Abstract
Objective
Castration of male apolipoprotein E-deficient (apoE-/-) mice reduces angiotensin II (Ang II)-induced abdominal aorta aneurysms (AAAs) to that of female mice. The purpose of this study was to determine whether this reduction is attributable to androgen-mediated regulation of aortic Ang II type 1A receptors (AT1aR).Methods and results
AT1aR mRNA abundance in the AAA-prone region of abdominal aortas was 8-fold greater compared to thoracic aortas of male but not female mice. AT1aR mRNA abundance decreased after castration in abdominal but not thoracic aortas of male mice. Dihydrotestosterone (DHT, 0.16 mg/d) administration to castrated male mice restored AT1aR mRNA abundance in abdominal aortas but had no effect in thoracic aortas. DHT also increased AT1aR mRNA abundance in abdominal aortas from female mice. Castrated male or female apoE-/- mice were administered DHT during infusion of saline or Ang II (1000 ng/kg/min for 28 days). DHT administration did not alter serum cholesterol concentrations, lipoprotein distributions, or atherosclerotic lesion areas in either male or female mice. However, administration of DHT increased AAA incidence in male (27% placebo versus 75% DHT) and female mice (28% placebo versus 64% DHT).Conclusions
Androgen promotes AT1aR mRNA abundance in abdominal aortas associated with increased Ang II-induced AAAs.Free full text

Androgen Increases AT1a Receptor Expression in Abdominal Aortas to Promote Angiotensin II-Induced AAAs in Apolipoprotein E Deficient Mice
Abstract
Objective
Castration of male apolipoprotein E deficient (apoE-/-) mice reduces angiotensin II (AngII)-induced abdominal aorta aneurysms (AAAs) to that of female mice. The purpose of this study was to determine whether this reduction is due to androgen-mediated regulation of aortic AngII type 1A receptors (AT1aR).
Methods and Results
AT1aR mRNA abundance in the AAA-prone region of abdominal aortas was 8-fold greater compared to thoracic aortas of male, but not female mice. AT1aR mRNA abundance decreased after castration in abdominal, but not thoracic aortas of male mice. Dihydrotestosterone (DHT, 0.16 mg/day) administration to castrated male mice restored AT1aR mRNA abundance in abdominal aortas, but had no effect in thoracic aortas. DHT also increased AT1aR mRNA abundance in abdominal aortas from female mice. Castrated male or female apoE-/- mice were administered DHT during infusion of saline or AngII (1,000 ng/kg/min for 28 days). DHT administration did not alter serum cholesterol concentrations, lipoprotein distributions, or atherosclerotic lesion areas in either male or female mice. However, administration of DHT increased AAA incidence in male (27% placebo vs. vs. 75% DHT) and female mice (28% placebo vs. 64% DHT).
Conclusions
Androgen promotes AT1aR mRNA abundance in abdominal aortas associated with increased AngII-induced AAAs.
Abdominal aortic aneurysms (AAAs) account for 2% of all deaths and are the tenth most common cause of mortality.1 The incidence and severity of abdominal aortic dilations are greater in males than females.2,3 Male gender has been consistently identified as a non-modifiable risk factor for AAA. However, the role of androgens as mediators of gender differences in AAA has not been investigated extensively.
Gender differences also impact AAA formation in experimental models of this disease. In aortic dilation promoted by transient intraluminal elastase infusion, male rats had larger and more frequent AAAs than females.4 Chronic infusion of angiotensin II (AngII) into hyperlipidemic mice resulted in AAA formation at a higher incidence in male compared to female mice.5–8 In agreement with a potential protection of female gender, estradiol administration to male apolipoprotein E (apoE) deficient mice reduced AngII-induced AAA formation.9 However, ovariectomy of apoE-/- mice did not significantly influence AAA formation, suggesting that endogenous ovarian hormones are not primary mediators of gender differences in AngII-induced AAAs.8 In contrast, removal of male sex hormones by orchiectomy of apoE-/- mice significantly reduced the incidence of AngII-induced AAAs to that observed in female mice.8 These data potentially implicate androgen as a primary mediator of gender differences in AngII-induced AAAs; however, mechanisms of androgen to promote AAA formation are unknown.
Androgen has been reported to increase the expression of each component of the renin-angiotensin system (RAS), including angiotensinogen, renin, ACE and AT1 receptors.10 Previous studies in our laboratory demonstrated that the AT1 receptor antagonist, losartan, abolished AngII-induced AAAs.11 AT1 receptors have a defined role in AngII-induced AAAs as demonstrated by the protective effect of losartan and AT1aR deficiency.12 Moreover, using bone marrow transplantation, AT1aR deficiency of recipient mice, but not in cells used to repopulate, reduced AngII-induced AAAs. Collectively, these results demonstrate that AngII induces AAA formation through stimulation of AT1aRs, and support a pivotal role on vascular wall cells in AAA formation.
Previous studies demonstrated that contractile responses to AngII were greater in abdominal than thoracic aortic ring segments from male C57BL/6 mice.13 This was associated with greater AT1 mRNA abundance in abdominal than thoracic aorta. Additional studies suggested that AT1b receptors in aorta were primarily responsible for AngII-mediated contractile responses of abdominal aortas, despite the presence of both AT1a and AT1b receptor subtypes.14,15 While these results support regional differences in abundance and subtypes of AT1 receptors along the length of the aorta in male mice, it is unknown if these regional differences in AT1 receptor expression are present in aortas from female mice, and whether these differences contribute to the differential sensitivity to AAA formation in male versus female mice.
In this study, we tested the hypothesis that androgen increases AT1a receptor expression in abdominal aortas to promote AngII-induced AAAs. We first examined the effect of castration during exogenous androgen or vehicle administration on AT1aR mRNA abundance in thoracic versus abdominal aortas from male and female apoE-/- mice. Then, we defined the effect of exogenous androgen administration to castrated male and female apoE-/- mice on AngII-induced atherosclerosis and AAA formation.
Methods
Animals
Male apoE-/- mice (12 wk old, backcrossed 10 times onto a C57BL/6 background) were purchased from Taconic Farms (Germantown, PA). Female apoE-/- mice (12 wk old, backcrossed 10 times onto a C57BL/6J background) were bred in house. All mice were maintained under barrier conditions. Water and normal laboratory diet were available ad libitum. Male and female mice were castrated as described previously8, and 1 week later implanted in the subcutaneous space with slow release pellets (Innovative Research Associates, Sarasota, FL) containing vehicle or dihydrotestosterone (DHT; 10 mg pellets/60 day sustained release; 0.16 mg/day). For studies examining the effects of castration on AT1aR mRNA abundance, castrated male mice administered vehicle or DHT were examined at 1 or 5 weeks after DHT administration (n = 10–13/treatment/time point). A group of ovariectomized female apoE-/- mice were included, with vehicle or DHT (0.16 mg/day) administration beginning 1 week after castration for a total duration of 5 weeks (n = 8/treatment group).
For studies examining the effects of DHT on AngII-induced atherosclerosis and AAAs in castrated male apoE-/- mice, 1 week after implantation of pellets (vehicle/DHT 0.16 mg/day; n = 12/group), Alzet minipumps (model 2004, Alzet, Cupertino, CA) delivering AngII (1,000 ng/kg/min) for 28 days were implanted in the subcutaneous space as described previously (total of 5 weeks of DHT administration).6,11 Saline-infused castrated male mice were not included in this study since results from this group have been reported previously.8 For ovariectomized female mice, 1 week after implantation of pellets (vehicle/DHT 0.16 mg/day), minipumps delivering saline (n = 6–8/pellet group) or AngII (1,000 ng/kg/min; n = 18–25/pellet group) were implanted for 28 day delivery. The experimental design of studies examining DHT regulation of AT1aR mRNA abundance in aortas and effect on AAA formation were matched for duration of DHT exposure prior to onset of AngII infusion (1 week DHT group) and for the total duration of DHT administration (5 week group). All procedures were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
For methods for blood pressure measurements, plasma and serum components, quantification of atherosclerosis and AAAs in mice, tissue composition and measurement of AT1aR mRNA abundance, please see www.ahajournals.org, Supplemental Methods.
Statistical Analyses
Data are represented as mean ± SEM. Parametric data were initially analyzed using ANOVA. If differences existed between groups, post hoc analyses were performed (Tukeys). The incidences of AAA were analyzed using Fisher’s exact test. P<0.05 was considered statistically significant. All statistical analyses were performed using SigmaStat (SPSS, Inc.).
Results
DHT selective augments AT1aR mRNA abundance in abdominal, but not thoracic aortas
AT1aR mRNA abundance was greater (7.6-fold) in abdominal than thoracic aortas of male, but not female apoE-/- mice (Figure 1A). AT1aR mRNA abundance progressively decreased after castration in abdominal aortas to levels observed in thoracic aortas. Administration of DHT for 1 week to castrated male apoE-/- mice resulted in complete restoration of AT1aR mRNA abundance in abdominal aortas, but had no effect in thoracic aortas. Longer exposures to DHT (5 weeks) also increased AT1aR mRNA abundance in abdominal aortas to levels not different from intact male mice. Interestingly, administration of DHT (total of 5 weeks) to ovariectomized female apoE-/- mice also resulted in increased AT1aR mRNA abundance in abdominal, but not thoracic aortas (Figure 1B).
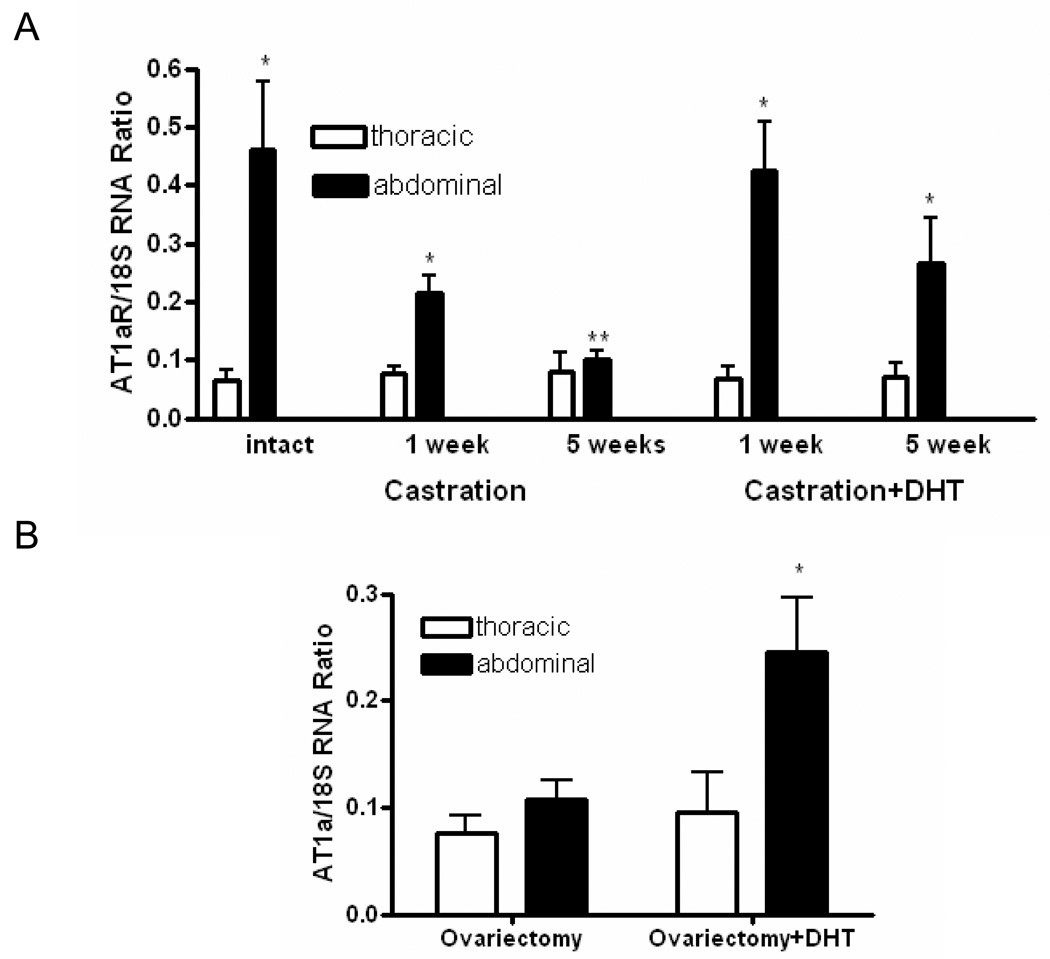
A, In males, AT1aR mRNA abundance was greater in abdominal than thoracic aortas. Castration decreased, while DHT restored AT1aR mRNA abundance in abdominal aortas. B, In females, DHT increased AT1aR mRNA abundance in abdominal aortas. Data are mean ± SEM; *, different from thoracic (P < 0.01); **, different from intact (P < 0.01).
To determine whether androgen regulation of AT1 receptors was restricted to the AT1aR subtype, we examined AT1b mRNA abundance in thoracic and abdominal aortas from male and female mice in each group. AT1bR mRNA abundance was greater in abdominal compared to thoracic aortas from male and female apoE-/- mice, with similar expression levels in aortas from male and female mice (please see www.ahajournals.org, Supplemental Figure IA). In addition, AT1bR mRNA abundance in abdominal aortas was not altered by castration, or by DHT administration to castrated male or female apoE-/- mice. We defined the contractile response to AngII in aortic rings from thoracic versus abdominal aortas of male and female apoE-/- mice. The contractile response to AngII was greater in abdominal than thoracic aortas from male and female apoE-/- mice, and was of similar magnitude in aortas from male and female mice (please see www.ahajournals.org Supplemental Figure IB). Moreover, there was no difference in AngII-induced contractile responses in castrated (5 weeks) male or female mice, or in mice administered DHT (5 weeks).
DHT administration to castrated male and female apoE-/- mice increases AngII-induced AAAs, but has no effect on atherosclerosis
In castrated male mice, body weight was not significantly altered by DHT administration (please see www.ahajournals.org, Supplemental Table I). Administration of DHT to male mice had no significant effect on baseline blood pressure (data not shown), and did not significantly alter the hypertensive response to AngII (please see www.ahajournals.org, Supplemental Table I). Moreover, DHT administration had no effect on total serum cholesterol concentrations (please see www.ahajournals.org online Supplemental Table I) or lipoprotein cholesterol distribution in AngII-infused castrated male mice (please see www.ahajournals.org, Supplemental Figure II). DHT was only detected in serum of mice implanted with DHT containing pellets (11 ± 5 pg/ml). Atherosclerotic lesion area was quantified on the intimal surface of the aortic arch and was not influenced by DHT administration in AngII-infused mice (placebo, 5.50 ± 0.79; DHT, 4.93 ± 1.06, P = 0.89).
In agreement with previous results8, castration reduced the incidence of AngII-induced AAAs in male mice (27%; Figure 2A). The incidence of AngII-induced AAAs was increased by administration of DHT compared to vehicle (75% vs. 27% respectively; P=0.03, Figure 2A). Furthermore, DHT administration resulted in more severe AAAs with significantly higher incidence of mortality due to ruptured aneurysms compared to vehicle (Figure 2B).
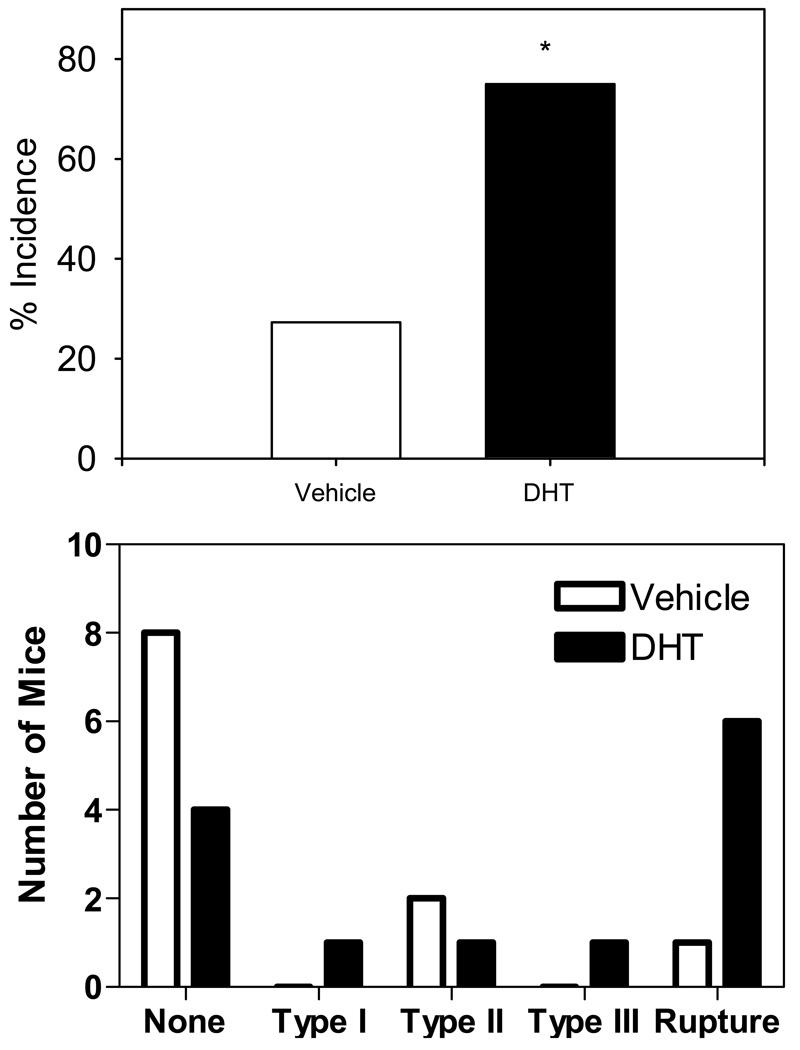
A. DHT increased the incidence of AAAs to 75% compared to vehicle (27%) (P = 0.03). B. DHT increased the severity and mortality of AngII-induced AAAs. * P=0.03 compared to placebo.
In ovariectomized female apoE-/- mice, body weight was increased in both saline- and AngII-infused female mice administered DHT (please see www.ahajournals.org, Supplemental Table I). Moreover, administration of DHT increased uterine wet weight in ovariectomized female mice (please see www.ahajournals.org, Supplemental Table I, saline and AngII-infused). Systolic blood pressure was not altered by administration of DHT in ovariectomized female apoE-/- mice. However, DHT administration significantly decreased AngII-induced hypertension (please see www.ahajournals.org, Supplemental Figure III). Total serum cholesterol concentrations (please see www.ahajournals.org online Supplemental Table 1) and lipoprotein cholesterol distributions (please see www.ahajournals.org, Supplemental Figure IV) were not altered by administration of DHT to female mice infused with either saline or AngII. Furthermore, in saline-infused females, atherosclerotic lesion surface area was not altered by administration of DHT (Figure 3A). Infusion of AngII increased the extent of atherosclerosis to a similar degree in both groups (Figure 3B).
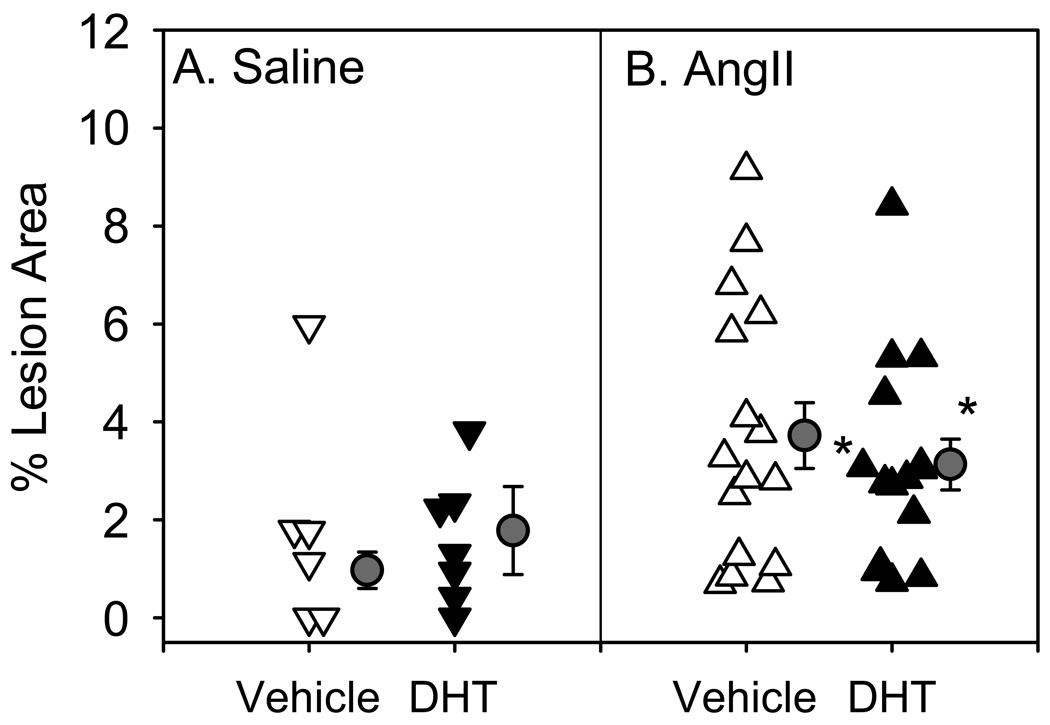
Infusion of AngII significantly increased atherosclerotic lesion area compared to saline-infused groups (* P<0.05 saline vs. AngII). However, DHT did not alter atherosclerotic lesion area compared to vehicle in either saline (A) or AngII-infused mice (B). Triangles represent the values from individual mice, circles represent mean, bars represent ± SEM.
AAAs were not detected in saline-infused female mice. Similar to male mice treated with DHT, female mice administered DHT had an increased incidence of AngII-induced AAAs compared to vehicle (66% vs. 22%, respectively, P = 0.03, Figure 4A). Furthermore, DHT increased the severity of AngII-induced AAAs (Figure 4B). AngII-induced AAAs have a highly heterogeneous morphological appearance throughout the regions of diseased aorta. After sectioning several AAAs from each group in their entirety, analysis using histological and immunostaining techniques revealed no overt differences in the characteristics of aneurysmal tissues between groups administered with vehicle versus DHT (data not shown).
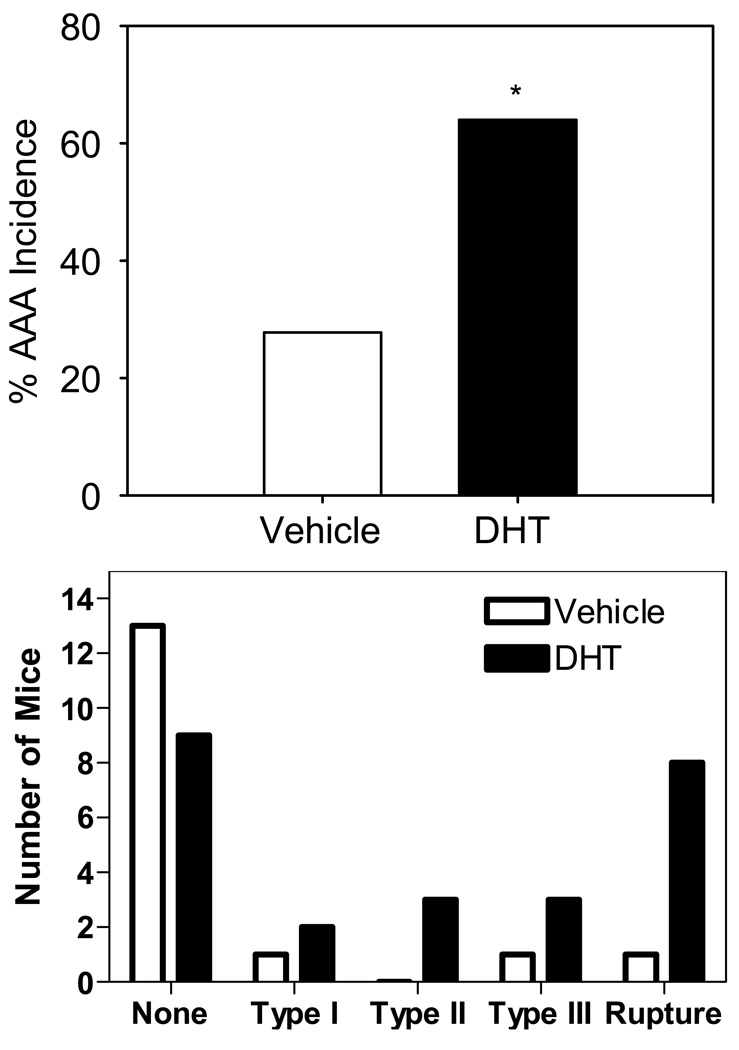
A. DHT administration significantly increased the incidence of AngII-induced AAA compared to vehicle (vehicle, 28%; AngII-infused 64%, P = 0.03). B. DHT increased the severity and mortality of AngII-induced AAAs.
Discussion
Results from this study demonstrate that male, but not female apoE-/- mice, exhibit regional differences in AT1aR mRNA abundance in aortas, with greater receptor expression in abdominal compared to thoracic aortas. Moreover, castration of male apoE mice resulted in a specific reduction in AT1aR mRNA abundance in abdominal aortas, that was restored by DHT administration. Interestingly, DHT also increased AT1aR mRNA abundance in abdominal aortas from female apoE-/- mice. These effects of androgen to promote AT1aR mRNA abundance specifically in abdominal aortas were associated with increased AAA incidence and severity in both male and female castrated apoE-/- mice. Interestingly, effects of castration and DHT to regulate AT1aR mRNA abundance and markedly influence AngII-induced AAAs were not mimicked in AngII-induced atherosclerosis.
Testosterone, the principal male sex hormone produced by the testes, is a substrate for aromatase to form estrogen, while DHT is not a substrate for this enzyme. Several cell types in male mice express aromatase, including vascular smooth muscle cells 21,22, a pivotal cell type implicated in AngII-induced AAAs.20 To avoid potential confounding effects from conversion of testosterone to estradiol, we administered DHT, the 5-∀ reductase metabolite of testosterone. DHT has higher affinity and longer duration of effect at the androgen receptor, and thus occupies most androgen receptor sites at steady state, even if testosterone concentrations predominate.23,24 The dose of DHT administered in these studies has been demonstrated previously to restore the weight of prostates in castrated male rats.25
The effect of androgen on AT1 receptor expression has been relatively unexplored. In rat epididymis, castration reduced AT1 receptor protein, that was restored when rats were treated with testosterone.26 Moreover, androgen was reported to increase AngII receptors in bovine adrenal glomerulosa cells.27 In contrast, neither castration alone or combined with androgen administration regulated AT1 receptor mRNA abundance in homogenates of renal cortex punches from male New Zealand genetically hypertensive rats 28, or in glomeruli from male rats 29. Our results demonstrate a specific effect of androgen to regulate AT1aR mRNA abundance in abdominal, but not thoracic aortas. Taken together, these results suggest that androgen exhibits tissue and/or cell-specific regulation of AT1 receptors. Interestingly, recent studies demonstrated that smooth muscle cells of the ascending and arch portions of the aorta are derived from murine neural crest, while smooth muscle cells of the abdominal aorta are derived predominately from splanchnic mesoderm.30 These differences in smooth muscle embryonic origins along the length of the aorta may have contributed to regional differences in AT1aR regulation by androgen.
In contrast to rodents, humans have one gene encoding AT1 receptors. Human vascular smooth muscle cells express androgen receptors 31, and would be anticipated to respond to androgen. In addition, incubation of androgen-dependent human prostate cancer cells with DHT increased AT1 receptor mRNA and protein 32, supporting androgen regulation of human AT1 receptors. However, it is unclear whether human aortic smooth muscle cells expressed along the length of the aorta respond differentially to androgen to increase AT1 receptor expression.
Previous investigators have demonstrated greater AngII-induced contractile responses in abdominal compared to thoracic aortic ring segments from male C57BL/6 mice.13 Further studies demonstrated a prominent role for AT1b receptors in AngII-induced contractile responses.15,33 Our results extend these findings by demonstrating greater AT1aR mRNA abundance in abdominal aortas from male, but not female mice. To define whether androgen effects were restricted to the AT1aR subtype, we measured AT1bR mRNA abundance, and the contractile response to AngII as an index of AT1b receptor function.13,15 While the contractile response to AngII exhibited a similar regional specificity to the abdominal aorta of male and female apoE-/- mice, AngII-induced contraction was not regulated by androgen, nor was AT1bR mRNA abundance. These results demonstrate that androgen specifically regulates aortic AT1a receptors. Given that AT1a and AT1b receptor subtypes exhibit differential cell and tissue distribution, differences in the promoters of these distinct genes may contribute to androgen-specific effects to increase AT1aR mRNA abundance.
We have reported previously that male mice are more susceptible to developing AngII-induced AAAs, and that castration of male mice significantly reduced the incidence of AngII-induced AAAs to that of female mice.7,8 In the current study, we confirmed that castration of male apoE-/- mice results in a low incidence of AngII-induced AAAs. Furthermore, we demonstrate that administration of DHT to castrated male or female apoE-/- mice, at a dose and duration of exposure that increased AT1aR mRNA abundance in abdominal aortas, significantly increases the incidence of AngII-induced AAAs. Our results do not specify the vascular wall cell type targeted by androgen to increase aortic AT1aR mRNA abundance. However, given that previous results demonstrate breaks in medial elastin in the abdominal aorta as an early event in AAA formation 20, smooth muscle cells are a likely target of androgen to promote AT1aR expression and AAA formation in male and female mice. In addition, since female mice also responded to DHT to increase AAA formation, these results demonstrate that females have sufficient androgen receptors to increase AT1aR mRNA abundance in abdominal aortas and promote AAA formation.
In relation to the human disease, in males and females the incidence of AAAs increases with age.34 However, androgen concentrations decline with age in males 35, suggesting that androgen may not be a mediator of increased AAA risk in aging males. Our results demonstrate that androgen mediates increased risk of AAA formation in male mice infused with AngII. It is unclear whether androgen similarly contributes to early events in aneurysm formation in human males, prior to an age when androgen concentrations decline. Aging and male sex may utilize distinct mechanisms to increase AAA risk. Alternatively, the magnitude of decline in circulating androgen concentrations with aging in males may not be sufficient to manipulate AT1 receptors in AAAs. If androgen exhibits similar effects in humans to increase AT1 receptor expression in abdominal aortas, then our results would suggest that androgen replacement therapy in aging males may increase AAA risk.
The role of androgens in development of atherosclerosis is controversial. We have demonstrated previously that castration increased the extent of atherosclerosis in both saline and AngII-infused male apoE-/- mice.8 However, there was no statistical interaction between castration of male mice and AngII infusion, suggesting distinct mechanisms augmented atherosclerosis. Androgen administration was also demonstrated to inhibit atherosclerosis in castrated male rabbits 36–38, as well as in LDL receptor-deficient male mice.39 In contrast, in male apoE-/- mice depletion of endogenous testosterone using a GnRH antagonist (cetrorelix) reduced atherosclerosis, while exogenous testosterone administration increased atherosclerosis.40,41 In the present study, the administration of exogenous DHT to castrated male apoE-/- mice did not alter atherosclerosis induced by hypercholesterolemia and when augmented with AngII. One possible explanation for the discrepancy in the effects of exogenous androgen administration, versus our previous results from castrated male mice, is the difference in study design, including the dose of AngII (1,000 ng/kg/min in this study versus 500 ng/kg/min in castrated male mice).8
Previous studies demonstrated that hypertension induced by norepinephrine did not increase atherosclerosis to the extent observed in apoE-/- mice infused with AngII.42 Additional studies have demonstrated that AT1aR deficiency, while not influencing blood pressure, inhibited the development of atherosclerosis in LDLr-/- mice.43,44,15,17,23 Similar to pressure-independent effects of AngII to increase atherosclerosis, in this study we found that while DHT either had no effect (males) or decreased (females) AngII-induced hypertension, it markedly promoted AAA formation in male and female mice. Several previous studies targeted at specific mechanisms have markedly altered the development of AngII-induced AAAs, without significantly affecting AngII-induced hypertension.8,43 Collectively, these results do not support a primary role for hypertension as a contributing factor to AngII-induced atherosclerosis or AAAs.
In conclusion, results from this study demonstrate that androgen positively regulates AT1aR mRNA abundance in abdominal aortas from male and female apoE-/-mice, and that this effect parallels AAA susceptibility. Androgen regulation was specific to the AT1aR subtype and to the abdominal aortic region, two features pivotal to AngII-induced AAAs. These results demonstrate that male sex hormones positively regulate AT1aR expression in a regional-specific manner to promote AngII-induced AAAs.
Acknowledgments
a) Sources of funding: This work was supported by a grant from the National Institutes of Health, P01 HL080100 (LC, AD) and a Pre-doctoral fellowship from the American Heart Association 0315062B (TH).
b) Acknowledgments: We acknowledge the excellent technical assistance of Jessica Moorleghen for experiments measuring aortic contractility, Deborah Howatt and Aaron Gay for lesion characterization, and the editorial assistance of Debra Rateri.
References
Full text links
Read article at publisher's site: https://doi.org/10.1161/atvbaha.107.160382
Read article for free, from open access legal sources, via Unpaywall:
https://www.ahajournals.org/doi/pdf/10.1161/ATVBAHA.107.160382
Free to read at intl-atvb.ahajournals.org
http://intl-atvb.ahajournals.org/cgi/content/abstract/28/7/1251
Free after 12 months at intl-atvb.ahajournals.org
http://intl-atvb.ahajournals.org/cgi/content/full/28/7/1251
Free after 12 months at intl-atvb.ahajournals.org
http://intl-atvb.ahajournals.org/cgi/reprint/28/7/1251.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
AT1b receptors contribute to regional disparities in angiotensin II mediated aortic remodelling in mice.
J R Soc Interface, 21(217):20240110, 28 Aug 2024
Cited by: 1 article | PMID: 39192727 | PMCID: PMC11350382
Aortic aneurysm: pathophysiology and therapeutic options.
MedComm (2020), 5(9):e703, 07 Sep 2024
Cited by: 0 articles | PMID: 39247619 | PMCID: PMC11380051
Review Free full text in Europe PMC
Hormonal influence: unraveling the impact of sex hormones on vascular smooth muscle cells.
Biol Res, 57(1):61, 04 Sep 2024
Cited by: 0 articles | PMID: 39227995 | PMCID: PMC11373308
Review Free full text in Europe PMC
Unleashing PD-1: a duel of immunity in aortic aneurysm formation.
J Clin Invest, 134(15):e182554, 01 Aug 2024
Cited by: 0 articles | PMID: 39087474 | PMCID: PMC11290959
Androgen aggravates aortic aneurysms via suppression of PD-1 in mice.
J Clin Invest, 134(15):e169085, 20 Jun 2024
Cited by: 1 article | PMID: 38900572 | PMCID: PMC11290977
Go to all (71) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Transient exposure of neonatal female mice to testosterone abrogates the sexual dimorphism of abdominal aortic aneurysms.
Circ Res, 110(11):e73-85, 26 Apr 2012
Cited by: 47 articles | PMID: 22539767 | PMCID: PMC3518797
Castration of male mice prevents the progression of established angiotensin II-induced abdominal aortic aneurysms.
J Vasc Surg, 61(3):767-776, 16 Jan 2014
Cited by: 31 articles | PMID: 24439319 | PMCID: PMC4099302
Associations of ApoAI and ApoB-containing lipoproteins with AngII-induced abdominal aortic aneurysms in mice.
Arterioscler Thromb Vasc Biol, 35(8):1826-1834, 04 Jun 2015
Cited by: 30 articles | PMID: 26044581 | PMCID: PMC4514578
Sex Chromosome Complement Defines Diffuse Versus Focal Angiotensin II-Induced Aortic Pathology.
Arterioscler Thromb Vasc Biol, 38(1):143-153, 02 Nov 2017
Cited by: 31 articles | PMID: 29097367 | PMCID: PMC5864127
Funding
Funders who supported this work.
NHLBI NIH HHS (5)
Grant ID: P01 HL080100-040001
Grant ID: P01 HL080100
Grant ID: P01 HL080100-01A10001
Grant ID: P01 HL080100-030001
Grant ID: P01 HL080100-020001




