Abstract
Free full text

Mice Heterozygous for Germline Mutations in Methylthioadenosine Phosphorylase (MTAP) Die Prematurely of T-cell Lymphoma
Abstract
Large homozygous deletions of 9p21 that inactivate CDKN2A, ARF, and MTAP are common in a wide variety of human cancers. The role for CDKN2A and ARF in tumorigenesis is well established, but whether MTAP loss directly affects tumorigenesis is unclear. MTAP encodes the enzyme methylthioadenosine phosphorylase, a key enzyme in the methionine salvage pathway. To determine if loss of MTAP plays a functional role in tumorigenesis, we have created an MTAP-knockout mouse. Mice homozygous for a MTAP null allele (MtaplacZ) have an embryonic lethal phenotype dying around day 8 post-conception. Mtap/MtaplacZ heterozygotes are born at Mendelian frequencies and appear indistinguishable from wild-type mice during the first year of life, but they tend to die prematurely with a median survival of 585 days. Autopsies on these animals reveal that they have greatly enlarged spleens, altered thymic histology, and lymphocytic infiltration of their livers, consistent with lymphoma. Immunohistochemical staining and FACS analysis indicate that these lymphomas are primarily T-cell in origin. Lymphoma infiltrated tissues tend to have reduced levels of Mtap mRNA and MTAP protein, and unaltered levels of methyldeoxycytidine. These studies show that Mtap is a tumor suppressor gene independent of CDKN2A and ARF.
Introduction
A quarter century ago, Toohey first recognized that certain murine malignant hematopoietic cell lines lacked methylthioadenosine phosphorylase (MTAP) activity (1). MTAP is a metabolic enzyme in the methionine salvage pathway that converts the polyaminebyproduct 5′-dideoxy-5′-methylthioadenosine (MTA) into adenine and methylthioribose-1-phosphate and is expressed in all tissues throughout the body (2, 3). Loss of MTAP has been shown to result in increased accumulation of MTA, a known inhibitor of S-adenosylmethionine-dependent methyltransferases (2, 4). In addition, loss of MTAP is known to affect the production of polyamines due to upregulation of ornithine decarboxylase (5).
Today we know that loss of MTAP is frequent in a large number of different human tumors including leukemias, lymphomas, mesothelioma, lung carcinoma, pancreatic carcinoma, squamous cell carcinoma, billiary tract cancer, glioblastoma, osteosarcoma, and neuroendocrine tumors (6–18). Loss rates range from 14% to 100% depending on the tumor type and the method used to assess MTAP loss.
MTAP is frequently inactivated in human tumors by large homozygous deletion of the 9p21 region where both MTAP and the CDKN2A/ARF tumor suppressor gene complex are located (19). Since these deletions generally inactivate CDKN2A/ARF as well as MTAP, it has been hypothesized that loss of MTAP in tumors was simply due to it being a co-incident bystander. However, there is a growing body of data that suggests this may not be the case. Re-expression of MTAP in MTAP-deleted MCF-7 breast cells results in loss of anchorage independent growth in vitro, and loss of tumor formation in vivo when these cells were injected subcutaneously in SCID mice (20). In addition, expression of MTAP in a MTAP-deleted melanoma cell line resulted in reduced invasion as measured in a Boyden Chamber assay (21). Finally, in non-small cell lung cancer and in astrocytomas, loss of MTAP has been observed in cells that retain CDKN2A/ARF (8, 9). Taken together, these observations suggest that MTAP may function as a tumor suppressor gene in its own right.
To test the idea that MTAP is a tumor suppressor gene, we have engineered a mouse with a germline mutation in the mouse MTAP gene (Mtap). We found that homozygosity for MTAP results in an early embryonic lethal phenotype, while heterozygosity often results in premature death by T-cell lymphoma. Our findings support the view that MTAP is a bona fide tumor suppressor gene.
Materials and Methods
Creation of MTAP-knockout mouse
RRK081 cells (SV129/Ola background) heterozygous for the MtaplacZ allele were obtained from Bay Genomics(http://baygenomics.ucsf.edu) and injected into C57BL6 blastocysts by microinjection. Eighty 3.5-day-old blastocyte embryos were then implanted into pseudo-pregnant C57BL6 mice. Four chimeric offspring were obtained and two exhibited germline transmission. Heterozygous offspring from these mice were then used for intercrosses to generate homozygous offspring, and were repeatedly backcrossed to C57BL6. Mice were fed Harlan Tekland 2018SX diet ad libitum. For survival analysis, mice were monitored daily and Kaplan-Meier analysis was performed using GraphPad Prism 4.0. All studies were approved by the Fox Chase Cancer Center animal use committee.
Genotyping
MtaplacZ was genotyped using two different methods. Initially genotyping was done at the RNA level. RNA was extracted from tail snips using RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Reverse transcription-PCR was done using the SuperScript One-step Reverse Transcription-PCR kit (Invitrogen) and oligos 5′CGGTGAAGATTGGAATAATTGGTG-3′ (sense), 5′-GACAGTATCGGCCTCAGGAAGATCG (antisense) and 5′-CTGTCAATGAATTGGTCAATGACCATG-3′ (antisense) for the amplification of Mtap+ and MTAPlacZ bands of 322 bp and 421 bp, respectively. After an initial reverse transcription phase at 52°C for 30 min, PCR amplification of cDNA was performed with an initial denaturation step at 94°C for 2 min, followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 30 s with a final extension at 72°C for 5 min.
Later, a DNA based assay was developed using primers 5′CAGGACAAATTGTGGGTC-TAAG-3′ (forward), 5′-GCAACAAGGACAGCCCAAGGAGATC-3′ (reverse 1) and 5′-ACTTGAGACCTTCTTTCTGGTCTTTC-3′ (reverse 2) to obtain either a Mtap band of 381 bp (with reverse 2) or a MtaplacZ band of 289 bp (with reverse 1). PCR reactions were carried out in a total volume of 25 μl reaction mixture containing 100 ng genomic DNA as a template, 1× PCR buffer (10 mM Tris-HCl, PH 8.0/50 mM KCl/1.5 mM MgCl2), 250 μM of each of fourdeoxynucleoside triphosphates, 10 ng each of sense and antisense primers, and 2.5 units of Taq DNA polymerase (Invitrogen). The thermal cycling conditions consisted of an initial denaturation at 94°C for 2 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 64°C for 30 s and extension at 72°C for 30 s.
Timed Matings
Female and male animals were paired in the evening and checked early in the morning for the presence of a vaginal plug. If a plug was observed, animals would be sacrificed after 7.5, 8.5, 9.5, and 12.5 days.
Immunohistochemistry
Autopsied materials were fixed in bufferedformalin and embedded in paraffin. The paraffin blocks werecut into 5-μm-thick sections that were placed on positivelycharged slides. The sections were dewaxed in xylene and hydratedthrough graded ethanol. Heat-induced antigen retrieval was thendone in 10 mmol/L sodium citrate (pH 6.0) in a microwave for10 min. The endogenous peroxidase activity was blocked by immersing the slides in 3% H2O2 in PBS for 30 min. After 30 min of incubation with goat blocking serum, slides were incubated witha 1:250 dilution of MTAP rabbit polyclonal antibodies (kindly provided by Dr. Schramm,(22)) or a 1:150 dilution of CD3 rabbit polyclonal antibodies (Dako, Carpinteria, CA) at 4°C overnight followed byincubation with goat anti-rabbit biotinylated secondary antibodyfor 30 min atroom temperature followed by streptavidin peroxidase for 30 min at room temperature. 3,3′-Diaminobenzidine (Sigma-Aldrich, St. Louis, MO) substratechromogen was applied for 4 min until the brown color developed. In the case of CD45, we used 1:200 dilution of mouse monoclonal biotinylated antibodies (BD Biosciences, San Jose, CA) and did not use secondary antibody. The slides were counterstained with hematoxylin and mountedwith Permount (Fischer Scientific, Pittsburgh, PA).
FACS analysis
FACS analysis on spleen from euthanized animals was performed as previously described (23). Analysis was performed using a BD Biosciences LSR II/DiVa flow cytometer, equipped with three laser excitation (405, 488, 630 nm). Staining combinations were performed on single cell suspensions of spleen and included Fl anti-CD8 (53–6), PE anti CD3 (500A–A2), APC anti-CD4 (GK1.5), Cy5PE TCR beta (H57–597). All reagents were made in the laboratory of Richard R. Hardy, except for DX5 and TCR-beta, which were purchased from eBioscience.
Southern Analysis
Genomic DNA was extracted using DNA isolation kit (Puregene) from spleens of diseased and normal animals. Approximately 20μg of genomic DNA was digested using restriction enzyme HindIII (for T cell lineage clonality assays of the TCRβ locus), and probed according to standard Southern blot protocol with TCRβ probe (3′Jβ2 probe for the Jβ2 cluster, (24)). Genomic DNA from tissue or cell lines with unrearranged germ line configuration of each antigen receptor locus was used as negative controls.
Real-time PCR
MTAP, p16 and β-actin were measured using Taqman technologyon anABI Prism 7900 Sequence Detection System. Total RNA was extracted using thePicoPure RNA kit (Molecular Devices, Sunnyvale, CA) according to the manufacturer’s instructions. First-strand cDNA was generated from total RNAfrom each sample using the High Capacity cDNA kit (Applied Biosystems, Foster City, CA)according to the manufacturer’s instructions. Reactions were prepared in triplicate for each gene using Taqman Gene Expression Master Mix and the following Taqman Gene Expression Assays (AppliedBiosystems): Mtap (Mm01257901_m1), Cdkn2a/Arf (Mm00494449_m1) and β-actin (Mm00607939_s1). Plates were loaded and reactions were cycled using Taqman universal cycling conditions according to the manufacturer’s instructions. During thermalcycling, the threshold cycle (Ct) was determined for each sample by taking the average of the three replicates. The average Ct value for the control gene β-actin was subtracted from the average Ct value for each target gene (Mtap and cdkn2a/Arf) to normalize the amount of sampleRNA added to the reaction. The comparative Ct (Ct) method was used to determine the level of the target gene mRNA in heterozygous animals samples relative to the level found in the normal sample (AppliedBiosystems User Bulletin #2, October 2001): relative quantification= 2−Ct, where Ct = average Ct (diseased) − average Ct (normal).
MTAP enzyme assay
MTAP enzyme activity in the liver extract was measured as described previously (20). A unit of MTA phosphorylaseactivity is defined as the enzyme amount that catalyzes theformation of 1 μmol of adenine/min under the conditionsof the assay described.
CGH analysis
According to the manufacturer’s protocol for Agilent OligonucleotideArray-based CGH for Genomic DNA Analysis Version 4.0 (Agilent Technologies, Santa Clara CA), three micrograms of high quality genomic DNA was digested with restriction endonucleases, AluI and RsaI and digested genomic DNA was labeled using Agilent Genomic DNA Labeling Kit PLUS. Test and reference DNA samples were labeled with either cyanine 5-or cyanine 3-dUTP, according to the manufacturer’s protocol. Cyanine 5- and cyanine 3-labeled DNA products were then purified using Microcon YM-30 filtration devices. The DNA yield and level of dye incorporation were measured using the ND-1000 Spectrophotometer. Appropriate cyanine 5- and cyanine 3-labeled DNA sample pairs were combined and then mixed with mouse Cot-1 DNA, Agilent 10X Blocking Agent, and Agilent 2X Hybridization Buffer. The labeled target solution was hybridized to Agilent’s 244K Mouse Genome CGH microarray (G4415A) using SureHyb chambers. After hybridization the microarrays were washed and dried according to the procedures described in Agilent’s protocol. Microarray slides were scanned immediately using an Agilent microarray scanner. Data for individual features on the microarray were extracted from the scan image using Agilent’s Feature Extraction (FE) Software. Output files from FE were subsequently imported into Agilent’s CGH data analysis software, CGH Analytics for DNA copy number analysis.
Methyldeoxycytidine Quantitation
Percent of methyldeoxycytidine present in genomic DNA was determined by LC-MS/MS as described (25).
Results
Homozygosity for MTAPlacZ causes early embryonic lethality
RRK081 embryonic stem cells, containing a gene-trap vector inserted between exon 3 and exon 4 of the mouse Mtap locus (MtaplacZ; Supplemental Figure 1), were injected into mouse blastocysts derived from C57BL/6 animals and the embryos were implanted in pseudopregnant females. Four chimeric offspring were obtained and these animals were then bred to C57BL/6 animals to establish germline transmission. Two of the chimeras exhibited germline transmission.
Heterozygous MtaplacZ/+ offspring were then intercrossed to determine the phenotype of a homozygous animal. We failed to obtain a single homozygous offspring out of 165 that were genotyped. Analysis of the heterozygous animals for MTAP enzyme activity in the liver showed that these animals had on average 31% activity compared to the wild-type animals (n=6 per group, p<0.0003), indicating that the insertion allele is a complete loss of function mutation. Taken together, these findings indicate that lack of MTAP activity results in an embryonic lethal phenotype.
We performed timed mating experiments to determine exactly when during development MtaplacZ homozygotes were being lost (Supplemental Table 1). We failed to observe any homozygous embryos present in the uteri at post-conception day 12.5 and day 9.5 mothers and also found a large number of empty uterine sacs. However, we were able to identify homozygous embryos present in the uteri of day 8.5 and day 7.5 animals. Homozygous embryos present at day 8.5, were much smaller and misformed compared to control heterozygous embryos (Fig. 1a). Both in size and appearance they appeared to resemble day 8 embryos (Theiler stage 12 as opposed to Theiler stage 13 (26)) and were probably in the process of reabsorption. Examination of day 7.5 embryos for MTAP expression by immunohistochemistry reveals that MTAP is expressed ubiquitously throughout the embryo at this time (Figure 1b). These results indicate that MtaplacZ/MtaplacZ homozygotes are lost early in embryonic development, around post-conception day 8.
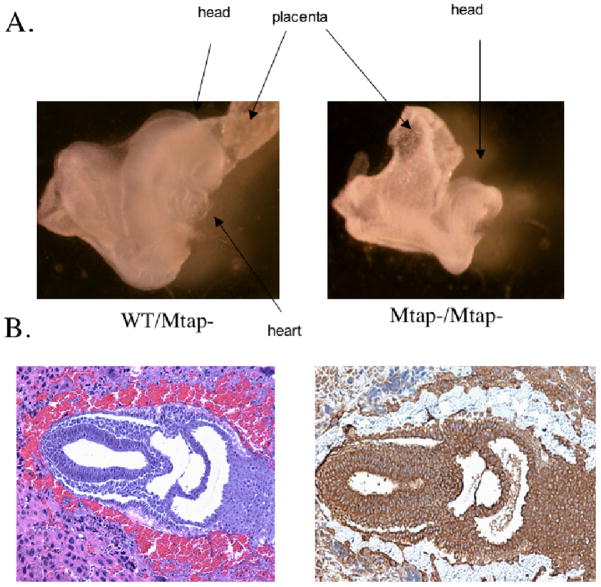
Mtap is essential during embryogenesis. A, Photograph of sibling day 8.5 MtaplacZ/+ and MtaplacZ/MtaplacZ embryos. Photographs taken with 40× magnification. B, Wild-type day 7.5 embryos sectioned and stained with H and E (left) or with an anti-MTAP antibody (right). Photographs taken at 400× magnification.
MtaplacZ heterozygotes die prematurely of severe lymphoproliferative disease
MtaplacZ/+ heterozygotes are born at Mendelian frequencies and have no obvious abnormalities. We observed no difference in weight gain between heterozygotes and control animals during the first six months of life (Supplemental Fig. 2a). Because MTAP is involved in methionine and polyamine metabolism, we examined the serum amino acid profiles of overnight fasted MtaplacZ/+ and +/+ animals. We observed no significant differences in the 27 compounds that were measured (Supplemental Fig. 2b).
To determine if there might be any long term detrimental effects of heterozygosity for MtaplacZ, we monitored a cohort of MtaplacZ/+ heterozygotes and sibling +/+ controls for up to 20 months and found that there was a significant difference in the survival of heterozygous animals compared to their wild-type siblings (Fig. 2a). Heterozygotes start dying as early as 200 days and have a median survival of 585 days. We performed necropsies on 21 of these animals and found that at least 60% of the animals showed marked splenomegaly, and often enlargement of the liver and thymus as well (Fig. 2b). Examination of spleen, liver, and thymus sections by H and E staining indicated that the animals suffered from severe lymphoproliferative disease resembling lymphoma (Fig. 2c). In addition, in one animal we also observed hepatocellular carcinoma in the liver.
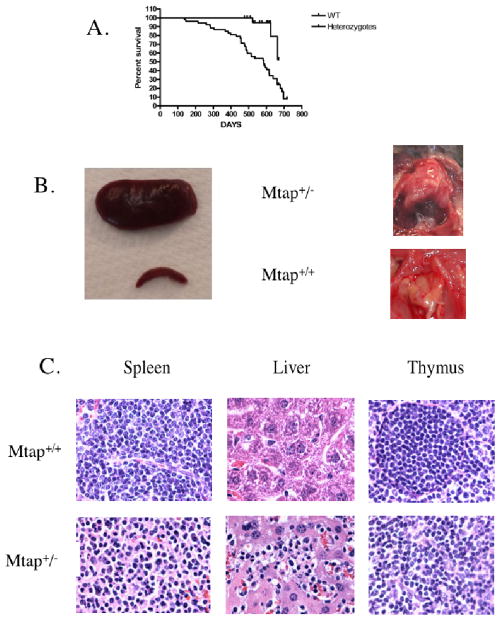
MtaplacZ/+ animals die prematurely of lymphoproliferative disease. A, Sibling wild-type (n=23) and heterozygous animals (n=57) were monitored daily and followed for up to 20 months. Tick marks indicate censored observations. Comparison of survival curves using a Logrank test gives a P<0.0005. B, Spleens and thymus at necropsy of an Mtap heterozygote and wild-type control. C, Pathology from a control and a heterozygous diseased animal. Sections of spleen, liver, and thymus were stained with Hematoxylin and Eosin and were viewed under 400× magnification. Note the lymphocyte infiltration in the liver and the loss of the follicular architecture in the thymus of heterozygous animals.
Characterization of lymphoma cells present in MtaplacZ heterozygotes
To more fully characterize the lymphoma present in MtaplacZ/+ animals, we performed immunohistochemical analysis of tissues from heterozygous and sibling wild-type animals. Spleen, thymus and liver were stained with the T-cell marker CD3 or the B-cell marker CD45. In the spleens of all of the 14 heterozygous animals diagnosed with lymphoma at autopsy, we observed increased CD3+ cells compared to control spleen and reduced numbers of CD45+ cells (Fig. 3a). Infiltration of CD3+ cells was also observed in the livers of some of the heterozygous animals (Supplemental Fig. 3). In animals with enlarged thymuses, we observed an expansion of the CD3+ population and a large decrease in the number of CD45+ staining cells (Figure 3b). The increase in CD3+ cells and the decrease in CD45+ cells indicates that MtaplacZ/+ animals are succumbing to T-cell lymphoma.
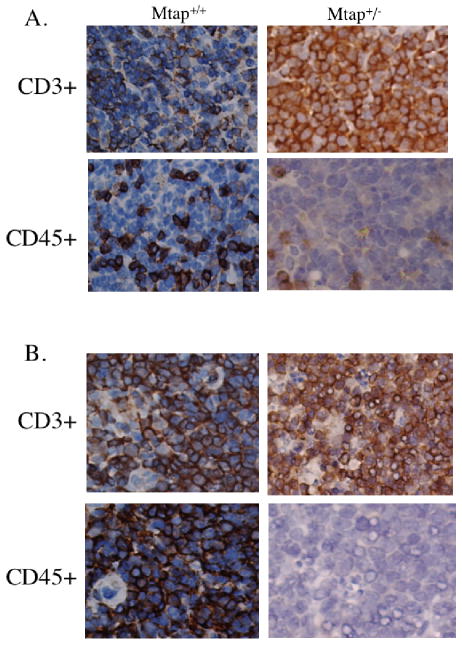
Proliferation of CD3+ T-cells in spleen and thymus. A, Spleen of an aged control (sacrificed at 525 days) and MtaplacZ/+ animal (deceased at 260 days) stained with either antibody to CD3+ or CD45+ viewed at 400× magnification. B, The thymus of the same two animals as above.
To further characterize the T-cell expansion, we examined the T-cell population in the spleen of aged heterozygous and control animals for CD4 and CD8 content using FACS analysis. For these experiments we sacrificed 11 MtaplacZ/+ heterozygotes (average age 713 days) and six +/+ controls (average age 684 days). In these sacrificed animals, we did not observe gross enlargement of the spleen as observed in the naturally deceased animals, and histopathological examination of the spleen showed that most of these animals had much milder lymphoid hyperplasia. However, despite this fact, we found that 4 of the 11 heterozygous animals had CD4+ levels above the 95% confidence range determined by the mean of the control animals (Fig. 4a). We did not observe any animals with CD8 levels outside the control range. These results indicate that heterozygous animals are predisposed to develop expansions of a CD4+ T-cell population.
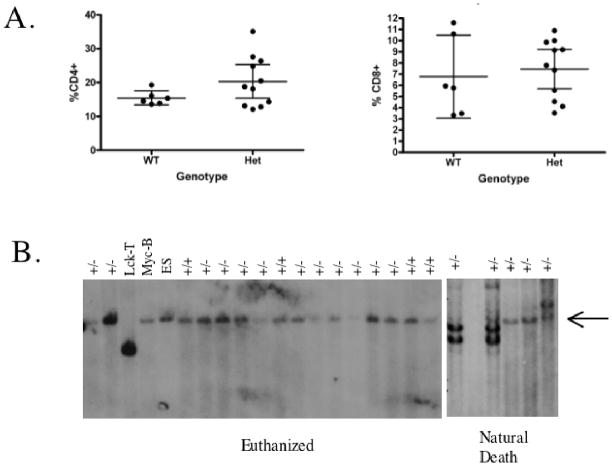
Evidence for T-cell clonality. A, FACS analysis of CD4+ and CD8+ present in the spleen of aged wild type and heterozygous animals. Horizontal bar shows mean percentage and error bars show 95% confidence intervals. B, Southern blot analysis of spleen DNA of representative heterozygous and wild type animals. The blot is probed with Jβ2 to detect TCRβ rearrangement. Arrow at right shows size of germline (unrearranged band). Controls include DNA from RRK081 embryonic stem cells (ES), DNA from a T-cell lymphoma from a Lck-T mouse, and DNA from a B-cell lymphoma from a Eμ-myc transgenic mouse.
To determine if the T-cell expansions were monoclonal, we performed southern blot analysis on spleen or thymus DNA isolated from 17 aged heterozygous and 4 sibling wild-type animals using a probe specific for the Db2-Jb2 cluster at the TCR-β locus (Fig. 4b). Five of the 17 heterozygous samples came from autopsy material derived from animals that had exhibited marked splenomegaly and had died of natural causes, while the remaining samples came from the healthy euthanized animals that had mild lymphoid hyperplasia used for the FACS analysis described above. In three of the five autopsy animals (60%) we found evidence of monoclonality, but we did not observe any evidence of monoclonality in the sacrificed animals. These findings show that the majority of heterozygous animals died of monoclonal T-cell lymphoma, but that the appearance of monoclonality occurs late in the disease process.
Loss of MTAP expression in lymphoma cells
We next examined if expression of the wild-type Mtap allele in MtaplacZ/+ mice was reduced in tissues showing lymphoproliferative disease. First, we performed immunohistochemistry using an anti-MTAP polyclonal antibody on thymus, spleen, and liver from an animal with severe lymphoma and compared staining to a wild-type healthy control animal (Fig. 5a). We observed significantly reduced MTAP staining in all three tissues compared to the control animal, showing that MTAP expression was reduced in all the lymphoid infiltrated tissue. In addition, we examined the spleens of nine other animals that died of lymphoma, and in all cases observed reduced MTAP staining compared to control animals (data not shown).
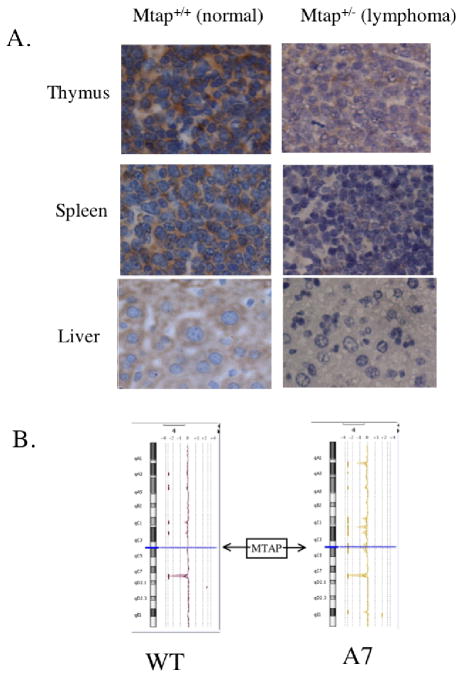
Evidence for loss of wild-type Mtap in lymphoma infiltrated tissue. A, Thymus, spleen and liver of an aged control (sacrificed at 525 days) and MtaplacZ/+ animal (deceased at 260 days) stained with antibody to MTAP viewed at 400× magnification. B, DNA from wild-type spleen and the enlarged spleen of a MtaplacZ/+ animal were hybridized of Agilent CGH chips and data was then analyzed using DNA analytics software. Regions of chromosome of copy number loss as determined by Z-score >3 are indicated by dark vertical bars on the left side. The location of the Mtap is shown by the arrow.
To determine if down-regulation of MTAP was caused by reduced Mtap mRNA, we quantitated Mtap RNA by qRT-PCR. Initially, we examined MTAP expression in 5 three-month old MtaplacZ/+ and five +/+ control animals that showed no evidence of lymphoid hyperplasia in the spleen. As expected, we found that the heterozygous animals had significantly lower Mtap mRNA than the +/+ controls (Fig. 6a). We then examined Mtap mRNA levels in the spleens of 13 older (sacrificed) MtaplacZ/+ animals with lymphoid hyperplasia. In 12 of the 13 cases we observed Mtap mRNA levels below the 95% confidence interval defined by the younger MtaplacZ/+ animals. To eliminate the possibility that Mtap mRNA levels decreased with age in healthy animals, we also measured Mtap mRNA in five older +/+ animals without evidence of lymphoid hyperplasia. We did not observe a difference in MTAP mRNA compared to the younger +/+ animals, indicating that MTAP expression is not lost simply due to aging.
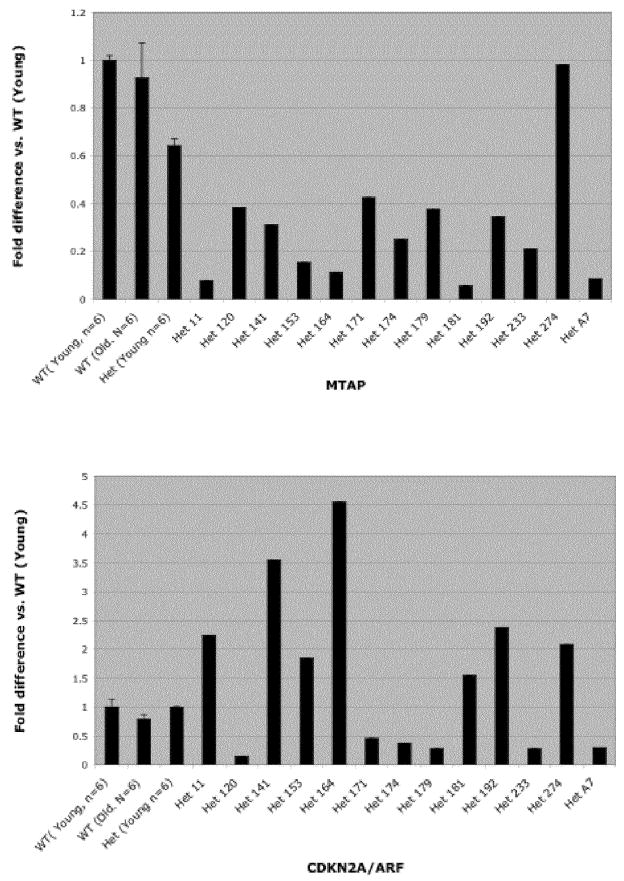
qRT-PCR of Mtap and Cdkn2a/Arf in aged MtaplacZ/+ animals. A, MTAP mRNA was quantitated by qRT-PCR (see Methods). Reference ranges were established using young wild-type animals, young heterozygous animals and old wild type animals and the 95% confidence intervals are shown (leftmost three columns). Other columns show relative Mtap mRNA levels in each of 13 aged heterozygotes with evidence of lymphoproliferative disease. B, Identical to A. except that Cdkn2a/Arf message was measured.
Since chromosomal deletion is a major mechanism for inactivation of Mtap in human tumors, we performed comparative genome hybridization analysis (CGH) on DNA isolated from the spleen of three MtaplacZ/+ animals exhibiting severe lymphoid hyperplasia. In only one of the three samples (A7) did we see evidence for loss of the Mtap region of mouse chromosome 4 (Fig. 5b). This finding suggests that the reduced expression of Mtap mRNA observed above may be occurring mostly through epigenetic mechanisms.
Loss of MTAP expression is independent from loss of CDKN2A expression
Since MTAP and CDKN2A/ARF are often inactivated together in human tumors by large deletions, we also examined Cdkn2a/Arf mRNA in the spleens of animals showing lymphoid hyperplasia by qRT-PCR. We found no difference in Cdkn2a/Arf mRNA levels in young sibling +/+ and MtaplacZ/+ animals, indicating that the MtaplacZ/+ insertion did not effect basal Cdkn2a/Arf expression. In older +/+ animals lacking hyperplasia, we observed a 20% decrease in Cdkn2a/Arf expression compared to the younger controls, suggesting some loss of Cdkn2a/Arf expression occurs as a result of aging. However, in the older MtaplacZ/+ animals we found large variability in Cdkn2a/Arf expression. In seven animals we observed significant elevations in Cdkn2a/Arf mRNA, while in seven others we observed reduced expression. The lack of correlation between Cdkn2a/Arf and Mtap mRNA levels in these samples suggests that loss of Mtap expression is driving lymphoma formation independent of Cdkn2a/Arf in these mice.
Loss of MTAP does not affect global deoxycytidine methylation
One possible mechanism by which loss of MTAP might promote tumorigenesis is by affecting cellular methylation reactions. To determine if loss of MTAP affected cellular methylation, we measured the amount of methyldeoxycytidine present in the DNA isolated from the enlarged spleens of Mtap heterozygotes and compared it to spleen DNA isolated from control animals. We did observe a slight decrease in the percentage of DNA incorporated methyldeoxycytidine in control vs. Mtap heterozygous animals, but the difference was not statistically significant (n=5, 4.3 ±0.08 vs. 4.04 ±0.45, P=0.33).
Discussion
In the experiments described above, we have characterized the phenotype of mice homozygous and heterozygous for a null allele of the Mtap locus. Aged mice heterozygous for MtaplacZ die prematurely with enlarged spleens, thymuses, and livers that have large numbers of CD3+ T-cells invading the affected organs. These findings show that germline heterozygosity for MtaplacZ predisposes animals to develop T-cell lymphoma, and suggests Mtap may be acting as a tumor suppressor gene. If Mtap were acting as a tumor suppressor gene, then according to Knudson’s two-hit hypothesis (27) tumor cells should inactivate the remaining wild-type gene. Supporting this idea, we observed reduced Mtap mRNA in 12 of 13 heterozygous animals examined with spleenic lymphoid hyperplasia. Interestingly, in the one animal that had high levels of Mtap mRNA, immunohistochemistry indicated a significant loss of Mtap protein in affected tissue (see Fig. 5a). This finding suggests that Mtap expression can be lost due to transcriptional as well as post-transcriptional mechanisms.
In human tumor derived cell lines the major mechanism of MTAP inactivation involves large homozygous deletions of the 9p21 region. These deletions generally affect both MTAP and the CDKN2A/ARF locus, located approximately 80 Kb away. Examination of Cdkn2a/Arf mRNA by qRT-PCR in the spleens of animals with lymphoid hyperplasia indicate that seven out of 12 animals that had low Mtap mRNA had elevated levels of Cdkn2a/Arf, strongly indicating that in MtaplacZ heterozygous mice, most Mtap inactivation is not occurring by large deletions that co-delete CDKN2A/ARF. Consistent with this idea, we only observed evidence for homozygous deletion in one of the three mice examined by CGH. These findings support the view that Mtap has tumor suppressor qualities independent of CDKN2A/ARF and that inactivation of the wild-type Mtap allele is occurring primarily by epigenetic mechanisms in these animals. Epigenetic silencing of MTAP via promoter hypermethylation has been previously demonstrated to occur in human gastric and lung cancers (28, 29).
In humans, loss of MTAP has been observed in a wide variety of tumors, including both solid and hematological malignancies. With the exception of a hepatocellular carcinoma observed in one animal, Mtap/MtaplacZ animals only developed T-cell lymphomas. In older heterozygous animals that have not yet died, we observed mild T-cell lymphoproliferative disease that was not clonal, suggesting that loss of Mtap might stimulate T-cell proliferation. The increased number of T-cells might increase the chances that a malignant clone may appear that would subsequently then kill the animals. The observation that germline mutations in tumor suppressor genes cause different phenotypes in mice and humans have been observed previously. For example, heterozygosity for Nf2 tumor suppressor gene results in increased risk of neurologic tumors in humans, but predispose mice to a wide variety of tumors including lymphoma, lung adenocarcinoma, hepatocellular carcinoma and sarcomas (30).
Despite the fact that human and mouse cells lacking MTAP appear to grow well in culture, mice homozygous for MtaplacZ are inviable and die early in embryogenesis, around post-conception day 8. Just before this time (post-conception day 7.5) MTAP appears to be expressed in all cells throughout the embryo. The day 8 homozygous embryos are severely deformed and much smaller than wild-type embryos suggesting that loss of MTAP may be affecting processes involved in mouse organogenesis. Early embryonic lethality in mice homozygous for null mutations has been observed for several mouse tumor suppressor genes including Rb, Wt1, Apc, Nf2 and Brca1 (30). Our findings are also consistent with those published by (31), which also found that Mtap was an essential gene in mice.
A possible mechanism by which loss of MTAP promotes tumorigenesis may be related to its effect on altered methionine metabolism and methylation. Tumor cells appear to have disrupted methionine methylation as manifested by their inability to grow in media in which methionine is replaced by its immediate precursor homocysteine (32). It has been hypothesized that the increased methionine requirements of tumor cells may be due to the high levels of transmethylation observed in tumor cells (33). Interestingly, drugs that inhibit DNA methylation reactions, such as ethionine or 5-Azacytidine, are also pro-tumorigenic. In theory, loss of MTAP could effect DNA methylation by two different mechanisms; either intracellular accumulation of the methyltransferase inhibitor MTA, or by reduction in the levels of S-adenosylmethionine due to the lack of a functional methionine salvage pathway. In the experiments reported here, we did not find a significant difference in the methylation status of enlarged spleen DNA isolated from Mtap+/− heterozygotes and Mtap+/+ control animals. However, these studies are limited by the fact that DNA samples were isolated from total tissue and presumably contain a mixture of Mtap−/+ stromal cells and Mtap−/− tumor cells. Although not statistically significant, it is interesting to note that on average the DNA from Mtap+/− spleens had slightly reduced methylation and more variability than DNA from the control animals.
In summary, our studies support the idea that Mtap, which encodes a basic metabolic enzyme, is a tumor suppressor gene that is essential for normal mouse development. The mice developed should be useful tools in elucidating the mechanism by which loss of Mtap contributes to tumorigenesis.
Acknowledgments
American Cancer Society Research Scholar Grant, RSG-03-157-01-GMC, NIH core grant CA06927, NCI grants HL057299, CA131024, and an appropriation from the Commonwealth of Pennsylvania. We acknowledge the contribution of the FCCC Genomics, Laboratory Animal, Transgenic, Sequencing, and Experimental Histopathology Facilities. We thank Drs. Joseph Testa and Maureen Murphy for control materials for clonality analysis.
References
Full text links
Read article at publisher's site: https://doi.org/10.1158/0008-5472.can-09-0145
Read article for free, from open access legal sources, via Unpaywall:
https://cancerres.aacrjournals.org/content/canres/69/14/5961.full.pdf
Free to read at cancerres.aacrjournals.org
http://cancerres.aacrjournals.org/cgi/content/abstract/69/14/5961
Free after 12 months at cancerres.aacrjournals.org
http://cancerres.aacrjournals.org/cgi/reprint/69/14/5961.pdf
Free after 12 months at cancerres.aacrjournals.org
http://cancerres.aacrjournals.org/cgi/content/full/69/14/5961
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1158/0008-5472.can-09-0145
Article citations
Deletions of CDKN2A and MTAP Detected by Copy-Number Variation Array Are Associated with Loss of p16 and MTAP Protein in Pleural Mesothelioma.
Cancers (Basel), 15(20):4978, 13 Oct 2023
Cited by: 2 articles | PMID: 37894345 | PMCID: PMC10605896
Enzyme-mediated depletion of methylthioadenosine restores T cell function in MTAP-deficient tumors and reverses immunotherapy resistance.
Cancer Cell, 41(10):1774-1787.e9, 28 Sep 2023
Cited by: 8 articles | PMID: 37774699 | PMCID: PMC10591910
MACHETE identifies interferon-encompassing chromosome 9p21.3 deletions as mediators of immune evasion and metastasis.
Nat Cancer, 3(11):1367-1385, 07 Nov 2022
Cited by: 37 articles | PMID: 36344707 | PMCID: PMC9701143
The Bidirectional Relationship Between Cancer Epigenetics and Metabolism.
Annu Rev Cancer Biol, 5(1):235-257, 30 Nov 2020
Cited by: 21 articles | PMID: 34109280 | PMCID: PMC8186467
The complexity of the serine glycine one-carbon pathway in cancer.
J Cell Biol, 219(1):e201907022, 01 Jan 2020
Cited by: 56 articles | PMID: 31690618 | PMCID: PMC7039202
Review Free full text in Europe PMC
Go to all (33) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Methylthioadenosine phosphorylase (MTAP) in hearing: gene disruption by chromosomal rearrangement in a hearing impaired individual and model organism analysis.
Am J Med Genet A, 143A(14):1630-1639, 01 Jul 2007
Cited by: 8 articles | PMID: 17534888
Independent Loss of Methylthioadenosine Phosphorylase (MTAP) in Primary Cutaneous T-Cell Lymphoma.
J Invest Dermatol, 136(6):1238-1246, 09 Feb 2016
Cited by: 7 articles | PMID: 26872600
[Methylthioadenosine phosphorylase and p16 as surrogate diagnostic markers for CDKN2A homozygous deletion in brain tumors].
Zhonghua Bing Li Xue Za Zhi, 53(5):439-445, 01 May 2024
Cited by: 0 articles | PMID: 38678323
MTAP loss: a possible therapeutic approach for glioblastoma.
J Transl Med, 20(1):620, 26 Dec 2022
Cited by: 3 articles | PMID: 36572880 | PMCID: PMC9791736
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (7)
Grant ID: CA06927
Grant ID: CA131024
Grant ID: R01 CA125195
Grant ID: R01 CA131024
Grant ID: P30 CA006927
Grant ID: R01 CA125195-03
Grant ID: R01 CA131024-01A2
NHLBI NIH HHS (3)
Grant ID: HL057299
Grant ID: R01 HL057299
Grant ID: R29 HL057299





