As shown in Fig. 3C, hNEAT1 RNA is restricted to a small number of large, distinct nuclear speckles in HeLa cell nuclei. Importantly, hNEAT1 accumulation colocalizes very well with both p54nrb and PSP1α (Fig. 3C). Further confocal z-section studies showed such colocalization occurs throughout the nucleus (Fig. S6 and Fig. S7). These results are in complete agreement with recent work in both mouse (Sasaki et al., 2009; Sunwoo et al., 2009) and human (Clemson et al., 2009) cells showing that NEAT1 RNA (MENepsilon/beta RNA in mouse) plays an essential role in the assembly and architecture of paraspeckles. To further examine the relationship between hNEAT1 RNA and other paraspeckle components, we carried out RNA immunoprecipitation experiments in HeLa cells. In further agreement with Clemson et al. (2009), we observed association of hNEAT1 RNA with p54nrb and PSF in HeLa cells (Fig. 3D, lanes 5 and 11).
Surprisingly, hNEAT1 RNA was undetectable in all hESCs we examined (H9 cells, Fig. 3D, Fig. 4A, B; H14 cells, Fig. 4C and H1 cells, Fig. S8). This absence of hNEAT1 was further confirmed using different PCR primers across hNEAT1 sequences (Fig. 4C and Fig. S8) and also using H9 cells that were cultured under different experimental conditions (Fig. 4B). Curiously, the noncoding NEAT2 RNA (MALAT-1), which lies immediately downstream of NEAT1 on chromosome 11 (Hutchinson et al., 2007), is expressed at high levels in hESCs (data not shown), suggesting independent regulation of expression of these ncRNAs.

hNEAT1 and paraspeckles appear upon hESC differentiation. A. Northern blot showing absence of hNEAT1 RNA in H9 cells. B. RT-PCR showing absence of hNEAT1 RNA in H9 cells (lane 1, H9 cells cultured in mTeSR; lane 4, H9 cells cultured in CM medium), but its presence in differentiated cells (Lane 3). C. RT-PCR showing hNEAT1 absence in undifferentiated H14 cells, but expression in differentiated H14 cells. D. H9 cells cultured in CM medium were induced with BMP4 for 5 days, and then fixed for immunostaining. Day 5 differentiated H9 cells show the trophoblast marker, Troma-1 and the loss of stem cell marker, SSEA4 (lower panel). p54nrb and PSP1α colocalize with each other and form multiple large accumulations (white arrows), indicating paraspeckles. E. Day 4 differentiated H14 cells show the p54nrb and PSP1α accumulations (white arrows) in the nuclei, indicating paraspeckles. F. hNEAT1 (in situ hybridization, green) is expressed in trophoblasts derived from H9 cells and colocalizes with p54nrb.
hNEAT1 is expressed after differentiation
To examine whether hNEAT1 expression correlates with paraspeckle formation during hESC differentiation, we differentiated H9 cells to trophoblasts using BMP4 treatment (Xu et al., 2002; Xu et al., 2005). The majority of H9 cells became trophoblasts after 4–5 days of BMP4 treatment as shown by the presence of the trophoblast marker, Troma-1, and the loss of the stem cell marker, SSEA-4 (Fig. 4D, lower panels). ADAR1 and the paraspeckle-related proteins, p54nrb, PSF and PSP1α were also detected in trophoblasts (Fig. 1D, lane 4). Importantly, we observed the return of paraspeckles in trophoblasts derived from H9 cells (Figs. 4D) and H14 cells (Fig. 4E and Fig. S9). PSP1α and p54nrb accumulated in numerous dots per trophoblast nucleus (Figs. 4D, 4E and S9), similar to the pattern observed in HeLa cells (Fig. 2, a–d) and other cell types (data not shown). In addition, PSF and PSP1α also showed colocalization throughout the nucleus (data not shown). Importantly, the expression of hNEAT1 RNA was detected during trophoblast differentiation (Fig. 4B, C and F). Taken together, these observations led us to speculate that the lack of paraspeckles in hESCs might mechanistically underlie the weak nuclear retention for mRNAs containing inverted Alu repeats.
hNEAT1 plays a critical role in both paraspeckle formation and function
Since the levels of Lin28 or Paics decrease significantly (data not shown), BMP4 induction is not an ideal model to address whether the appearance of paraspeckles during differentiation leads to the re-establishment of the nuclear retention system. However, to address the functional connection between paraspeckles and retention, we used several independent approaches to knock down hNEAT1 expression in HeLa cells. Following treatment of cells with phosphorothioate-modified hNEAT1 antisense oligodeoxynucleotides hNEAT1 RNA was typically reduced 60%–70% in HeLa cells after 48 hr treatment as shown by in situ hybridization (Fig. 5A and Fig. S10) and RT-PCR (Fig. 5C). After treatment with antisense oligos, the nuclear hNEAT1 foci were largely reduced (Fig. 5A and Fig. S10), and the appearance of PSP1α and p54nrb became more uniform, showing fewer and smaller paraspeckles per nucleus. Fig. 5B shows typical staining patterns of hNEAT1 and p54nrb, and of hNEAT1 and PSP1α, after hNEAT1 knockdown in HeLa cells. These experiments again confirm that paraspeckle-specific accumulations of p54nrb and PSP1α require the presence of hNEAT1. In other experiments we used an siRNA directed against hNEAT1 RNA and based on one shown to be effective by Clemson et al. (2009). Figure 5F shows that siRNA is also effective at reducing hNEAT1 levels in cells.
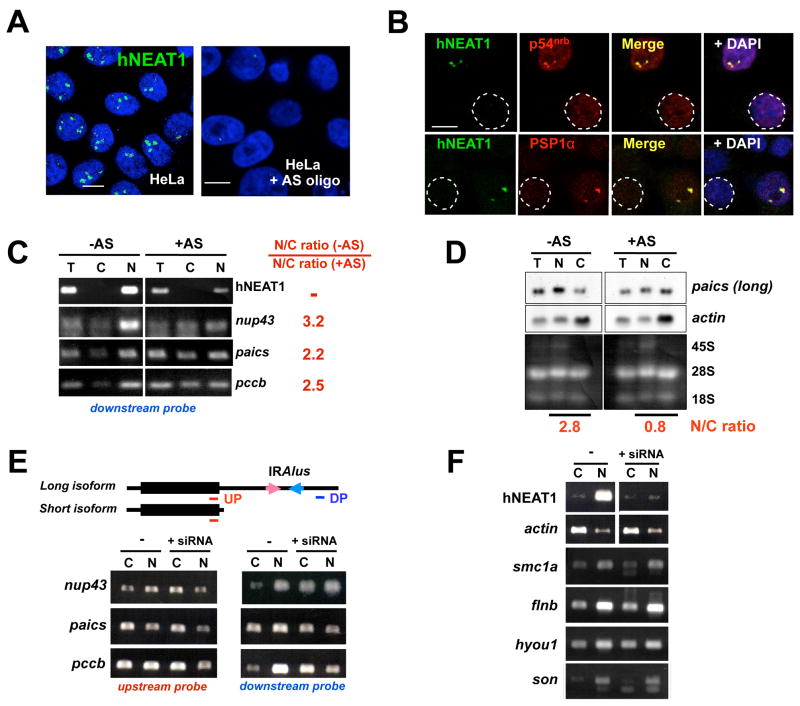
Role of hNEAT1 in paraspeckle function. A. hNEAT1 RNA in situ hybridization indicates efficient knockdown of hNEAT1 levels after 48 hr treatment with pooled antisense (AS) oligodeoxynucleotides. B. hNEAT1 RNA correlates with paraspeckle formation. A typical staining pattern of hNEAT1 and p54nrb, (upper panel) and of hNEAT1 and PSP1α (lower panel) in hNEAT1 knockdown HeLa cells. Circled nuclei show that fewer paraspeckles are apparent in cells in which hNEAT1 has been knocked down. C. IRAlus-containing mRNAs escape nuclear retention in hNEAT1 knockdown HeLa cells. Nuclear and cytoplasmic RNAs were isolated from untreated and hNEAT1 AS-treated HeLa cells and analyzed by RT-PCR. PCR primers are located in the downstream of IRAlus in the 3′-UTRs to detect only full-length mRNAs (Fig. S3 and Fig. 5E, DP). In untreated HeLa cells, the majority of full-length Nup43, Paics, and Pccb mRNAs are retained in the nucleus (left panel), while significantly more is exported to the cytoplasm in hNEAT1 knockdown cells (right panel). Bands were quantitated using ImageJ software. In each case, omission of RT led to no signal (data not shown). T, total RNA; C, cytoplasmic RNA; N, nuclear RNA. D. Northern blotting. Nuclear and cytoplasmic RNAs were isolated from untreated and hNEAT1-AS treated HeLa cells and resolved on a denaturing agarose gel. In untreated HeLa cells, the majority of Paics mRNA is retained in the nucleus (left panel), while more Paics is exported to the cytoplasm in the hNEAT1 knockdown cells (right panel). The membrane was stripped and re-probed for actin mRNA, which shows no change in its cellular distribution after AS treatment. E. IRAlus-containing mRNAs escape nuclear retention in HeLa cells after anti-hNEAT1 siRNA treatment. The upper panel shows two isoforms of Nup43, Paics, and Pccb: the long isoform has one pair of IRAlus in its 3′-UTR, while the short one lacks IRAlus. The red line indicates the location of the upstream probe (UP) which recognizes both isoforms, and the dark blue line indicates the location of the downstream probe (DP) which only recognizes the long IRAlus-containing isoform. F. Controls. The same mRNA samples from Panel E were used. Smc1a, flnb, hyou1, and son are mRNAs that do not contain IRAlus in their 3′-UTRs, but are normally enriched in the nucleus. These mRNAs show no change in their N/C distribution after hNEAT1 knockdown.
Finally, if hNEAT1 RNA were essential for paraspeckle-associated nuclear retention, we would expect to observe that mRNAs with IRAlus would have a different fate in HeLa cells after hNEAT1 knockdown. We chose three mRNAs that contain IRAlus in their 3′-UTRs and which are expressed well in HeLa cells (nup43, paics and pccb, see Fig. S3). For each of these, hNEAT1 knockdown resulted in less efficient retention in the nucleus (Fig. 5C, D and E), while the nuclear/cytoplasmic distribution of actin mRNA remained unchanged (Fig. 5D and F ). For the experiments shown in Fig. 5E we used two sets of RT-PCR primers, one specific for mRNAs with extended 3′-UTRs that contain IRAlus (“downstream probe”), and another set that detects both short and long isoforms (“upstream probe”). Note that the longer isoform of each of the IRAlus-containing mRNA examined changes its N/C ratio after knockdown of hNEAT1 RNA, regardless of whether the knockdown was achieved using antisense DNA oligonucleotides (Fig. 5C and D) or siRNA (Fig. 5E). As the short isoforms are more efficiently exported to the cytoplasm (see Fig. 1C; Prasanth et al., 2005; Chen et al., 2008), and since the upstream primers detect both long (nuclear retained) and short (more efficiently exported) mRNA isoforms, the results obtained using them show more modest effects of hNEAT1 knockdown (Fig. 5E). We conclude from these experiments that noncoding hNEAT1 RNA not only orchestrates paraspeckle assembly, but also influences the nuclear retention of structured or edited mRNAs. Lastly, we have identified a number of mRNAs that are inefficiently exported to the cytoplasm but which do not show evidence of editing and which do not contain IRAlus in their 3′-UTRs. Several of these are shown in Fig. 5F. For these mRNAs, hNEAT1 knockdown does not at all alter their N/C distribution, consistent again with a connection between editing-associated retention and paraspeckles.
Discussion
Nuclear retention of structured or edited mRNAs occurs in paraspeckles and requires hNEAT1 RNA
There is growing evidence that gene expression can be regulated by the retention of mature mRNAs in the nucleus. Retention correlates with RNA duplex formation and with A-to-I editing. We reported earlier that nuclear dsRNAs that are promiscuously edited by ADAR can be bound by a complex containing the nuclear protein p54nrb, which displays a strong binding affinity for RNAs with inosines in them (Zhang and Carmichael, 2001). More recently, we showed that many mRNAs with inverted Alu repeats in their 3′-UTRs may be inefficiently exported to the cytoplasm owing to nuclear sequestration in prominent nuclear p54nrb-containing complexes (Chen et al., 2008) which have now been shown to be paraspeckles. Such retention has been confirmed by another group, which further demonstrated that this phenomenon can provide the cell an important reservoir of mRNAs that can be mobilized for rapid export to the cytoplasm following cellular stress (Prasanth et al., 2005). However, nuclear retention is not the only fate of mRNAs with IRAlus. It was recently reported that some mRNAs with IRAlus could be detected in the cytoplasm where they were associated with translating polysomes (Hundley et al., 2008). Indeed, we also have also found this to be the case for a subset of cells expressing transcripts containing IRAlus (Chen et al., 2008; and data not shown). Within the same culture, there is strong nuclear retention in some cells, but only partial or even poor retention in a smaller fraction (Chen et al., 2008). While we do not yet understand the underlying basis for this alternative fate for mRNAs with IRAlus, it is possible that retention can be modulated or regulated in response to as yet obscure factors (Chen and Carmichael, 2008). Also, this variable phenomenon leads to the types of results we have shown in Figs. 1C, 5C, 5D and 5E, where retention is not complete.
Here we have examined in greater detail the mechanistic connection between dsRNA formation, ADAR editing and nuclear retention. Interestingly, human embryonic stem cells express ADAR1 and the paraspeckle-associated proteins p54nrb, PSF and PSP1α. However, paraspeckles are not formed and mRNAs with IRAlus in their 3′-UTRs are efficiently edited and bind to p54nrb, but are exported to the cytoplasm. These results demonstrate that retention requires more than editing and p54nrb binding. It may require paraspeckles, which are dependent on hNEAT1 for their assembly. These results suggest an important biological function for an abundant nuclear-retained noncoding RNA. Fig. 6 depicts our current working model for the nuclear retention machinery in differentiated and undifferentiated hESCs.
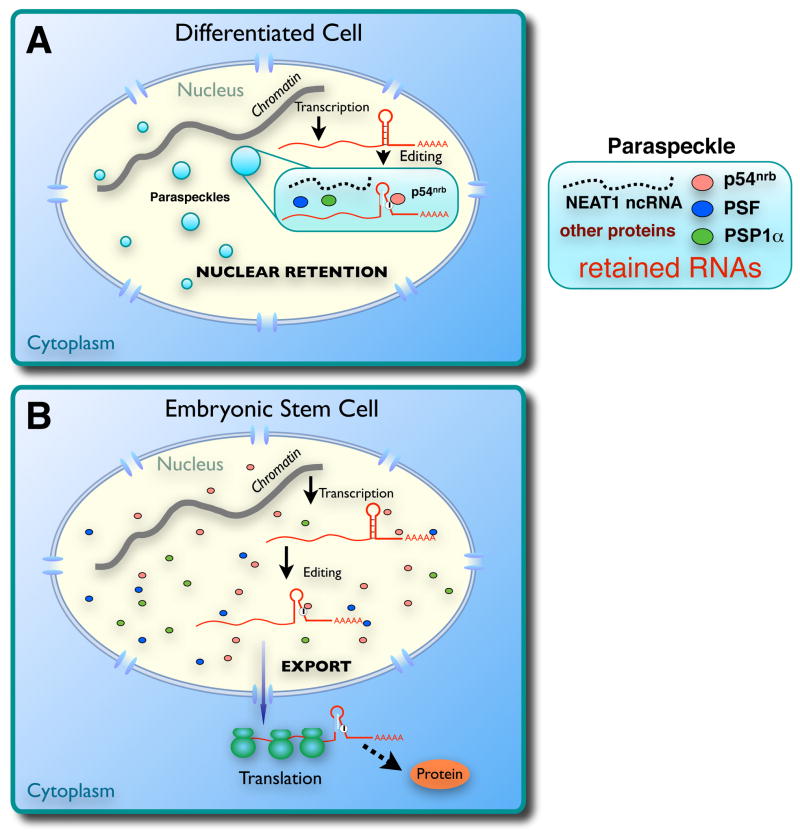
A. A model for nuclear retention of promiscuously edited RNAs in differentiated cells. Nuclear noncoding hNEAT1 RNA plays a critical role in paraspeckle formation and function. B. Altered gene regulation in human ES cells. In these cells, edited mRNAs are exported to the cytoplasm because of a defect in paraspeckle formation and function.
Paraspeckle associated proteins are multifunctional
A role in nuclear retention most likely represents only one of a multitude of functions of the p54nrb protein. While p54nrb, PSF and PSP1α accumulate in paraspeckles, these proteins are also found elsewhere throughout the nucleoplasm and thus may have functions apart from retention. These proteins can heterodimerize with each other and, since each contains two tandem RNA recognition motifs (RRMs) of unknown binding specificity, it is quite possible that there are numerous important RNA targets in addition to edited RNAs. In addition, both p54nrb and PSF are also known to be DNA-binding proteins and there have been reports of important roles for these factors in the regulation of both pre-mRNA splicing and transcription (Basu et al., 1997; Emili et al., 2002; Gozani et al., 1994; Ishitani et al., 2003; Kameoka et al., 2004; Kaneko et al., 2007; Lindsey et al., 1995; Mathur et al., 2001; Patton et al., 1993; Straub et al., 1998; Urban et al., 2000; Yang et al., 1993; Yang et al., 1997; Zhang et al., 1993; Zhang and Carmichael, 2001). Thus, while paraspeckle-associated proteins are multifunctional, NEAT1 may have only one function. Paraspeckles may represent subnuclear compartments that utilize a noncoding RNA to recruit and organize a group of multifunctional nuclear proteins for a specialized function, mRNA nuclear retention. We still do not know how hNEAT1 association with these factors leads to formation of paraspeckles and results in the nuclear retention of a subset of mRNAs and edited transcripts. Since p54nrb, PSF and PSP1α form heterodimers, it is possible that if one polypeptide were to contact hNEAT1 RNA while the other were to contact an mRNA target, then retention might result from tethering to hNEAT1 RNA, which might itself be anchored in the nucleus via interactions that have not yet been elucidated. In addition, it is possible that paraspeckles may be devoid of export factors. If this were the case, RNAs directed to paraspeckles would be unable to access the export machinery. Cleavage of retained RNAs to remove the anchoring sequence(s), as described in the work of Prasanth et al. (2005), would release the mRNAs from paraspeckles, making them available for export.
Human embryonic stem cells have altered dsRNA response pathways
Until now, nothing has been reported regarding dsRNA response pathways in hESCs. hESCs are the only cells we have found that lack hNEAT1 RNA and efficiently export mRNAs with IRAlus from the nucleus to the cytoplasm. Some of these exported mRNAs are edited, while others may not be. Although further studies will be required to determine the nature and abundance of cytoplasmic mRNAs with hairpin structures in hESCs, these cells are clearly unusual in that they tolerate cytoplasmic dsRNA owing to the lack of an efficient IFN/PKR response system (data not shown). This allows the translation of mRNAs that are more restricted to the nucleus in other cells. Significantly, one such mRNA encodes LIN28. LIN28 is known to be a regulator of developmental timing in C. elegans (Ambros and Horvitz, 1984; Horvitz et al., 1983) and colocalizes with mRNP complexes, P-bodies, and stress granules in pluripotent mouse P19 cells (Balzer and Moss, 2007). This protein also is one of four that together can reprogram somatic cells to pluripotent stem cells (Yu et al., 2007). In recent independent studies, this protein has been shown to affect microRNA processing in hESCs (Newman et al., 2008; Piskounova et al., 2008; Rybak et al., 2008; Viswanathan et al., 2008) and to enhance the translation of the mRNAs for specific cell cycle regulatory factors in mouse ES cells (Xu et al., 2009). We speculate that, like Lin28, there may be additional mRNAs important for hESC growth or function, whose expression may be enabled by the lack of the nuclear retention system described here. Further, since differentiation would demand that such genes be downregulated, this would be consistent with the induction of hNEAT1 expression and the formation of paraspeckles that we have oberved as hESCs are induced to develop into trophoblasts.
Of potential relevance to the results described here is the fact that the chromatin of pluripotent stem cells is unusually active in transcription, including substantial sense-antisense transcription (Efroni et al., 2008). This may result at least partly from an altered state of chromatin organization, with many chromosomal domains that are condensed in differentiated cells not being silenced in ES cells (Bernstein et al., 2006; Meshorer and Misteli, 2006; Meshorer et al., 2006). Thus, hESCs may express an unusually high amount of dsRNA within the nucleus, and some of this RNA may be exported into the cytoplasm.
There may be yet another rationale for a less efficient nuclear retention system in hESCs. We previously noted that many mRNAs with IRAlus in their 3′-UTRs undergo alternative polyadenylation to produce some mRNAs lacking IRAlus but others containing them (Chen et al., 2008). This phenomenon is in fact seen with the genes described in the studies reported here. mRNAs lacking IRAlus are efficiently exported to the cytoplasm while those containing IRAlus are largely confined to the nucleus. But the longer UTRs may contain specific microRNA binding sites that can be used for gene regulation only if these longer mRNAs reach the cytoplasm. This concept is illustrated by the Lin28 mRNA. In hESCs, the let-7 miRNA binding sites within the Lin28 3′-UTR are located immediately upstream of the IRAlus. If alternative polyadenylation were to remove not only the IRAlus but also the microRNA binding sites, then gene regulation in the cytoplasm would be quite different.
Finally, dsRNA editing may serve a critical but still obscure function in hESCs. ADAR1−/− homozygous mice die by embryonic day 11.5 with defects in erythropoiesis in the liver and with widespread apoptosis (Wang et al., 2000; Wang et al., 2004). Therefore, ADAR1 appears to be important for the viability and the development of non-neuronal tissues in the mouse. Recently, it has been reported that ADAR1 is essential for the maintenance of hematopoiesis and suppression of interferon signaling in the mouse (Hartner et al., 2009). In that system, ADAR1 is essential for maintenance of both fetal and adult hematopoietic stem cells, and loss of ADAR1 in hematopoietic stem cells led to global upregulation of type I and II interferon-inducible transcripts, followed by rapid apoptosis. We do not yet know whether the phenotypes summarized above are related to the regulatory pathway we have described here.
In conclusion, we report here the first description of the functional role of a long nuclear noncoding RNA in post-transcriptional regulation of gene expression. The significance of this RNA retention system is further highlighted by the finding that hESCs lack it. These cells express paraspeckle protein components, but not hNEAT1 RNA. Thus, we speculate that absence of NEAT1 RNA might serve as a marker of pluripotency. Further studies are needed to identify not only the mRNAs that are affected by this retention system in differentiated cells, but also those that are allowed to efficiently enter the cytoplasm in pluripotent stem cells.
Experimental Procedures
Cell culture, hES cell culture and transfection
HeLa and HEK293 cells were cultured in DMEM supplemented with 10% fetal bovine serum. Human ES cells were maintained on plates coated with growth-factor-depleted Matrigel (BD Biosciences) in either serum-free, defined mTeSR medium (StemCell Technologies Inc) or fibroblast-conditioned medium (CM) with mitotically inactivated mouse embryo fibroblasts supplemented with 4 ng/ml human bFGF (Life Technologies) (Xu et al., 2001). Cell cultures were regularly evaluated for Oct3/4 and Sox2 expression every 3–4 weeks and cells were passaged every 6–7 days. Differentiation of hESCs to trophoblasts was as previously described (Xu et al., 2002; Xu et al., 2005).
RNA nuclear retention analysis
Nuclear and cytoplasmic RNA isolation in HeLa cells was performed as described (Chen et al., 2008), and in hESCs was carried out with modifications detailed in a supplemental file. In all analyses, cell-equivalent amounts of cytoplasmic and RNA samples were used.
For northern blotting, Dig labeled Lin28, Paics, Adar1 and U6 probes were made by the DIG-High Prime DNA Labeling and Detection Starter Kit (Roche); Dig labeled antisense tRNAlys was made using T7 RNA polymerase with the DIG Northern Starter Kit (Roche), and the actin probe used was provided with the kit. Total, cytoplasmic and nuclear RNA were isolated from 107 cells. Nuclear/cytoplasmic ratios were normalized to actin mRNA.
RNA in situ hybridization and immunofluorescence microscopy
To detect hNEAT1 RNA, cells were rinsed briefly in PBS and then fixed in 3.6% formaldehyde plus 10% acetic acid in PBS (pH 7.4) for 15 min at RT. Cells were permeabilized in PBS containing 0.2–0.5% Triton X-100, and 5 mM VRC (Invitrogen) on ice for 5 min. Cells were washed in PBS 3×10 min and rinsed once in 2×SSC prior to hybridization. Hybridization was carried out using nick-translated cDNA probes (nick-translation kit; VYSIS Inc.) in a moist chamber at 37°C for 12–16 hr as described (Spector et al., 1998). A partial hNEAT1 clone was a gift from Dr. K. Prasanth. For colocalization studies, after RNA-FISH cells were again fixed for 5 min in 2% formaldehyde, and IF and imaging were performed as described (Chen et al, 2008). Antibodies used in IF are listed in a supplemental file. Images were taken with a Zeiss LSM 510 microscope.
Immunoprecipitation (IP) and RNA-protein-complex IP
HeLa cells and H9 cells on 10 cm dishes were rinsed twice with ice-cold PBS before harvesting in 10 mL ice-cold PBS by scraping. Cell pellets were resuspended in 1 mL IP buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 0.05% Igepal, 1 mM PMSF, proteinase inhibitor cocktail), and subjected to 2 rounds of gentle sonication and centrifuged to obtain cell extracts. For RNase treatment, 200 μg/mL RNaseA was added to cell extracts and incubated at 4°C for 2 hr. For IP, the RNaseA treated and non-treated cell extracts were incubated with 40 μL Protein A/G PLUS-Agarose beads (Santa Cruz Biotechnology) and 2.5 μg mouse anti-p54nrb antibody or anti-PSF antibody at 4°C for 2 hr. The beads were washed five times with IP buffer, resuspended in 2x SDS sample buffer and boiled for 5 min before loading on SDS PAGE. RNA-Protein-Complex IP were carried out in HeLa and H9 cells as previously described (Chen et al, 2008).
Antisense-oligonucleotide and dsRNA treatment
To knockdown the expression of hNEAT1 RNA, phosphorothioate-modified oligodeoxynucleotides (Seq 1: GGTTCTCGGAAAACTGGTGA and Seq 2: GTAACAGAATTAGTTCTTACCA) were synthesized at University Core DNA Services, University of Calgary. In addition, hNEAT1 siRNA sense: 5′-/5Phos/rGrUrGrArGrA rArGrU rUrGrC rUrUrA rGrArA rArCrU rUrUC C-3′, and antisense: 5′-rGrGrA rArArG rUrUrU rCrUrA rArGrC rArArC rUrUrC rUrCrArCrUrU-3′ (Clemson et al., 2009) were synthesized at Integrated DNA Technologies. Pooled DNA oligonucleotides or annealed dsRNAs were introduced to HeLa cells using Lipofectamine RNAiMax (Invitrogen). Optimal Lipofectamine RNAiMax/oligonucleotide ratios were empirically determined.
RT-PCR
After treatment of RNA samples with DNase I (DNA-free™ kit; Ambion), cDNA was transcribed with SuperScript II (Invitrogen) using oligo (dT) or random hexamers. Primers are listed in supplementary material.
RNA editing analysis
Total RNAs were isolated from H9 cells and treated with DNase I (Ambion, DNA-free™ kit). The AluSc on Lin28 mRNA was reverse transcribed with ThermoScript (Invitrogen) with a gene-specific primer located downstream of the IRAlus. The resultant cDNA was amplified by PCR subcloned using the TOPO-TA cloning kit (Invitrogen). The editing frequency was estimated by sequencing 20 individual clones containing the appropriately sized inserts (Agencourt Bioscience Corp).
Acknowledgments
We thank K. Prasanth for a probe for hNEAT1, K. Morris for help throughout the work and Li Yang for useful advice throughout. We also thank S. Garren, A. Günzl, Y. Huang, K. Morris and D. Moschenross for helpful comments on the manuscript. Human embryonic cell lines H1, H9 and H14 were obtained from the WiCell Research Institute and the CT Stem Cell Core Facility. This work was supported by grant CA04382 from the National Cancer Institute and an award from the State of Connecticut Stem Cell Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Akhmedov AT, Lopez BS. Human 100-kDa homologous DNA-pairing protein is the splicing factor PSF and promotes DNA strand invasion. Nucleic Acids Res. 2000;28:3022–3030. [Europe PMC free article] [Abstract] [Google Scholar]
- Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226:409–416. [Abstract] [Google Scholar]
- Athanasiadis A, Rich A, Maas S. Widespread A-to-I RNA editing of Alu-containing mRNAs in the human transcriptome. PLoS Biol . 2004;2:e391. Epub 2004 Nov 2009. [Europe PMC free article] [Abstract] [Google Scholar]
- Balzer E, Moss EG. Localization of the developmental timing regulator Lin28 to mRNP complexes, P-bodies and stress granules. RNA Biol. 2007;4:16–25. [Abstract] [Google Scholar]
- Bass BL. RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem. 2002;71:817–846. [Europe PMC free article] [Abstract] [Google Scholar]
- Bass BL, Weintraub H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell. 1988;55:1089–1098. [Abstract] [Google Scholar]
- Basu A, Dong B, Krainer AR, Howe CC. The intracisternal A-particle proximal enhancer-binding protein activates transcription and is identical to the RNA- and DNA-binding protein p54nrb/NonO. Mol Cell Biol. 1997;17:677–686. [Europe PMC free article] [Abstract] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. [Abstract] [Google Scholar]
- Blow M, Futreal PA, Wooster R, Stratton MR. A survey of RNA editing in human brain. Genome Res. 2004;14:2379–2387. [Europe PMC free article] [Abstract] [Google Scholar]
- Bonin M, Oberstrass J, Lukacs N, Ewert K, Oesterschulze E, Kassing R, Nellen W. Determination of preferential binding sites for anti-dsRNA antibodies on double-stranded RNA by scanning force microscopy. RNA. 2000;6:563–570. [Europe PMC free article] [Abstract] [Google Scholar]
- Chen LL, Carmichael GG. Gene regulation by SINES and inosines: Biological consequences of A-to-I editing of Alu element inverted repeats. Cell Cycle. 2008;7:3294–3301. [Abstract] [Google Scholar]
- Chen LL, DeCerbo JN, Carmichael GG. Alu element-mediated gene silencing. EMBO J. 2008;27:1694–1705. [Europe PMC free article] [Abstract] [Google Scholar]
- Clemson CM, Hutchinson JN, Sara SA, Ensminger AW, Fox AH, Chess A, Lawrence JB. An architectural role for a nucleaar noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell. 2009;33:717–726. [Europe PMC free article] [Abstract] [Google Scholar]
- Efroni S, Duttagupta R, Cheng J, Dehghani H, Hoeppner DJ, Dash C, Bazett-Jones DP, Le Grice S, McKay RD, Buetow KH, et al. Global transcription in pluripotent embryonic stem cells. Cell Stem Cell. 2008;2:437–447. [Europe PMC free article] [Abstract] [Google Scholar]
- Emili A, Shales M, McCracken S, Xie W, Tucker PW, Kobayashi R, Blencowe BJ, Ingles CJ. Splicing and transcription-associated proteins PSF and p54nrb/nonO bind to the RNA polymerase II CTD. RNA. 2002;8:1102–1111. [Europe PMC free article] [Abstract] [Google Scholar]
- Fox AH, Bond CS, Lamond AI. P54nrb forms a heterodimer with PSP1 that localizes to paraspeckles in an RNA-dependent manner. Mol Biol Cell. 2005;16:5304–5315. [Europe PMC free article] [Abstract] [Google Scholar]
- Fox AH, Lam YW, Leung AK, Lyon CE, Andersen J, Mann M, Lamond AI. Paraspeckles. A novel nuclear domain. Curr Biol. 2002;12:13–25. [Abstract] [Google Scholar]
- Gozani O, Patton JG, Reed R. A novel set of spliceosome-associated proteins and the essential splicing factor PSF bind stably to pre-mRNA prior to catalytic step II of the splicing reaction. EMBO J. 1994;13:3356–3367. [Europe PMC free article] [Abstract] [Google Scholar]
- Hartner JC, Walkley CR, Lu J, Orkin SH. ADAR1 is essential for the maintenance of hematopoiesis and suppression of interferon signaling. Nat Immunol. 2009;10:109–115. [Europe PMC free article] [Abstract] [Google Scholar]
- Horvitz HR, Sternberg PW, Greenwald IS, Fixsen W, Ellis HM. Mutations that affect neural cell lineages and cell fates during the development of the nematode Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol. 1983;48:453–463. [Abstract] [Google Scholar]
- Hundley HA, Krauchuk AA, Bass BL. C. elegans and H. sapiens mRNAs with edited 3′ UTRs are present on polysomes. RNA. 2008;14:2050–2060. [Europe PMC free article] [Abstract] [Google Scholar]
- Hutchinson JN, Ensminger AW, Clemson CM, Lynch CR, Lawrence JB, Chess A. A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics. 2007;8:39. [Europe PMC free article] [Abstract] [Google Scholar]
- Ishitani K, Yoshida T, Kitagawa H, Ohta H, Nozawa S, Kato S. p54(nrb) acts as a transcriptional coactivator for activation function 1 of the human androgen receptor. Biochem Biophys Res Commun. 2003;306:660–665. [Abstract] [Google Scholar]
- Kameoka S, Duque P, Konarska MM. p54(nrb) associates with the 5′ splice site within large transcription/splicing complexes. EMBO J. 2004;23:1782–1791. [Europe PMC free article] [Abstract] [Google Scholar]
- Kaneko S, Rozenblatt-Rosen O, Meyerson M, Manley JL. The multifunctional protein p54nrb/PSF recruits the exonuclease XRN2 to facilitate pre-mRNA 3′ processing and transcription termination. Genes Dev. 2007;21:1779–1789. [Europe PMC free article] [Abstract] [Google Scholar]
- Kim DD, Kim TT, Walsh T, Kobayashi Y, Matise TC, Buyske S, Gabriel A. Widespread RNA editing of embedded alu elements in the human transcriptome. Genome Res. 2004;14:1719–1725. [Europe PMC free article] [Abstract] [Google Scholar]
- Kumar M, Carmichael GG. Antisense RNA: function and fate of duplex RNA in cells of higher eukaryotes. Microbiol Mol Biol Rev. 1998;62:1415–1434. [Europe PMC free article] [Abstract] [Google Scholar]
- Levanon EY, Eisenberg E, Yelin R, Nemzer S, Hallegger M, Shemesh R, Fligelman ZY, Shoshan A, Pollock SR, Sztybel D, et al. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat Biotechnol. 2004;22:1001–1005. [Abstract] [Google Scholar]
- Li SX, Tong YP, Xie XC, Wang QH, Zhou HN, Han Y, Zhang ZY, Gao W, Li SG, Zhang XC, et al. Octameric structure of the human bifunctional enzyme PAICS in purine biosynthesis. J Mol Biol. 2007;366:1603–1614. [Abstract] [Google Scholar]
- Lindsey LA, Crow AJ, Garcia-Blanco MA. A mammalian activity required for the second step of pre-messenger RNA splicing. J Biol Chem. 1995;270:13415–13421. [Abstract] [Google Scholar]
- Mathur M, Tucker PW, Samuels HH. PSF is a novel corepressor that mediates its effect through Sin3A and the DNA binding domain of nuclear hormone receptors. Mol Cell Biol. 2001;21:2298–2311. [Europe PMC free article] [Abstract] [Google Scholar]
- Meshorer E, Misteli T. Chromatin in pluripotent embryonic stem cells and differentiation. Nature Rev Mol Cell Biol. 2006;7:540–546. [Abstract] [Google Scholar]
- Meshorer E, Yellajoshula D, George E, Scambler PJ, Brown DT, Misteli T. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev Cell. 2006;10:105–116. [Europe PMC free article] [Abstract] [Google Scholar]
- Myojin R, Kuwahara S, Yasaki T, Matsunaga T, Sakurai T, Kimura M, Uesugi S, Kurihara Y. Expression and functional significance of mouse paraspeckle protein 1 on spermatogenesis. Biol Reprod. 2004;71:926–932. [Abstract] [Google Scholar]
- Newman MA, Thomson JM, Hammond SM. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA. 2008;14:1539–1549. [Europe PMC free article] [Abstract] [Google Scholar]
- Nishikura K. Modulation of double-stranded RNAs in vivo by RNA duplex unwindase. Ann NY Acad Sci. 1992;660:240–250. [Abstract] [Google Scholar]
- Patton JG, Porro EB, Galceran J, Tempst P, Nadal-Ginard B. Cloning and characterization of PSF, a novel pre-mRNA splicing factor. Genes Dev. 1993;7:393–406. [Abstract] [Google Scholar]
- Piskounova E, Viswanathan SR, Janas M, LaPierre RJ, Daley GQ, Sliz P, Gregory RI. Determinants of microRNA processing inhibition by the developmentally regulated RNA-binding protein Lin28. J Biol Chem. 2008;283:21310–21314. [Abstract] [Google Scholar]
- Polson AG, Crain PF, Pomerantz SC, McCloskey JA, Bass BL. The mechanism of adenosine to inosine conversion by the double-stranded RNA unwinding/modifying activity: a high-performance liquid chromatography-mass spectrometry analysis. Biochemistry. 1991;30:11507–11514. [Abstract] [Google Scholar]
- Prasanth KV, Prasanth SG, Xuan Z, Hearn S, Freier SM, Bennett CF, Zhang MQ, Spector DL. Regulating gene expression through RNA nuclear retention. Cell. 2005;123:249–263. [Abstract] [Google Scholar]
- Richards M, Tan SP, Tan JH, Chan WK, Bongso A. The transcriptome profile of human embryonic stem cells as defined by SAGE. Stem Cells. 2004;22:51–64. [Abstract] [Google Scholar]
- Rybak A, Fuchs H, Smirnova L, Brandt C, Pohl EE, Nitsch R, Wulczyn FG. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol. 2008;10:987–993. [Abstract] [Google Scholar]
- Saha S, Murthy S, Rangarajan PN. Identification and characterization of a virus-inducible non-coding RNA in mouse brain. J Gen Virol. 2006;87:1991–1995. [Abstract] [Google Scholar]
- Sasaki YTF, Ideue T, Sano M, Mityama T, Hirose T. MENepsilon/beta noncoding RNAs are essential for structural integrity of paraspeckles. Proc Natl Acad Sci USA. 2009;106:2525–2530. [Europe PMC free article] [Abstract] [Google Scholar]
- Spector DL, Goldman RD, Leinwand LA. Cells: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1998. [Google Scholar]
- Straub T, Grue P, Uhse A, Lisby M, Knudsen BR, Tange TO, Westergaard O, Boege F. The RNA-splicing factor PSF/p54 controls DNA-topoisomerase I activity by a direct interaction. J Biol Chem. 1998;273:26261–26264. [Abstract] [Google Scholar]
- Sunwoo H, Dinger ME, Wilusz JE, Amaral PP, Mattick JS, Spector DL. MENepsilon/beta nuclear-retained non-coding RNAs are up-regulated upon muscle differentiation and are essential components of paraspeckles. Genome Res. 2009;19:347–359. [Europe PMC free article] [Abstract] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. [Abstract] [Google Scholar]
- Urban RJ, Bodenburg Y, Kurosky A, Wood TG, Gasic S. Polypyrimidine tract-binding protein-associated splicing factor is a negative regulator of transcriptional activity of the porcine p450scc insulin-like growth factor response element. Mol Endocrinol. 2000;14:774–782. [Abstract] [Google Scholar]
- Viswanathan SR, Daley GQ, Gregory RI. Selective Blockade of MicroRNA Processing by Lin-28. Science. 2008;320:97–100. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang Q, Carmichael GG. Effects of length and location on the cellular response to double-stranded RNA. Microbiol Mol Biol Rev. 2004;68:432–452. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang Q, Khillan J, Gadue P, Nishikura K. Requirement of the RNA editing deaminase ADAR1 gene for embryonic erythropoiesis. Science. 2000;290:1765–1768. [Abstract] [Google Scholar]
- Wang Q, Miyakoda M, Yang W, Khillan J, Stachura DL, Weiss MJ, Nishikura K. Stress-induced apoptosis associated with null mutation of ADAR1 RNA editing daminase gene. J Biol Chem. 2004;279:4952–4961. [Abstract] [Google Scholar]
- Xu B, Zhang K, Huang Y. Lin28 modulates cell growth and associates with a subset of cell cycle regulator mRNAs in mouse embryonic stem cells. RNA. 2009;15:357–361. [Europe PMC free article] [Abstract] [Google Scholar]
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. [Abstract] [Google Scholar]
- Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP, Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. [Abstract] [Google Scholar]
- Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–190. [Abstract] [Google Scholar]
- Yang YS, Hanke JH, Carayannopoulos L, Craft CM, Capra JD, Tucker PW. NonO, a non-POU-domain-containing, octamer-binding protein, is the mammalian homolog of Drosophila nonAdiss. Mol Cell Biol. 1993;13:5593–5603. [Europe PMC free article] [Abstract] [Google Scholar]
- Yang YS, Yang MC, Tucker PW, Capra JD. NonO enhances the association of many DNA-binding proteins to their targets. Nucleic Acids Res. 1997;25:2284–2292. [Europe PMC free article] [Abstract] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. [Abstract] [Google Scholar]
- Zhang WW, Zhang LX, Busch RK, Farres J, Busch H. Purification and characterization of a DNA-binding heterodimer of 52 and 100 kDa from HeLa cells. Biochem J. 1993;290:267–272. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhang Z, Carmichael GG. The fate of dsRNA in the nucleus. A p54(nrb)-containing complex mediates the nuclear retention of promiscuously A-to-I edited RNAs. Cell. 2001;106:465–475. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.molcel.2009.06.027
Read article for free, from open access legal sources, via Unpaywall:
http://www.cell.com/article/S1097276509004651/pdf
Subscription required at www.molecule.org
http://www.molecule.org/cgi/content/reprint/35/4/467
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.molcel.2009.06.027
Article citations
NEAT1 modulates the TIRR/53BP1 complex to maintain genome integrity.
Nat Commun, 15(1):8438, 30 Sep 2024
Cited by: 0 articles | PMID: 39349456 | PMCID: PMC11443056
siRNAs Targeting Non-Human Species-Specific lncRNAs Trigger Cell Death in Human Colorectal Cancer Cells.
J Cancer, 15(18):5956-5967, 23 Sep 2024
Cited by: 0 articles | PMID: 39440066 | PMCID: PMC11493012
Nucleolar stress induces nucleolar stress body formation via the NOSR-1/NUMR-1 axis in Caenorhabditis elegans.
Nat Commun, 15(1):7256, 23 Aug 2024
Cited by: 0 articles | PMID: 39179648 | PMCID: PMC11343841
Genetics of cell-type-specific post-transcriptional gene regulation during human neurogenesis.
Am J Hum Genet, 111(9):1877-1898, 20 Aug 2024
Cited by: 0 articles | PMID: 39168119
Spatial Visualization of A-to-I Editing in Cells Using Endonuclease V Immunostaining Assay (EndoVIA).
ACS Cent Sci, 10(7):1396-1405, 08 Jul 2024
Cited by: 0 articles | PMID: 39071059 | PMCID: PMC11273454
Go to all (418) article citations
Other citations
Wikipedia
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Nucleotide Sequences
- (1 citation) ENA - CN349329
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A NEAT way of regulating nuclear export of mRNAs.
Mol Cell, 35(4):395-396, 01 Aug 2009
Cited by: 9 articles | PMID: 19716782
Protein arginine methyltransferase CARM1 attenuates the paraspeckle-mediated nuclear retention of mRNAs containing IRAlus.
Genes Dev, 29(6):630-645, 01 Mar 2015
Cited by: 62 articles | PMID: 25792598 | PMCID: PMC4378195
Alu element-mediated gene silencing.
EMBO J, 27(12):1694-1705, 22 May 2008
Cited by: 228 articles | PMID: 18497743 | PMCID: PMC2435129
Paraspeckles: nuclear bodies built on long noncoding RNA.
J Cell Biol, 186(5):637-644, 31 Aug 2009
Cited by: 274 articles | PMID: 19720872 | PMCID: PMC2742191
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (4)
Grant ID: CA04382
Grant ID: R01 CA045382
Grant ID: R01 CA045382-22
Grant ID: R01 CA045382-23