Abstract
Free full text

Costimulation as a platform for the development of vaccines: a peptide-based vaccine containing a novel form of 4-1BBL eradicates established tumors
Abstract
Vaccines represent an attractive treatment modality for the management of cancer primarily because of their specificity and generation of immunological memory important for controlling recurrences. However, the efficacy of therapeutic vaccines may require formulations that not only generate effective immune responses, but also overcome immune evasion mechanisms employed by progressing tumor. Costimulatory molecules play critical roles in modulating innate, adaptive, and regulatory immunity, and have potential to serve as effective immunomodulatory components of therapeutic vaccines. In this study, we tested the function of a novel soluble form of 4-1BBL costimulatory molecule in modulating innate, adaptive, and regulatory immunity, and assessed its therapeutic efficacy in the HPV-16 E7-expressing TC-1 cervical cancer and survivin-expressing 3LL lung carcinoma mouse models. Vaccination with 4-1BBL activated DCs and enhanced antigen uptake, generated CD8+ T cell effector/memory responses, and endowed T effector cells refractory to suppression by CD4+CD25+FoxP3+ T regulatory cells. Immunization with 4-1BBL in combination with an E7 peptide or survivin protein resulted in eradication of TC-1 and 3LL tumors, respectively. 4-1BBL was more effective than TLR agonists LPS, MPL, CpG, and an agonistic 4-1BB Ab as a component of E7 peptide-based therapeutic vaccine for the generation of immune responses and eradication of TC-1 established tumors in the absence of detectable toxicity. Therapeutic efficacy was associated with reversal of tumor mediated nonresponsiveness/anergy as well as establishment of long-term CD8+ T cell memory. Potent pleiotropic immunomodulatory activities combined with lack of toxicity highlight the potential of 4-1BBL molecule as an effective component of therapeutic cancer vaccines.
Introduction
Regardless of many advances in vaccinology, the therapeutic potential of cancer vaccines remains to be realized. This is partially due to an array of immunoevasive and suppressive mechanisms employed by progressing tumors (1, 2). Therefore, the success of therapeutic vaccines will require formulations that are not only effective in generating new immune responses and/or boost the existing ones, but also in their ability to overcome immune evasion mechanisms. In this context, the discovery and development of novel adjuvants with potent immunomodulatory activities on cells of innate, adaptive, and regulatory immunity without adverse toxicity at therapeutic doses is of significant importance in the field of cancer immunotherapy.
Costimulatory signals transduced via CD28 and TNFR family members play paramount roles in modulating innate, adaptive, and regulatory immunity (3), and as such agonistic ligands for this class of immunomodulatory receptors have potential to serve as effective components of therapeutic cancer vaccines. Consistent with this notion is the demonstrated efficacy of agonistic Abs against costimulatory receptors in various therapeutic preclinical tumor settings (4–7). The use of agonistic Abs, however, may be associated with severe toxicity as demonstrated in selected settings in rodents (8, 9) and human (10). We hypothesized that signaling by natural ligands may have better efficacy and safety as compared with agonistic Abs and herein tested this notion using 4-1BB ligand (4-1BBL), a member of the TNFR family, as the immunomodulatory component of vaccines. The choice of 4-1BBL was because of the pleiotropic effects of 4-1BB signaling on various cells of innate (11), adaptive (5, 12), and regulatory immunity (13, 14) as well as the robust therapeutic efficacy of agonistic 4-1BB Abs in various rodent cancer models (4, 5). Inasmuch as the natural 4-1BBL functions as a cell membrane-bound protein and has no activity in soluble form (15), we recently generated a novel form of this ligand by fusing the extracellular domain of murine 4-1BBL to the C-terminus of a modified core-streptavidin (SA-4-1BBL) (16). The choice of streptavidin (SA) is because it exists as stable tetramers and oligomers (17), and as such serves as a chaperon to enable the chimeric SA-4-1BBL to exists as multivalent tetramers and oligomers (18, 19) with the ability to cross-link 4-1BB receptor on immune cells for potent signal transduction (16).
In the present study, we demonstrated that soluble SA-4-1BBL protein served as an effective immunomodulatory component of vaccines by activating DCs and enhancing antigen uptake, stimulating primary T cell responses and maintaining long-term memory, and licensing T effector (Teff) cells to overcome the suppressive effect of CD4+CD25+FoxP3+ T regulatory (Treg) cells. A single vaccination with SA-4-1BBL mixed with survivin protein or a dominant CD8+ T cell epitope for E7 was effective in eradicating established 3LL and TC-1 tumors, respectively, without detectable toxicity. Importantly, SA-4-1BBL had better activity than TLR agonists, LPS, MPL, CpG, and an agonistic Ab to 4-1BB in modulating various immune responses and eradicating TC-1 tumors. Collectively, our findings provide a strong rationale for further developing this novel form of soluble 4-1BBL as an immunomodulatory component of therapeutic vaccines against cancer and infections.
Materials and Methods
Mice
C57BL/6.SJL and C57BL/6 mice were bred in our animal facility at the University of Louisville. 4-1BB knockout (KO) mice were generously provided by Dr. A.T. Vella of University of Connecticut, Farmington, CT, with permission from Dr. B.S. Kwon of University of Ulsan, Korea. All animals were cared for in accordance with institutional and NIH guidelines.
Reagents
Construction, expression, purification, and characterization of SA-4-1BBL (endotoxin level 0.004 EU/μg protein) were recently described (16). Anti-41BB agonistic Ab (clone 3H3) was kindly provided by Dr. R. Mittler (Emory University, Atlanta, GA) (9). Fluorochrome-conjugated Abs (anti-CD8-PerCP, anti-I-A/I-E-PE, anti-CD86-APC, anti-IFN-γPE, anti-CD45.1/2-APC,) and isotype controls were purchased from BD-PharMingen and eBioscience. HPV16 E7 peptide (E749-57 RAHYNIVTF) and CpG oligonucleotide (G*GGGGACGATCGTCG*G*G*G*G*G; *represents phosphorothioate linkage) were purchased from CPC Scientific (San Jose, CA) and Operon Biotechnologies, (Alameda, CA), respectively. LPS and MPL were purchased from Sigma-Aldrich (St. Louis, MO) and InVivogen, (San Diago, CA), respectively.
Intracellular cytokine staining
LNs were processed into single-cell suspensions and stimulated with PMA/ionomycin for intracellular cytokine staining as described (20).
In vivo antigen uptake by DCs
Twenty-five μg of FITC-labeled ovalbumin mixed with 25 μg of SA-4-1BBL or equimolar quantity of SA in PBS was injected s.c. into the right flank of C57BL/6 mice. Animals injected with OVA-FITC alone served as control. Draining LNs were harvested 3 hrs later and processed into a single-cell suspension. After blocking Fc-receptors, cells were stained with APC-conjugated anti-mouse CD11c Ab. FITC+CD11c+ cells were analyzed using flow cytometry.
T cell proliferation and suppression assays
The effect of soluble SA-4-1BBL protein on of CD8+ T cell proliferation was determined as previously described (16). For suppression assay, CD4+CD25+ Treg and CD4+CD25− Teff cells were sorted from WT and 4-1BB KO C57BL/6 mice by flow cytometry. Treg cells were cocultured at various ratios with a fixed number of Teff cells (2.5×104 cells/well) in U-bottom 96-well plates in the presence of anti-CD3 Ab, irradiated (2000 cGy) syngeneic splenocytes (1×105 cells/well), 1 μg/ml SA-4-1BBL or equimolar (0.4 μg/ml) quantity of SA protein. Cells were cultured for 3 days, pulsed with [3H]-thymidine during the last 16 hrs of culture, harvested, and analyzed for proliferation as described (16).
In vivo cytotoxicity assay
B6.SJL (CD45.1) spleen cells were labeled with 2.5 μM CFSE (CFSEhigh) and 0.25 μM CFSE (CFSElow). CFSEhigh cells were then pulsed with either 2 μg/ml of E749-57 or a survivin peptide as a control for 90 min at 37°C in a 5% CO2 incubator. After extensive washing, CFSEhigh and CFSElow cells were mixed at a 1:1 ratio, and injected i.v. into C57BL/6 (CD45.2) mice 5 days after vaccination. Spleens were removed 48 hrs later, processed into single cell suspension, stained with APC-labeled CD45.1 Ab and analyzed by flow cytometry to determine the ratio of CFSElow/CFSEhigh target cells. The percentage of in vivo killing was calculated by the formula [1−((CFSElow/CFSEhigh for test)/(CFSElow/CFSEhigh for naive))] × 100.
Tumor models and vaccination
Hundred-thousand TC-1 or 3LL cells were injected s.c. into the right flank of C57BL/6 mice. Tumor growth was monitored 2–3 times/week using caliper. Animals bearing tumors were euthanized when tumors reached a size of 15 mm in diameter or earlier if tumors ulcerated or animal showed signs of discomfort. For TC-1 therapeutic studies, mice were immunized s.c. 10 days post-tumor challenge with 50 μg of E749-57 peptide alone or in combination with 25 μg of SA-4-1BBL, 10 μg of SA as control protein, 25 μg of LPS, 25 μg of MPL, 10 μg of CpG or 100 μg of 3H3 Ab against 4-1BB. The quantities of LPS, MPL, and CpG used represent optimum doses established by published literature (21) and studies in this report. For tumor recurrence studies, tumors were removed surgically when they reached an average size of 4 mm in diameter. The mice were left to recover from surgery for 48 hrs and then vaccinated s.c. with 50 μg of E749-57 plus 25 μg SA-4-1BBL or an equimolar control SA (10 μg) protein. For 3LL tumors, s.c. vaccination was performed using 25 μg of SA-4-1BBL mixed with 50 μg of recombinant mouse survivin on day 6 post-tumor challenge.
Analysis of tumor infiltrating CD8+ T cells
Mice bearing TC-1 tumors of ~3 mm in diameter were injected s.c. with 50 μg of E749-57 peptide mixed with 25 μg SA-4-1BBL, 25 μg MPL, 25 μg LPS, or 10 μg CpG. Tumors were harvested 7 days later, digested in 2 mg collagenase-P/ml, and 1 mg DNase I/ml in PBS for 2 hrs at 37°C with occasional shaking. The resultant cells were washed, and stained with anti-mouse CD3 and CD8. Anti-CD45.2 Ab was used to selectively exclude CD45 negative tumor cells from analysis.
Statistical analyses
Statistical analyses were performed using Student’s t-test, ANOVA, or Log-rank test using SPSS software. For each test p value less than 0.05 and 0.001 were considered significant (*) and very significant (**), respectively.
Results
SA-4-1BBL has pleiotropic effects on cells of innate, adaptive, and regulatory immunity
The 4-1BB receptor is constitutively expressed on a subpopulation of immature DCs and CD4+CD25+FoxP3+ Treg cells (14, 16), and inducibly expressed on activated CD4+ and CD8+ T cells (22, 23). Therefore, we first investigated the effect of SA-4-1BBL treatment on each of these cell populations. In vitro treatment of bone-marrow derived primary immature DCs (BM-DCs) or the JAWS II DC line with SA-4-1BBL for 48 hrs resulted in the activation of both cell types as assessed by the upregulated expression of CD86 and MHC class II molecules, and this effect was comparable to that obtained using the TLR4 agonist LPS (Fig. 1A). However, SA-4-1BBL was more effective than MPL, a modified version of LPS, and TLR9 agonist CpG in upregulating various activation markers on BM-DCs (Supplementary Fig. S1; Table S1).
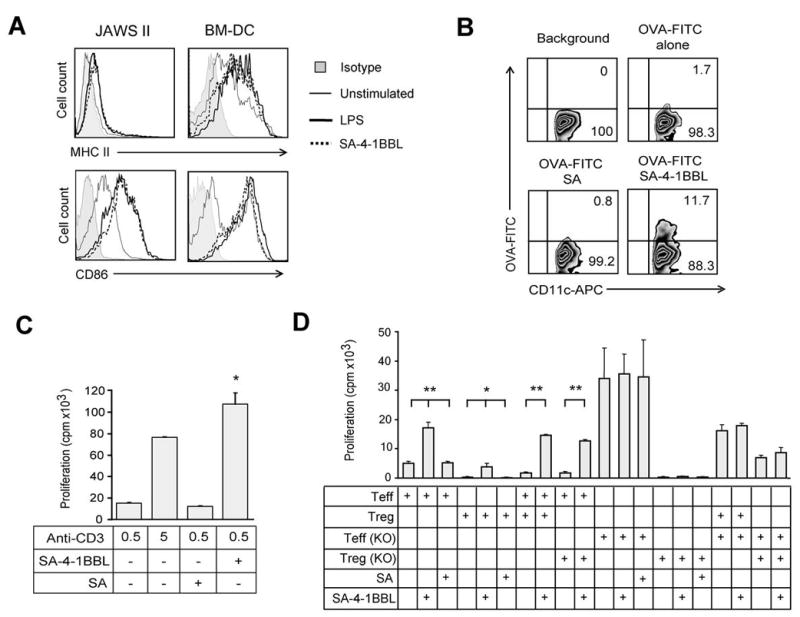
Pleiotropic activities of SA-4-1BBL on various cells of the immune system. A, SA-4-1BBL activates BM-DCs and JAWS II DCs in vitro. Expression of MHC class II and CD86 molecules on cells stimulated with SA-4-1BBL (5 μg/ml) or LPS (5 μg/ml) for 48 hrs. B, SA-4-1BBL enhances antigen uptake by DCs in vivo. C57BL/6 mice were injected s.c. with OVA-FITC (25 μg) and SA-4-1BBL (25 μg) or an equimolar quantity (10 μg) of SA as control. Draining LN cells were harvested 3 hrs later, stained with an APC-anti-CD11c Ab, and analyzed in flow cytometry. C, SA-4-1BBL induces CD8+ T cell proliferation in vitro. Sorted CD8+ T cells were cultured with the indicated quantities of an anti-CD3 Ab, irradiated splenocytes, and soluble SA-4-1BBL (1 μg/ml) or SA for 3 days. *p<0.05, SA-4-1BBL vs. all the other groups. D, SA-4-1BBL renders Teff cells resistant to Treg cell suppression. Sorted CD4+CD25- Teff and CD4+CD25+ Treg cells from WT or 4-1BB KO C57BL/6 mice were cocultured at 2:1 Teff:Treg ratio in the presence of irradiated splenocytes and SA-4-1BBL (1 μg/ml) or SA as indicated. *p<0.05, **p<0.001. Data are representative of a minimum of two independent experiments for each panel.
It has been shown that activation of immature DCs with an agonistic Ab to CD40 or TLR agonists result in enhanced antigen uptake followed by maturation (24). To test if 4-1BB stimulation also enhances antigen uptake by DCs, we injected mice with SA-4-1BBL and FITC-ovalbumin (OVA-FITC) as a cognate antigen. There was a significant increase (>11%) in antigen uptake by DCs isolated from mice treated with SA-4-1BBL as compared with DCs from mice treated with OVA-FITC alone (1.7%) or antigen with control SA (0.85%) (Fig. 1B).
We have recently demonstrated that SA-4-1BBL as a soluble protein has robust costimulatory activity on CD4+ T cells (16). To test if the costimulatory activity of SA-4-1BBL also applies to CD8+ T cells, flow-sorted cells were used in a CD3 Ab-based proliferation assay. At suboptimal doses of anti-CD3 Ab stimulation, SA-4-1BBL showed potent costimulatory activity on CD8+ T cells (Fig. 1C).
CD4+CD25+FoxP3+ Treg cells constitutively express the 4-1BB receptor (14, 25, 26). We have recently shown that 4-1BB signaling into Treg cells results in their proliferation without a major effect on their suppressive function in the absence of the ligand (16). However, in the presence of 4-1BBL, Treg cells failed to suppress Teff cells and the lack of suppressive effect was associated with the 4-1BB signaling into Teff cells, rather than Treg cells (16). To further confirm this finding, we performed CD3 Ab-based suppression studies using Teff and Treg cells obtained from 4-1BB WT and KO mice. Costimulation with SA-4-1BBL was effective in blocking the suppressive function of Treg cells on WT Teff cells, but not KO Teff cells, demonstrating the importance of 4-1BB signaling into Teff cells for overcoming the suppressive function of Treg cells (Fig. 1D). The Treg cells from KO mice showed better suppressive activity (Fig. 1D). Taken together, these data demonstrate that SA-4-1BBL has pleiotropic effects on cells of innate (DCs), adaptive (Teff cells), and regulatory (Treg cells) immunity, and as such has potential to serve as an effective immunomodulatory component of therapeutic cancer vaccines.
SA-4-1BBL serves as an effective immunomodulatory component of a therapeutic cancer vaccine
To test if the significant immunomodulatory activities of SA-4-1BBL translate into therapeutic efficacy, the TC-1 cell line expressing c-Ras and HPV-16 E7 and E6 oncogenes was used as a transplantable tumor model for cervical cancer. Immunization of naive mice with a synthetic E749-57 peptide representing the dominant CD8+ T cell epitope in C57BL/6 mice (27) in combination with varying doses of SA-4-1BBL generated in vivo killing responses, in which 50 μg of the peptide and 25 μg of SA-4-1BBL produced the most pronounced effect (Fig. 2A). The killing activity of this vaccine regimen was further confirmed using E7 expressing TC-1 cells as targets in an in vitro cytotoxicity assay (Supplementary Fig. S2). Consequently, this vaccine formulation was tested in two different therapeutic tumor settings for efficacy. A single s.c. injection of the vaccine formulation was effective in eradicating 10-day established TC-1 tumors in 75% of mice and retarding the tumor growth for the rest with more than 80% survival for 90 days observation period (Fig. 2B; Supplementary Fig. S3). Vaccination of 4-1BB KO mice with E749-57 peptide + SA-4-1BBL did not show better efficacy than E749-57 peptide alone (data not shown), demonstrating that vaccine effect is primarily mediated through 4-1BB signaling. Furthermore, the surviving mice did not develop tumors when re-challenged with live TC-1 cells 60 days after initial tumor inoculation, demonstrating long-term immunologic memory. Importantly, the therapeutic efficacy of SA-4-1BBL-based vaccine was also demonstrated in the 3LL mouse lung carcinoma model where a recombinant survivin protein was used as a bona fide self TAA antigen (Fig. 2C).
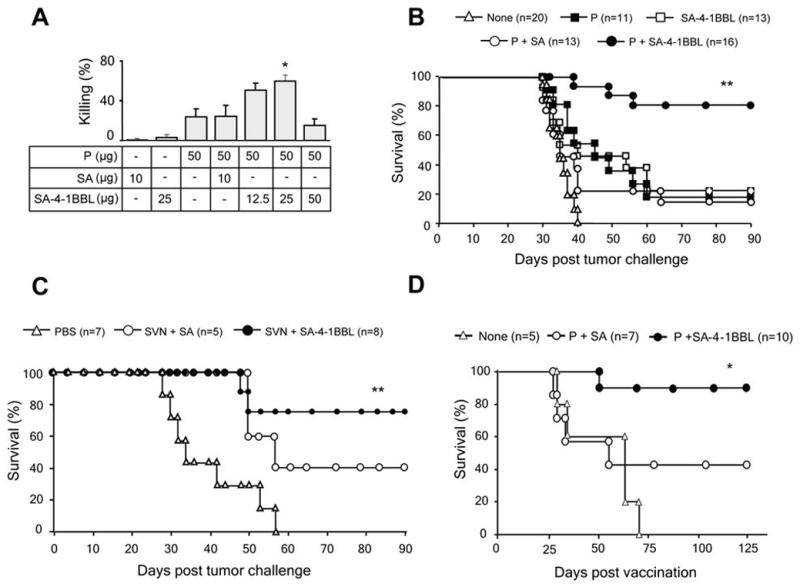
SA-4-1BBL serves as an effective immunomodulatory component of a therapeutic cancer vaccine. A, determining the optimal dose of SA-4-1BBL for in vivo killing response. C57BL/6 mice (n=4 per group) were immunized s.c. with 50 μg of E749-57 peptide (P) and indicated doses of SA-4-1BBL or SA. In vivo E749-57-specific killing was determined 7 days later. B, vaccination with SA-4-1BBL is effective in eradicating established TC-1 tumors. C57BL/6 mice were challenged with live TC-1 cells and vaccinated once s.c. 10 days later with the peptide (50 μg) alone or in combination with SA-4-1BBL (25 μg) or an equimolar quantity of SA (10 μg). Tumor-free mice in the P + SA-4-1BBL group were rechallenged with live TC-1 cells 60 days after initial tumor challenge and monitored for tumor growth. **p<0.0001 for P+SA-4-1BBL vs. other groups. C, Vaccination with SA-4-1BBL and survivin as an autologous TAA is effective in eradicating established 3LL tumors. C57BL/6 mice were challenged with live 3LL cells and vaccinated once s.c. 6 days later with PBS or a recombinant survivin protein (50 μg) mixed with SA-4-1BBL (25 μg) or an equimolar quantity of SA (10 μg) as control. **p<0.001 for SVN+SA-4-1BBL vs. other groups. D, Vaccination with SA-4-1BBL prevents tumor recurrence. C57BL/6 mice with ~4 mm tumors were subjected to tumor resection and vaccinated 48 hrs later with a single s.c. injection of P (50 μg) with SA-4-1BBL (25 μg) or SA (10 μg). *p<0.008 for SA-4-1BBL vs. other groups.
Therapeutic cancer vaccines are well-suited for the control/prevention of recurrences in patients undergone tumor resection and chemotherapy as such patients may have minimal levels of immunosuppressive/regulatory mechanisms that curb the efficacy of vaccines (28). Hence, we tested the efficacy of our vaccine formulation for preventing/controlling recurrences in a surgical tumor removal model in mice. A single s.c. vaccination with SA-4-1BBL and E749-57 peptide 2 days after resection of established tumors was effective in preventing recurrences in 90% of animals as compared to 42.8% for the peptide alone and 0% for control groups (Fig. 2D). Tumor-free animals shown in Fig. 2B retained long-term peptide-specific CD8+ Teff memory responses as demonstrated by in vivo killing (Figure 3A). This effector memory response could further be boosted by revaccination, resulting in higher killing (Fig. 3A) and IFN-γ production (Fig. 3B). Consistent with the role of 4-1BB signaling in development and maintenance of memory, we observed a significant increase in total memory CD8+CD44high T cell pool in long-term animals as compared with naive mice (39.1% vs. 14.7%) (Fig. 3C). Importantly, we did not detect any sign of acute toxicity recently reported for agonistic Abs to 4-1BB (9) in vaccinated mice as assessed by sizes of lymphoid tissues, lymphocyte proliferation, systemic cytokines, and gross pathology (Schabowsky et al., manuscript submitted).
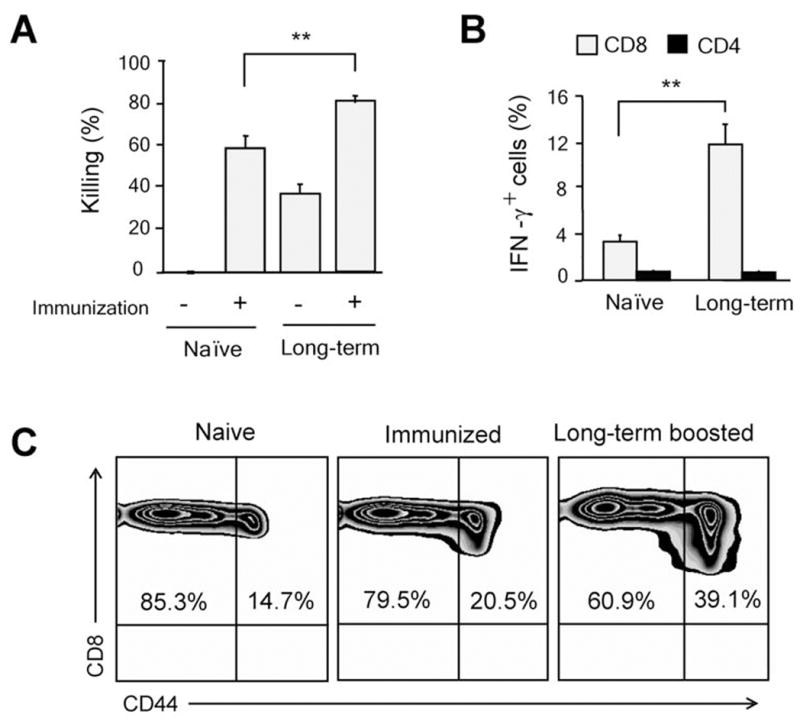
SA-4-1BBL-treated long-term animals maintain E7-specific CD8+ T cell effector and memory pool. A, long-term surviving animals develop E749-57-specific CD8+ T cell memory responses as determined by in vivo killing response (**p<0.001), B, higher intracellular IFN-γ expression (**p<0.001), and C, increased percentages of total memory CD44+CD8+ T cells. Data in panels (A), (B), and (C) were obtained from mice boosted 7 days earlier, except panel (A) naïve immunization (-) where in vivo killing response was assessed in naïve unvaccinated animals. A minimum of 3 mice/group were used for experiments in panels A-C.
SA-4-1BBL vaccination reverses the tumor mediated immune suppression
It has previously been reported that the initial antigen-specific killing response mounted by the host gradually decreases due to induction of antigen specific anergy as a function of tumor growth in the TC-1 model (29). To confirm this finding and test the efficacy of our vaccine to boost tumor-specific CD8+ T cell responses in mice with established TC-1 tumors, we performed E7 peptide-specific in vivo killing studies in unvaccinated tumor animals with small and large tumors. There was a significant reduction in the peptide-specific in vivo killing response in mice bearing large tumors as compared with those with smaller tumors (Fig. 4A). Importantly, vaccination of mice bearing large tumors with SA-4-1BBL and E7 peptide restored the killing response to levels comparable to those observed for mice without tumors (Fig. 4B).
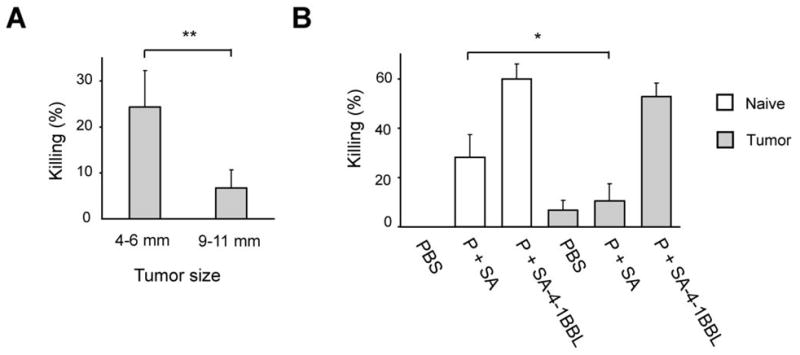
SA-4-1BBL vaccination reverses immune nonresponsiveness imposed by large tumors. A, mice (n=4 per group) with tumors of various sizes demonstrate a progressive decrease in in vivo CD8+ T cell killing activity as a function of tumor size. Mice were injected with syngeneic CFSE-labeled splenocytes pulsed with E749-57 peptide without SA-4-1BBL treatment to measure spontaneous cytotoxicity generated by TC-1 cells (**p<0.001). B, SA-4-1BBL restores the killing activity of CD8+ T cells in TC-1 tumor bearing mice. Naïve and tumor-bearing animals (n=4-6) (9-11 mm in diameter) were immunized s.c. with 50 μg P in combination with 25 μg SA-4-1BBL or SA. Peptide specific in vivo killing response was determined 7 days later (n=3). *p<0.05 for P+SA naive mice vs. P+SA tumor bearing mice.
SA-4-1BBL has better efficacy than TLR agonists as the immunomodulatory component of a therapeutic cancer vaccine
There has been significant interest in using TLR agonists as adjuvants for therapeutic vaccines due to their stimulatory effects on innate immune responses (30). Inasmuch as 4-1BB signaling directly modulates innate, adaptive, and regulatory immunity, we hypothesized that SA-4-1BBL may have better immunostimulatory efficacy than TLR agonists. Initially, we compared the efficacy of SA-4-1BBL to MPL, a detoxified form of LPS used in the clinic (31). In a dose response study where 50 μg E749-57 peptide was used for vaccination in combination with various doses of SA-4-1BBL and MPL, we determined 25 μg/injection to be the optimal dose for both MPL and SA-4-1BBL for the generation of in vivo peptide-specific killing response (Fig. 5A). These doses of SA-4-1BBL and MPL were used for the rest of studies. SA-4-1BBL showed significantly better in vivo killing activity than MPL over various peptide doses tested (Fig. 5B). The better effect of SA-4-1BBL in generating in vivo killing responses translated to a better therapeutic efficacy against TC-1 tumors. A single vaccination with SA-4-1BBL and E749-57 peptide resulted in the eradication of tumors in 75% of mice as compared with 30-42% achieved using LPS or MPL as components of the vaccine (Fig. 5C). Furthermore, administration of E749-57 peptide with 10 μg of CpG, a dose that showed efficacy in previously published studies (21, 32), resulted in a survival rate (40%) comparable to those achieved using LPS and MPL. Importantly, vaccination with 50 μg of the peptide and 100 μg of an agonistic 4-1BB Ab (3H3) (9) was less effective than peptide and SA-4-1BBL in eliminating TC-1 tumors (Supplementary Fig. S4).
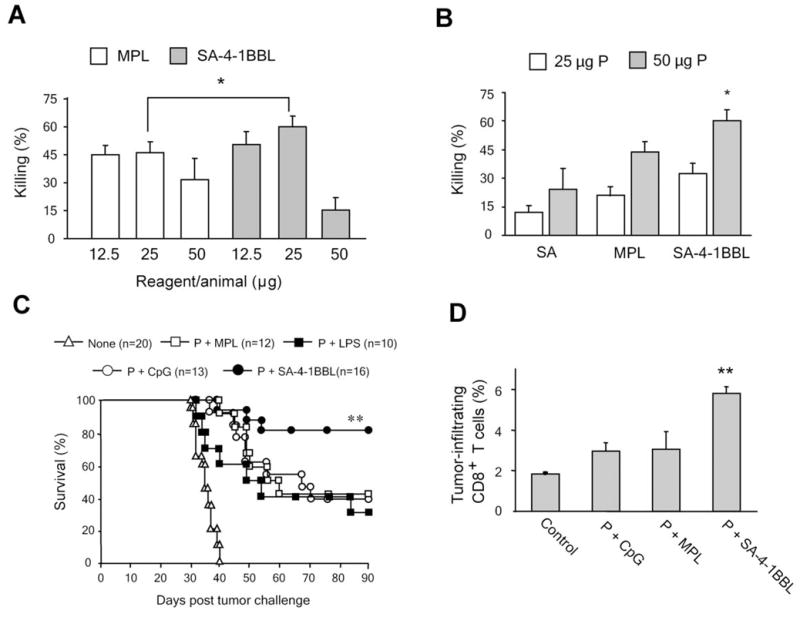
SA-4-1BBL has better efficacy than TLR agonists as immunomodulatory component of a therapeutic cancer vaccine. Vaccination with SA-4-1BBL generates better in vivo killing responses than MPL. A, C57BL/6 mice (n=4 per group) were immunized s.c. with a constant dose (50 μg) of E749-57 peptide and varying doses of SA-4-1BBL or MPL to determine the most optimum doses. B, C57BL/6 mice (n=4-8 per group) were immunized s.c. with constant doses (25 μg of each) of SA-4-1BBL or MPL and varying doses of E749-57 to obtain the most optimum dose of the peptide. Peptide-specific killing response was determined 7 days later. *p<0.05, SA-4-1BBL vs. all the other groups. C, SA-4-1BBL is more effective than TLR agonists in eradicating established TC-1 tumors. C57BL/6 mice were challenged with live TC-1 cells and vaccinated 10 days later with 50 μg P alone or in combination with 25 μg SA-4-1BBL, LPS, or MPL, or 10 μg CpG. **p<0.0001 for P+SA-4-1BBL vs. other groups. D, vaccination with SA-4-1BBL results in significantly higher infiltration of CD8+ T cells into tumor as compared with TLR agonists. Tumor bearing animals (n=3-4 per group) were vaccinated s.c. with 50 μg E749-57 in combination with SA-4-1BBL, MPL, or CpG at similar doses indicated in (C). Tumor-infiltrating cells were harvested 7 days post vaccination and analyzed using flow cytometry. **p<0.001, SA-4-1BBL vs. all the other groups.
The better efficacy of the SA-4-1BBL vaccine correlated with significantly higher percentages of tumor-infiltrating CD8+ T cells as compared with MPL or CpG, as determined by flow cytometry (Fig. 5D) and immunofluorescence microscopy (Supplementary Fig. S5).
SA-4-1BBL is more effective than TLR agonists in generating memory recall responses
Given the demonstrated role of 4-1BB signaling in the maintenance of CD8+ T cell memory pool and generation of secondary responses (33), we tested the efficacy of SA-4-1BBL to generate CD8+ T cell memory responses in two different settings. First, memory and recall responses were investigated in long-term surviving mice that had undergone successful immunotherapy using E749-57 peptide in combination with SA-4-1BBL, LPS, MPL, or CpG. The long-term tumor-free mice were tested 90 days after single vaccination for in vivo killing of the syngeneic target cells pulsed with E749-57 peptide. Mice vaccinated with 4-1BBL retained a significantly higher killing response than all others groups, indicating the existence of long-term effector memory response (Fig. 6A). The better efficacy of SA-4-1BBL in generating/maintaining long-term CD8+ T cell memory was also demonstrated in a second setting where naïve mice that had been vaccinated 75 days earlier were rechallenged with the same vaccine formulation and tested for E749-57 peptide-specific in vivo killing responses 7 days later (Fig. 6B).
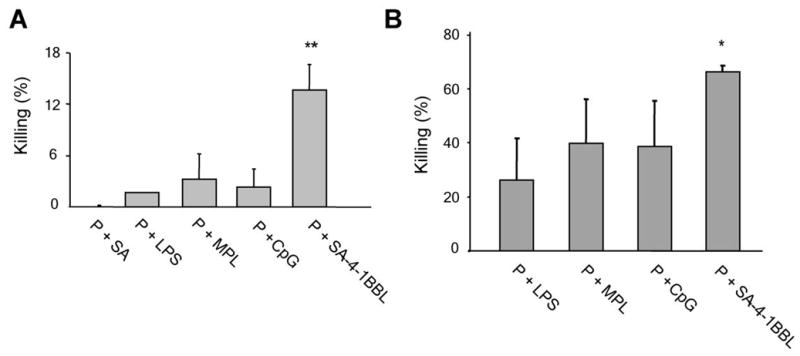
SA-4-1BBL generates more effective memory recall responses than TLR agonists. A, immunotherapy with SA-4-1BBL generates better long-term memory responses than TLR agonists. C57BL/6 mice (n=3 per group) that had undergone effective immunotherapy with E749-57 in combination with SA-4-1BBL, MPL, or CpG were maintained for 90 days post tumor challenge. These animals were then directly challenged with E749-57-pulsed target cells to determine in vivo killing response without a booster vaccination. **p<0.001, SA-4-1BBL vs. all the other groups. B, SA-4-1BBL generates better recall responses than TLR agonists in a prime-boost setting. Naïve C57BL/6 mice (n=3-4 per group) were immunized s.c. with E749-57 (50 μg) in combination with 25 μg SA-4-1BBL, LPS, or MPL, or 10 μg CpG. These animals were boosted with the same regimen 75 days later and E749-57-specific in vivo killing response was determined. *p<0.05, SA-4-1BBL vs. all the other groups.
Discussion
The role of immunosurveillance against spontaneous tumors and the potential for immunological control of cancer have been well established. However, the translation of existing extensive knowledge in cancer immunobiology into successful TAA-based therapeutic cancer vaccines in the clinic remains to be realized. Although the exact nature of cellular and molecular mechanisms responsible for this deficiency is not known and are most likely complex, the weak immunogenic features of TAAs, self tolerance to these antigens, and various direct and indirect immunoevasive mechanisms employed by the progressing tumor are some possibilities (1, 25, 29). Therefore, the success of therapeutic vaccines most likely will depend on their ability to induce strong immune responses against tumors as well as overcome various immune evasion mechanisms. This may require vaccine formulations that are designed to contain immunomodulators having pleiotropic effects on various cells of the innate, adaptive, and regulatory immunity. We herein report that a novel form of soluble 4-1BBL molecule chimeric with core streptavidin, SA-4-1BBL (16), has potential to serve such an immunomodulator. SA-4-1BBL i) activated DCs and enhanced antigen uptake, ii) generated primary and memory CD8+ T cell responses, and iii) endowed Teff cells refractory to the suppressive function of CD4+CD25+FoxP3+ Treg cells. Importantly, these pleiotropic immunomodulatory functions of SA-4-1BBL translated into its efficacy as a component of TAA antigens in eradicating established tumors in two different tumor models; a xenogeneic TAA peptide (E7)-based vaccination in TC-1 cervical cancer model and a self TAA (survivin)-based vaccination in 3LL lung carcinoma model.
Engagement of 4-1BB with its ligand, 4-1BBL, expressed by activated APCs results in T cell activation, clonal expansion, survival, and the establishment and maintenance of long-term memory (5, 22, 34). Therefore, 4-1BB signaling has extensively been exploited for improving the efficacy of various immunotherapeutic approaches against cancer and infections. Signaling via 4-1BB using an agonistic Ab was shown to enhance the CD8+ T cell response as well as broaden their repertoire to subdominant influenza epitopes (35). Importantly, 4-1BB stimulation was shown to be sufficient for the generation of primary CD8+ T cell responses (33). Consistent with these findings, we demonstrated that vaccination with the SA-4-1BBL and E749-57 peptide generated effective primary and memory CD8+ T cell responses in naïve mice as well as in mice challenged with TC-1 tumors expressing E7 antigen, and these responses were more pronounced than those generated using TLR agonists MPL and CpG. Unlike MPL and CpG that primarily target DCs, the better efficacy of SA-4-1BBL may be due to its direct effects on the function of both DCs and T cells. Following vaccination, SA-4-1BBL may first interact with constitutively expressed 4-1BB on DCs and stimulate these cells for antigen uptake and upregulation of various immunostimulatory molecules for the generation of primary CD8+ T cell responses. At the second stage, SA-4-1BBL may interact with 4-1BB upregulated on the surface of antigen-experienced CD8+ T cells for expansion, survival, and establishment of long-term memory. This notion is consistent with our data demonstrating the robust function of soluble SA-4-1BBL in directly activating DCs and T cells. Therefore, the inability of TLR agonists to directly activate and prolong survival of antigen-specific CD8+ T cells (5) may explain the better activity of SA-4-1BBL that potentiates both primary and memory responses.
Immunization with a single dose of SA-4-1BBL and a synthetic peptide representing the dominant CD8+ T cell epitope for E7 resulted in the eradication of 10-day established tumors with tumor-free survival in 75% of mice. In addition, vaccination after surgical removal of tumors protected 90% of mice from recurrences. The therapeutic efficacy was associated with a strong peptide-specific in vivo killing response and a high frequency of CD8+ T cells expressing the signature cytokine IFN-γ for the Th1 response. TC-1 tumors were shown to induce anergy in CD8+ T cells as a means of immune evasion (29, 36). Consistent with this finding, we demonstrated gradual decrease of peptide-specific in vivo killing responses as a function of tumor size. Importantly, vaccination with SA-4-1BBL and E749-57 peptide resulted in the recovery of peptide-specific in vivo killing responses in animals with large tumor burdens. Our results are consistent with previous studies demonstrating that signaling via 4-1BB receptor using an agonistic Ab prevents and reverses established anergy of CD8+ T cells in the P815 mastocytoma tumor and bone marrow transplantation models (36).
Importantly, vaccination with SA-4-1BBL and E749-57 peptide was more effective than TLR agonists LPS/MPL and CpG, two benchmark adjuvants used in various preclinical and clinical vaccine settings (2), as well as an agonistic 4-1BB Ab in eradicating the TC-1 tumors. The better therapeutic efficacy was associated with the ability of SA-4-1BBL to induce better CD8+ T cell primary, recall, and memory responses as well as their infiltration into the tumor. It has recently been shown that TC-1 tumors may exploit the regulatory function of CD4+CD25+FoxP3+ Treg cells for immune evasion (37). Important in this context are the recent findings of den Haan et al. demonstrating that vaccination with LPS or Poly (I:C) not only activate the immune system but simultaneously induces Ag specific, IL-10-producing Treg cells that strongly suppress CD8+ T cell responses (38). In addition, plasmacytoid dendritic cells activated by CpG induce the conversion of CD4+CD25- T cells into CD4+CD25+ regulatory T cells (39), and CpG can induce CD19+ splenic dendritic cells to acquire potent T cell suppressive function through the production of idoleamine 2,3-dioxygenase (40). Therefore, the better efficacy of SA-4-1BBL based therapeutic cancer vaccine in the present study may not only be due to its ability to generate an effective tumor-specific CD8+ T cell response, but also curb the regulatory immunity, such as reversal of CD8+ T cell anergy and modulation of regulatory T cell functions.
The development of potent adjuvants without adverse toxicity is crucial to the success of therapeutic vaccines. Our findings support the notion that select natural ligands to costimulatory molecules may have the potential to serve as effective adjuvants as components of therapeutic vaccines, provided that soluble, active forms of these molecules are generated. The robust effect of SA-4-1BBL in modulating innate, adaptive, and regulatory immunity with therapeutic consequences in the absence of detectable toxicity is consistent with this notion. Our data further demonstrate the utility of streptavidin as a fusion partner for the generation of costimulatory molecules with potent immunological activities in soluble forms (18). Testing this novel form of SA-4-1BBL in clinical trials will assess its therapeutic potential, and if effective, this molecule may serve as safe and effective alternatives to TLR agonists and agonistic Abs as adjuvants for the development of therapeutic vaccines against cancer and chronic infections.
Acknowledgments
We thank O. Grimany for technical help with recombinant proteins.
Grant support: Supported in parts by NIH R43 CA 109866 (HS), R43 AI071618 (HS, ESY) R41 CA121665 (HS), KLCRP (HS), KSTC-145-402-22 (HS), and JBCC Research Fellowship (RS).
Footnotes
Disclosure of Potential Conflict of Interest
SA-4-1BBL is licensed from UofL by ApoImmune, Inc., Louisville, KY, for which HS serves as CSO and HS and ESY have significant equity interest.
References
Full text links
Read article at publisher's site: https://doi.org/10.1158/0008-5472.can-08-3141
Read article for free, from open access legal sources, via Unpaywall:
https://aacrjournals.org/cancerres/article-pdf/69/10/4319/2611932/4319.pdf
Free to read at cancerres.aacrjournals.org
http://cancerres.aacrjournals.org/cgi/content/abstract/69/10/4319
Free after 12 months at cancerres.aacrjournals.org
http://cancerres.aacrjournals.org/cgi/content/full/69/10/4319
Free after 12 months at cancerres.aacrjournals.org
http://cancerres.aacrjournals.org/cgi/reprint/69/10/4319.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Oncolytic Adenovirus Armed with a Novel Agonist of the CD137 Immune Checkpoint Stimulator Suppresses Tumor Growth.
Vaccines (Basel), 12(3):340, 21 Mar 2024
Cited by: 1 article | PMID: 38543974 | PMCID: PMC10974162
A novel agonist of 4-1BB costimulatory receptor shows therapeutic efficacy against a tobacco carcinogen-induced lung cancer.
Cancer Immunol Immunother, 72(11):3567-3579, 22 Aug 2023
Cited by: 1 article | PMID: 37605009 | PMCID: PMC10991934
4-1BBL as a Mediator of Cross-Talk between Innate, Adaptive, and Regulatory Immunity against Cancer.
Int J Mol Sci, 22(12):6210, 09 Jun 2021
Cited by: 8 articles | PMID: 34207500 | PMCID: PMC8227424
Review Free full text in Europe PMC
Meeting Report: Translational Advances in Cancer Prevention Agent Development Meeting.
J Cancer Prev, 26(1):71-82, 01 Mar 2021
Cited by: 4 articles | PMID: 33842408 | PMCID: PMC8020174
Vaccination Strategies for the Control and Treatment of HPV Infection and HPV-Associated Cancer.
Recent Results Cancer Res, 217:157-195, 01 Jan 2021
Cited by: 18 articles | PMID: 33200366 | PMCID: PMC8564785
Go to all (53) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Protein structures in PDBe
-
(2 citations)
PDBe - 1BBLView structure
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
4-1BB ligand as an effective multifunctional immunomodulator and antigen delivery vehicle for the development of therapeutic cancer vaccines.
Cancer Res, 70(10):3945-3954, 20 Apr 2010
Cited by: 40 articles | PMID: 20406989 | PMCID: PMC2872136
Prime-boost vaccination with SA-4-1BBL costimulatory molecule and survivin eradicates lung carcinoma in CD8+ T and NK cell dependent manner.
PLoS One, 7(11):e48463, 08 Nov 2012
Cited by: 18 articles | PMID: 23144888 | PMCID: PMC3493554
SA-4-1BBL as the immunomodulatory component of a HPV-16 E7 protein based vaccine shows robust therapeutic efficacy in a mouse cervical cancer model.
Vaccine, 28(36):5794-5802, 04 Jul 2010
Cited by: 19 articles | PMID: 20603135 | PMCID: PMC2921468
SA-4-1BBL as a novel adjuvant for the development of therapeutic cancer vaccines.
Expert Rev Vaccines, 13(3):387-398, 01 Mar 2014
Cited by: 8 articles | PMID: 24521311 | PMCID: PMC4721633
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (5)
Grant ID: R43 CA109866
Grant ID: R43 CA109866-01A1
Grant ID: R41 CA121665-01A2
Grant ID: R41 CA121665
Grant ID: R43 CA 109866
NIAID NIH HHS (2)
Grant ID: R43 AI071618
Grant ID: R43 AI071618-01A1





