Abstract
Free full text

Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation
Abstract
The extracellular matrix consists of structural macromolecules and other proteins with regulatory functions. An important family of the latter class of molecules found in most tissues is the small leucine-rich repeat proteins (SLRPs). We have previously shown that the SLRP fibromodulin binds directly to C1q and activates the classical pathway of complement. In the present study we further examine the interactions between SLRPs and complement. Osteoadherin, like fibromodulin, binds C1q and activates the classical pathway strongly while moderate activation is seen in the terminal pathway. This can be explained by the interaction of fibromodulin and osteoadherin with factor H, a major soluble inhibitor of complement. Also, chondroadherin was found to bind C1q and activate complement, albeit to a lesser extent. Chondroadherin also binds factor H. We confirm published data showing that biglycan and decorin bind C1q but do not activate complement. In this study a similar pattern is seen for lumican although its affinity for C1q is lower than for biglycan and decorin. Furthermore, using electron microscopy and radiolabeled SLRPs, we demonstrate two different classes of SLRP binding sites on C1q, to head and stalk respectively, where only binding to the head appears to be activating. We propose a role for SLRPs in the regulation of complement activation in diseases involving the extracellular matrix, particularly those characterized by chronic inflammation such as rheumatoid arthritis, atherosclerosis, osteoarthritis and chronic obstructive lung disease.
1. Introduction
The complement system makes up an essential part of the innate immune system in the first-line defense against pathogens. The classical pathway of complement is triggered by binding of particular ligands to the C1-complex consisting of the recognition protein C1q, and two copies each of the proteolytic subunits C1s and C1r (Gaboriaud et al., 2004). The N-terminal heads of C1q are responsible for C1-activation via interaction with, for instance, clustered IgG and IgM antibodies, C-reactive protein (CRP), DNA and lipopolysaccharide (LPS; for review see (Gaboriaud et al., 2004)). The alternative pathway is initiated by autoactivation of the unstable complement factor C3 and its subsequent deposition on activating pathogen surfaces, while the lectin pathway is triggered when mannose-binding lectin (MBL) binds to carbohydrate-rich structures, on for example, bacteria leading to a similar series of reactions as in the classical pathway (Walport, 2001a; Walport, 2001b).
Complement activation triggers a plethora of effects involving the innate as well as the adaptive immune system. For instance, C3a and, especially, C5a stimulate chemotaxis of immune cells while the pore-forming membrane attack complex (MAC) disrupts membrane integrity. Activation of complement is tightly regulated by a number of soluble or membrane-bound inhibitors, examples of which are factor H (FH) and C4b-binding protein (C4BP). In recent years, pathologic complement activation has been implicated in numerous diseases, such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), hemolytic uremic syndrome and glomerulonephritis (Morgan and Walport, 1991).
Cartilage mainly consists of extracellular supramolecular assemblies, with cells constituting less than 10 % of the volume. One of the most abundant structures of the extracellular matrix (ECM) is the highly negatively charged proteoglycan (aggrecan), which is found nestled in networks of collagen fibers. The latter contains many other bound molecules, which maintain and stabilize the interactions with surrounding structures. Among these molecules are members of the small leucine-rich repeat protein (SLRP) family, such as decorin, biglycan, fibromodulin (FMOD), lumican, PRELP (proline-arginine-rich-end-leucine-rich repeat protein), chondroadherin (CHAD) and osteoadherin (OSAD) (Fig. 1; for review see (Heinegård et al., 2002)). All SLRPs contain a central leucine-rich repeat (LRR) region with disulphide bridges on both ends. The LRR-region is normally glycosylated and is flanked by N- and/or C-terminal regions, which can be highly charged. In these peripheral domains the SLRPs often show the lowest, and often no, homology.
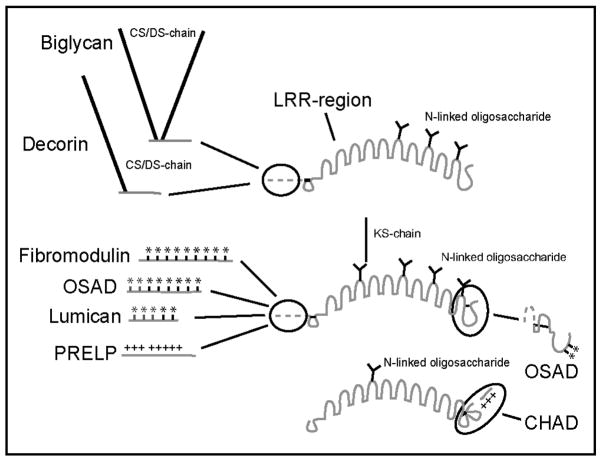
All proteins contain an LRR-region, which in all but one case (CHAD) includes varying numbers of glycosylation substitution sites. Asterisks indicate tyrosine sulfation sites and + indicates positively charged arginine- or lysine rich patches. CS: chondroitin sulfate, DS: dermatan sulfate, KS: keratan sulfate.
Some cartilage proteins have already been found to interact with complement factors. For example, C1q binds to decorin (Groeneveld et al., 2005; Krumdieck et al., 1992), biglycan (Groeneveld et al., 2005), fibronectin (Bing et al., 1982; Sorvillo et al., 1985), laminin (Bohnsack et al., 1985) and FMOD (Sjöberg et al., 2005). However, of these only FMOD gives rise to complement activation. Like hIgG, FMOD binds to the globular head domains of C1q, while the other known C1q-ligands in the ECM bind the collagen-like tail. FMOD also binds TGF-β1 and is thus suggested to modulate its activity in vivo (Hildebrand et al., 1994; Soo et al., 2000). OSAD is a close structural relative of FMOD with high sequence homology and has been reported to be expressed primarily in bone, but may also be found in hypertrophic cartilage (Sommarin et al., 1998). The protein contains a tyrosine sulfate rich moiety in the N-terminal domain, with a number of sulfated residues similar to that of FMOD. OSAD has been implicated in TGF-β and BMP-2 signaling (Rehn et al., 2006) and binds to αvβ3 integrin (Lucchini et al., 2004; Wendel et al., 1998). A study on OSAD in rat primarily showed expression in bone marrow and in bone directly under articular cartilage forming in the femoral head (Shen et al., 1999). Furthermore, CHAD was recently demonstrated to promote rapid spreading of synovial fibroblasts from osteoarthritis (OA) patients, implicating a role in OA pathology (Schedel et al., 2004).
The overall rationale for this study was to determine how the presence of SLRPs in the extracellular matrix can favor or block complement activation, presumably contributing to pathology when they enhance activation. We have previously reported that FMOD binds directly to the globular head domain of C1q and that this interaction gives rise to pronounced activation of the classical pathway of complement (Sjöberg et al., 2005). We now present detailed data demonstrating that several other SLRPs bind C1q and that one of them, OSAD, also is a very potent complement activator. We hypothesize that these interactions contribute to the chronic inflammation seen in diseases such as RA, OA and atherosclerosis.
2. Materials and Methods
2.1. Proteins & sera
C1q was purified from human serum as described previously (Tenner et al., 1981). Serum depleted of C1q was purchased from Quidel and pooled human serum was from Innovative Research. FH was isolated from human plasma (Blom et al., 2003a). The H384 and Y384 variants of FH were expressed as constructs consisting of complement control protein (CCP) domains 6–8, then purified as described previously (Clark et al., 2006). C3-met, which corresponds functionally to C3b, was prepared by treatment of C3 with methylamine as described in (Blom et al., 2003b). The stalk- and head- portions of C1q were prepared by partial digestion of intact C1q, according to published protocols (Paques et al., 1979; Reid and Porter, 1976) using pepsin (for the preparation of the stalk, Worthington) and collagenase (for the head, from Clostridium histolyticum, Worthington (CLSPA grade)). FMOD was isolated from both bovine and human articular cartilage (Heinegård et al., 1986). Biglycan, decorin and PRELP were prepared by standard techniques from bovine cartilage and lumican from cornea (for reference see (Heinegård et al., 2002)). Chondroadherin was a recombinant protein expressed without a tag in EBNA 293 cells (Mansson et al., 2001). OSAD was a recombinant protein similarly expressed in EBNA 293 cells with a histidine tag in its N-terminal for efficient purification (Önnerfjord et al., 2004). To account for differences between recombinantly expressed SLRPs and those purified from tissue, we tested purified (human & bovine) and recombinant CHAD & OSAD in parallel during the initial experimental stages. Insignificant differences in activating and binding capabilities were seen (not shown). All preparations contained picomolar concentrations of LPS as determined with limulus test (Hycult Biotechnology). We confirmed that such low amounts of LPS do not cause complement activation in our experimental set up.
2.2. 125I Labeling of SLRPs
Labeling was typically done using 30 μg of a particular SLRP, as a 1 mg/ml solution in Tris-buffered saline, pH 8.0 containing 4.0 M guanidine-HCl. The SLRPs were mixed with 5 μl of 125I (Perkin Elmer) with stirring, and the labeling reaction was started by adding 5 μl of 2 mg/ml chloramine T in 50 mM sodium phosphate buffer, pH 7.5. The reaction was quenched by adding 5 μl of 2 mg/ml Na2S2O5 in the same buffer. The following reaction times were found to be appropriate: biglycan, FMOD, OSAD; 30 s; decorin, lumican; 3 min; CHAD; 6 min. After quenching, the labeled SLRPs were diluted 33-fold into TBS/1 % (w/v) BSA (Sigma) and the free 125I was removed by size-exclusion chromatography (PD-10 columns, GE Healthcare). Resins had been pre-treated with 10 % (w/v) BSA to minimize non-specific adsorption of the labeled SLRPs. The SLRP-containing fractions were recovered and diluted 1:1 with 100 % glycerol and stored at −20 °C.
2.3. Complement activation assay
All incubation steps were made with 50 μl solution for 1 h at room temperature, except when stated otherwise. Every step was followed by extensive washing with 50 mM Tris-HCl, 150 mM NaCl, 2 mM CaCl2, 0.1 % Tween; pH 7.5. Microtiter plates (Maxisorp, Nunc) were coated over night at 4°C with either SLRPs (2.5 μg/ml), aggregated human IgG (5 μg/ml, Immuno), mannan (100 μg/ml, Sigma, for lectin pathway) or zymosan (50 μg/ml, Sigma, for alternative pathway) diluted in 75 mM Na2CO3; pH 9.6. The wells were blocked for 2 h with 200 μl of 1 % BSA in phosphate buffered saline (PBS); blocking solution. Dilutions of human serum or serum deficient in C1q in GVB++ (2.5 mM veronal buffer pH 7.3, 150 mM NaCl, 0.1 % gelatin, 1 mM MgCl2, 0.15 mM CaCl2) or Mg-EGTA (2.5 mM veronal buffer pH 7.3, 70 mM NaCl, 140 mM glucose, 0.1% gelatin, 7 mM MgCl2, 10 mM EGTA) were added to the plates and incubated for 20 min (for detection of C1q, C3b and C4b) or 45 min (C9) at 37°C, followed by incubation with specific rabbit polyclonal antibodies against C1q (Dako), C3d (Dako), C4b (Dako), or goat polyclonal antibodies against C9 (Advanced Research Technology) diluted in blocking solution. Horseradish peroxidase (HRP) -conjugated secondary antibodies against rabbit or goat immunoglobulins (Dako) were diluted in blocking solution and allowed to bind. Bound enzyme was quantified using the 1,2-phenylenediamine dihydrochloride (OPD)/H2O2 colorimetric assay (Dako), which was quenched with 0.5 M H2SO4 followed by determination of A490 values.
2.4. Direct binding assays
Unless stated otherwise, all incubations were for 1 h at room temperature in 50 μl solution followed by washing as described above for the activation assay. SLRPs were coated in microtiter plate wells as described above. Aggregated human IgG (2.5 μg/ml, Immuno) and CRP (5 μg/ml, Calbiochem) were used as positive controls for C1q- and FH-binding respectively. The wells were then blocked with 200 μl blocking solution and incubated at room temperature for 2 h. C1q at concentrations ranging between 1 μg/ml and 20 μg/ml, FH at 4 or 8 μg/ml or polymorphic variants of FHCCP6-8 at 1, 3 or 9 μg/ml, in binding buffer (50 mM HEPES, pH 7.4, 100 mM NaCl, 2 mM CaCl2, 50 μg/ml BSA) were then allowed to incubate in the wells for 2 h. For ionic strength dependence experiments, binding buffers with additional NaCl were used. Specific polyclonal antibodies, rabbit anti-human C1q antibody (Dako) or goat anti-human FH antibody (Quidel), respectively, diluted in blocking solution, were then added to each well. This was followed by 30 min incubation with rabbit or goat anti-rabbit IgG-HRP conjugate (Dako), also diluted in blocking solution. Bound C1q or FH was measured using the colorimetric assay described for the complement activation assay.
2.5 Radioassays
Intact and truncated C1q species were coated in microtiter plate wells by diluting stock solutions to 10 μg/ml with 75 mM sodium carbonate buffer, pH 9.6, then delivering 50 μl volumes into the wells and incubating overnight at 4°C. Following washing and blocking (as per the above activation assay protocol), 125I-labeled SLRPs were added to the wells. The SLRPs had been diluted in binding buffer (see above) to 50,000 counts per 50 μl. Following 4 h incubation at room temperature, the plates were washed (as above) and the quantities of isotope labeled proteins retained were quantified using a gamma-counter. The data were normalized for each SLRP. Hence values were expressed as the proportion of counts retained in each well, relative to the number of counts added.
2.6. Surface plasmon resonance (Biacore)
Interactions between C1q and the following proteins: OSAD, decorin, PRELP, CHAD and lumican, were analyzed using surface plasmon resonance (Biacore 2000, Biacore). The individual flow cells of a CM5 sensor chip were activated, each with 20 μl of a mixture of 0.2 M 1-ethyl-3-(3 dimethylaminopropyl) carbodiimide and 0.05 M N-hydroxy-sulfosuccinimide at a flow rate of 5 μl/min, after which OSAD, decorin, PRELP, CHAD and lumican (20 μg/ml in 10 mM Na-acetate buffer, pH 4.0) were injected over individual flow cells to reach 2000 resonance units (RU). Not reacted groups were blocked with 20 μl of 1 M ethanolamine, pH 8.5. An attempt to immobilize biglycan under the same conditions failed. A negative control was prepared for each chip by activating and subsequently blocking the surface of flow cell 1. The association kinetics was studied for various concentrations of purified C1q (0.003–0.2 mg/ml) in 10 mM Hepes, pH 7.5 supplemented with 150 mM NaCl, 2.5 mM CaCl2, 0.005 % Tween 20. Protein solutions were injected for 200 s during the association phase at a constant flow rate of 30 μl/min. The sample was injected first over the negative control surface and then over immobilized SLRPs. Signal from the control surface was subtracted. The dissociation was followed for 200 s at the same flow rate. In all experiments, two 10 μl injections of 2 M NaCl, 100 mM HCl, 5 mM EDTA were used to remove bound ligands during a regeneration step. The BiaEvaluation 3.0 software (Biacore) was used to analyze sensorgrams obtained. For each SLRP, response units obtained at plateau of sensorgrams were plotted against concentrations of injected C1q and used for calculation of equilibrium affinity constants.
2.7. Electron microscopy
The location of individual SLRP molecules on C1q was analyzed by negative staining and transmission electron microscopy as described previously (Engel and Furthmayr, 1987). SLRP samples were conjugated with 5 nm colloidal thiocyanate gold (Baschong et al., 1985). C1q aliquots were mixed with the SLRP-Au conjugates and incubated for 1 h at 4°C. Five μl aliquots were adsorbed onto carbon-coated grids for 1 min, washed with two drops of water, and stained on two drops of 0.75 % uranyl formate. The grids were rendered hydrophilic by glow discharge at low pressure in air. Specimens were observed in a JEOL JEM 1230 electron microscope operated at 80 kV accelerating voltage, and images were recorded with a Gatan Multiscan 791 CCD camera.
3. Results
3.1. SLRPs activate complement to varying degrees
We assessed the capability of seven different SLRPs to activate complement in vitro. The proteins were coated on to microtiter plate wells, serial dilutions of normal human serum (NHS) were added and the amounts of deposited complement components were measured. FMOD and OSAD were very potent activators of the early classical pathway, as shown by appreciable deposition of C1, C4b and C3b (Fig. 2, panels A to F). Deposition levels similar to or higher than those for the positive control, hIgG, were seen for FMOD and OSAD. With regard to C9, which represents the amount of MAC formed on the plate surface, we found that both FMOD and OSAD yielded only moderate levels of deposition (Fig. 2G & H). The known C1q ligands decorin and biglycan also triggered some C1-deposition (Fig. 2A & B). However, since both of these ligands failed to activate the subsequent components of complement, we believe that this signal only corresponds to binding of C1 but not its activation (Fig. 4 and (Groeneveld et al., 2005)). CHAD consistently activated complement, albeit at a very low level. Like FMOD and OSAD, CHAD showed stronger activation of early (Fig. 2A and C) compared to later events in the pathway (Fig. 2E & G). Intriguingly, at the highest serum concentration used, PRELP consistently gave rise to C9-deposition at levels comparable to those seen for FMOD and OSAD (Fig. 2H).
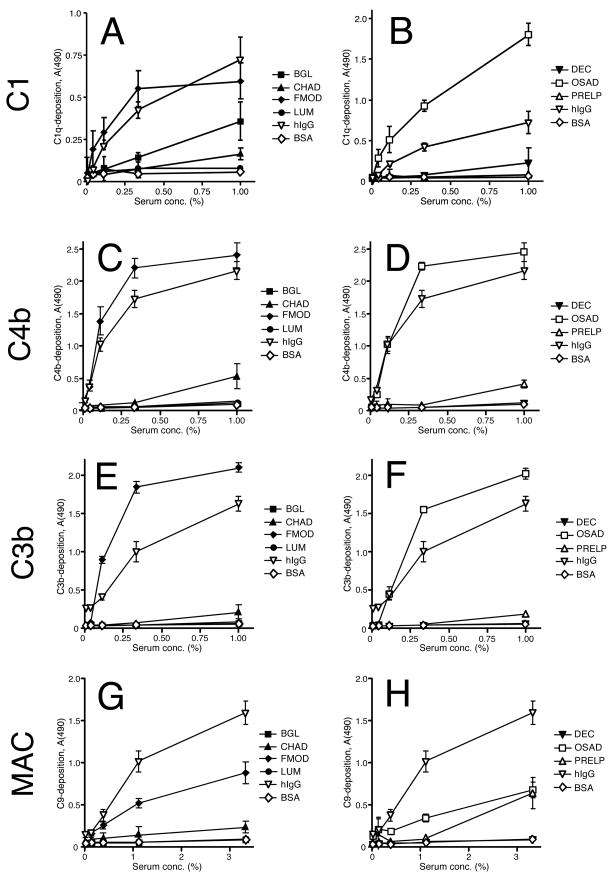
SLRPs and controls were coated onto microtiter plate wells over night followed by blocking of the plate to prevent further protein binding to the plastic surface. Increasing concentrations of normal human serum (NHS) were added and incubated at 37°C. Specific antibodies were used to detect deposition of complement components indicating complement activation. The pattern of C1 (A, B), C4b (C, D), C3b (E, F) and C9 (G, H) deposition is plotted in the figure. The data were collected from two representative duplicate experiments (n=4). Error bars show standard deviation. Statistics were performed for all data (two-tailed Student’s T-test): In panels A & B, all points above 0.1 % NHS were significantly different (p < 0.01) from BSA except for one point each on decorin (highest NHS concentration) and lumican (second highest NHS concentration). In C & D, significance was seen for all NHS concentrations on FMOD & OSAD (p < 0.001) and for the highest concentration on PRELP (p < 0.001). In E & F, all data points for FMOD and OSAD showed significance (p < 0.001 and p < 0.01, respectively) while calculations for only the highest serum concentration point on CHAD, lumican and PRELP showed significance (p < 0.05 for the former two and p < 0.001 for the latter). In G & H, significance was seen for all data points on FMOD (p < 0.05) and OSAD (p < 0.001) as well as the highest concentration points on CHAD and PRELP (p < 0.01).
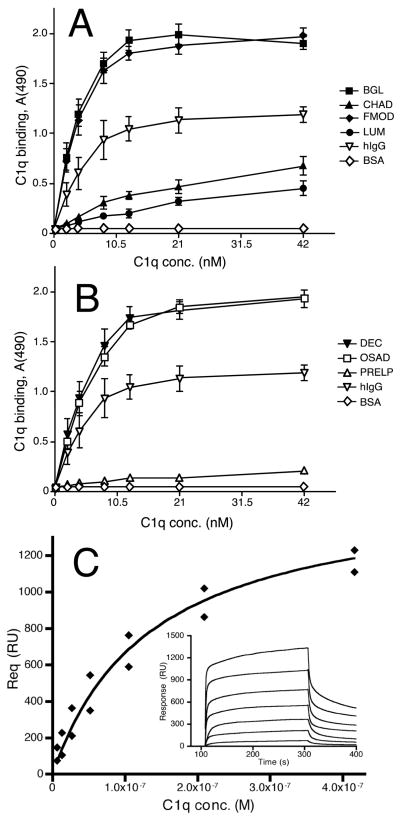
A, B Plates were coated with SLRPs or controls, blocked and then incubated with C1q at the concentrations indicated. Bound C1q was detected using specific polyclonal antibodies. The data were collected from two representative duplicate experiments (n=4). Error bars denote standard deviation. The difference between C1q binding on SLRPs and BSA is statistically significant (p < 0.01; two-tailed Student’s T-test) for all data points above 0 μg/ml C1q except for the two lowest C1q concentrations on PRELP. C C1q was injected at least twice at increasing concentrations (6.5 nM–416 nM) over the Biacore CM5-chip with bound OSAD and in parallel over a control flow cell that was used to subtract nonspecific signal. The amount of bound C1q was measured in arbitrary response units (RU, inset). The response obtained for each concentration of C1q at equilibrium (Req) was plotted against concentration of C1q, which allowed for estimation of KD presented in Table 1.
We next investigated the ability of the SLRPs to activate the alternative and lectin pathways of complement. We confirmed our previously published data regarding the activation properties of FMOD (Sjöberg et al., 2005), since this SLRP did not trigger lectin pathway activation, but to some extent activated the amplification loop of the alternative pathway (Fig. 3A & B). A similar pattern was seen for OSAD, although somewhat higher levels of C4b and C3b were recorded for alternative pathway activation (Fig. 3A & B). When compared to zymosan, FMOD and OSAD showed only low levels of activation. Like FMOD and OSAD, neither CHAD nor PRELP activated the lectin pathway as compared to mannan.
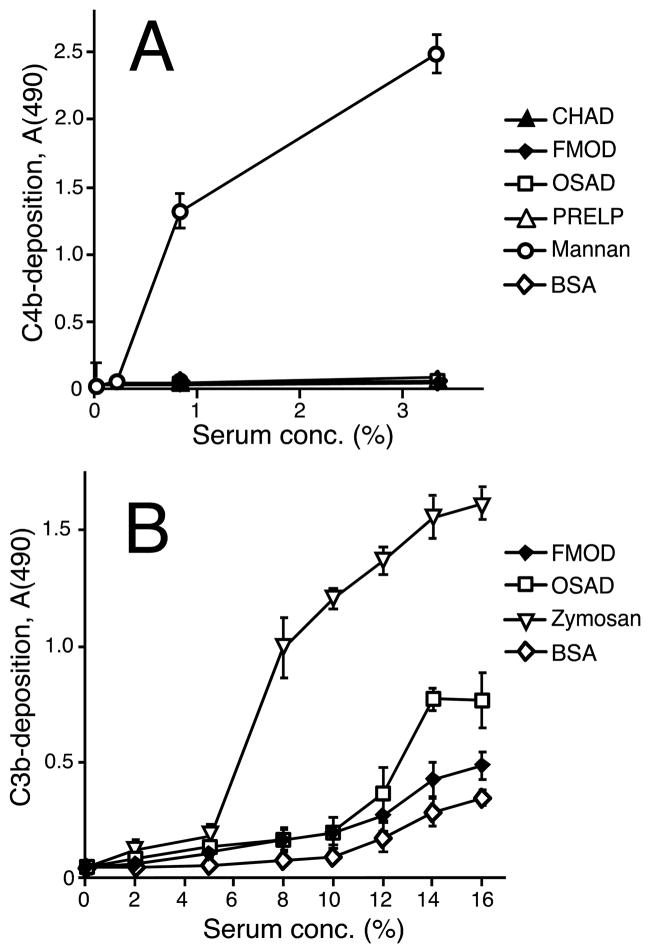
SLRPs and controls were coated onto microtiter plate wells then the plates were blocked for further direct binding. Human serum diluted to various concentrations was incubated at 37°C. Specific antibodies against C3b and C4b were added to detect complement activation on the surfaces. In A, the lectin pathway was assayed by using C1q-deficient serum, which eliminates classical pathway contribution. Mannan was used as the positive control for C4b-deposition via the lectin pathway. B shows complement activation as C3b-deposition from NHS in a buffer specific for the alternative pathway. Here, zymosan was used as the positive control. In both panels the data are from two representative duplicate experiments (n=4). Error bars show standard deviation. In B the differences between activation on both FMOD & OSAD and BSA are statistically significant for all serum dilutions above 5 % (p < 0.05; two tailed Student’s T-test).
Importantly, we determined that our SLRP preparations contained only picomolar quantities of LPS that were below the threshold concentration needed to capture C1q and cause complement activation via the classical or alternative pathways in our experimental system (not shown). Furthermore, serum from which all antibodies were removed by affinity chromatography using protein A and G Sepharose yielded identical results as untreated serum (not shown). This demonstrated that the serum used in this study did not contain autoantibodies against SLRPs, which could otherwise have given rise to false signal intensity.
3.2. Binding of SLRPs to C1q
A solid phase, ELISA-type approach was used for the initial investigation of the interaction between purified C1q, a component of the C1 complex, and the SLRPs: biglycan, CHAD, decorin, FMOD, lumican, OSAD and PRELP. Aggregated human IgG and BSA were used as positive and negative controls, respectively. The SLRPs, hIgG and BSA were coated in microtiter plate wells and soluble C1q was then allowed to interact. The highest binding in a dose dependent manner was seen for biglycan, decorin, FMOD and OSAD (Fig. 4A,B), indicating that these SLRPs bound C1q more efficiently than the other proteins examined, including hIgG. Compared to these ligands, CHAD and lumican bound C1q appreciably less efficiently, while PRELP showed only negligible binding.
Biglycan, decorin, FMOD and OSAD showed similar binding (Fig. 4A,B) with saturation observed at C1q concentrations of around 6 μg/ml. The saturation point of the titration of aggregated hIgG with C1q also appeared to be close to 6 μg/ml. C1q appeared to bind more strongly to these five ligands, as indicated by the slopes of the curves, than to CHAD and lumican, for which absorbance values continued to increase with increasing C1q concentration up to the maximum concentration tested (20 μg/ml). PRELP showed insignificant C1q binding even at the highest concentration. Furthermore, FMOD in a solution efficiently prevented C1q binding to immobilized FMOD and the same was true for the other SLRPs that bound C1q appreciably (not shown).
Furthermore, interactions between C1q and SLRPs bound to the surface of the CM5 chip were studied using surface plasmon resonance. Since C1q may engage in interactions with SLRPs via multiple binding sites, calculations of association and dissociation rate constants were not feasible. Instead, C1q was injected over SLRPs, exemplified by OSAD in Fig. 4C, until saturation was reached (Fig 4C, inset). Equilibrium dissociation constant (KD) was derived from a binding curve showing response at equilibrium (Req) plotted against concentration (steady state affinity model). FMOD displayed the highest affinity (lowest KD) followed by OSAD (Fig. 4C), decorin and lumican (Table I). No interaction was detected between PRELP and C1q, while the interaction between CHAD and C1q although detectable was too weak to allow calculation of KD.
Table 1
AP: alternative pathway, CP: classical pathway, LP: lectin pathway
| SLRP Subclass | Name | Direct Binding | Activation | ||||
|---|---|---|---|---|---|---|---|
| C1q | KD (nM)* | FH | CP | AP | LP | ||
| I | Biglycan | +++ (tail) | n.d. | - | - | - | - |
| Decorin | +++ (tail) | 267 | - | - | - | - | |
| Fibromodulin | +++ (head) | 87 § | +++ | +++ | + | - | |
| II | Lumican | + (tail) | 590 | - | - | - | - |
| Osteoadherin | +++ (head) | 135 | + | +++ | ++ | - | |
| PRELP | + | no binding | - | + | - | - | |
| IV | Chondroadherin | + (head) | weak binding | ++ | - | - | - |
3.3. Binding of SLRPs to Heads and Tails of C1q
To substantiate the above findings, a “reverse” binding strategy was employed, where the binding of soluble SLRPs to immobilized C1q was investigated. Rather than using an array of antibodies with potentially different affinities and binding sites for detection, the SLRPs were labeled with 125I. We normalized the data, i.e. binding for each SLRP was expressed in terms of the number of counts retained in the wells, relative to the total number added (Fig. 5A). The counts obtained for full length C1q for each SLRP were then set to 1. Using this approach, it was possible to examine the relative interactions of each SLRP with C1q heads and tails (Fig. 5A). However, it is important to note that normalization abolished differences between absolute signal intensities of the various SLRPs.

A Wells of microtiter plates were coated with intact C1q, C1q head, C1q tail or BSA, blocked and then incubated with the 125I-labeled SLRPs indicated (50,000 counts per well). After washing, the numbers of counts retained were determined and values are expressed as the proportion of counts added. For each SLRP, the data were normalized against the average number of counts retained for intact C1q. The displayed data represent the mean of quadruplicate determinations (n=4), with error bars representing the standard deviation. Asterisks indicate significance (two-tailed Student’s T-test) accordingly: *, p < 0.05; ***, p < 0.001, ns - not significant. B SLRPs were coated as in the direct C1q-binding assay and C1q was added at 5 μg/ml in buffers with varying ionic strength or with 150 mM NaCl and 5 mM EDTA. Bound C1q was detected with specific antibodies. The wells for each SLRP were developed separately to allow for proper comparison between the different buffer conditions. The data were collected from two representative duplicate experiments (n=4) and error bars show standard deviation. In B all changes, as compared to the respective levels for 150 mM, are statistically significant except for the effect of EDTA on C1q binding to biglycan and decorin (two-tailed Student’s T-test).
All tested SLRPs bound both C1q head and tail regions to rather similar degree. However, FMOD appeared to bind clearly more efficiently to the globular head domains than to the collagenous stalk, while the reverse was true for lumican. Nonetheless, we can conclude that, in general, all SLRPs tested bind to C1q via structures that are present in the preparations of both the head and tail regions defined by proteolytic digestions using two different types of proteinases (Fig. 5A & B). To examine the nature of this interaction further, electron microscopy images of complexes formed between C1q and gold-labeled SLRPs were prepared. OSAD (Fig. 6B) and CHAD (Fig. 6C) appeared to bind only to the head region of C1q, as seen for FMOD earlier (Sjöberg et al., 2005). In contrast, decorin (Fig. 6D), biglycan (Fig. 6E) and lumican (Fig. 6F) showed binding primarily, if not exclusively, to the stalk region. Virtually all gold-particles could be found in contact with C1q. For each SLRP, at least 300 individual C1q interactions were examined visually. Ninety to 95 % of all molecules bound C1q heads or tail in the manner shown in the representative figure.
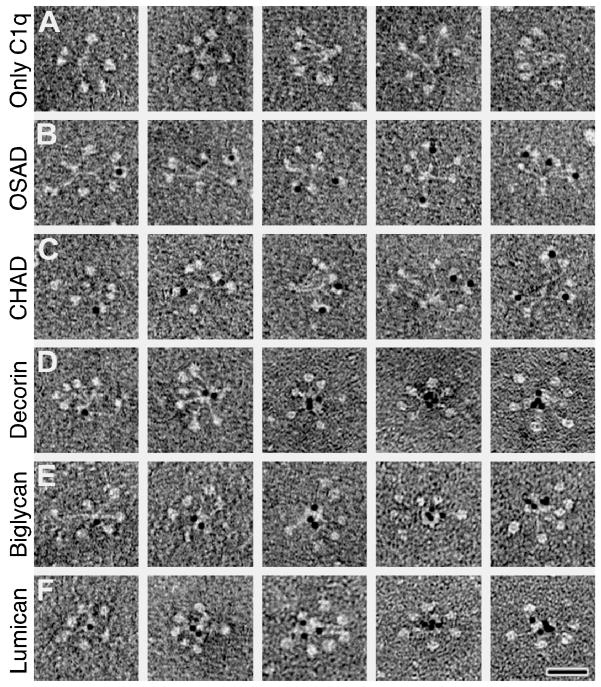
The complexes between C1q and gold-labeled SLRPs were visualized by negative staining. A Single molecules of C1q without any ligand. B Single molecules of C1q with one to three bound gold-labeled OSAD molecules. C Single molecules of C1q with one to three bound gold-labeled CHAD molecules. D Single molecules of C1q with one to four bound gold-labeled decorin molecules. E Single molecules of C1q with one to four bound gold-labeled biglycan molecules. F Single molecules of C1q with one to four bound gold-labeled lumican molecules. Ninety to 95 % of all interactions occurred according to the patterns presented (n ≥ 300 for each respective interaction). Scale bar is 25 nm.
All the interactions between SLRPs and C1q investigated were sensitive to increases in ionic strength, suggesting that binding includes electrostatic interactions (Fig. 5B). While the interactions between biglycan, decorin, lumican and C1q were entirely abolished at 300 mM NaCl, binding of C1q to FMOD, OSAD and particularly CHAD was still apparent at this ionic strength. When adding 5 mM EDTA to binding buffer at physiological ionic strength, a significant but minor decrease in interaction was detected for CHAD, FMOD, lumican and OSAD (Fig. 5B).
3.4. Binding of SLRPs to FH
Next, because of weak deposition of C9 in the complement activation assay, an investigation was made into whether the complement inhibitor FH interacts directly with the panel of SLRPs. Since FMOD is known to bind FH (Sjöberg et al., 2005), we hypothesized that other SLRPs may also do so. Indeed, using the solid phase approach in microtiter plates, we detected direct binding of FH to CHAD and OSAD (Fig. 7A). Although weak, the OSAD-FH interaction is significant as compared to the background level, both at 4 and 8 μg/ml FH. Neither biglycan, decorin, lumican nor PRELP bound FH (Fig. 7A).
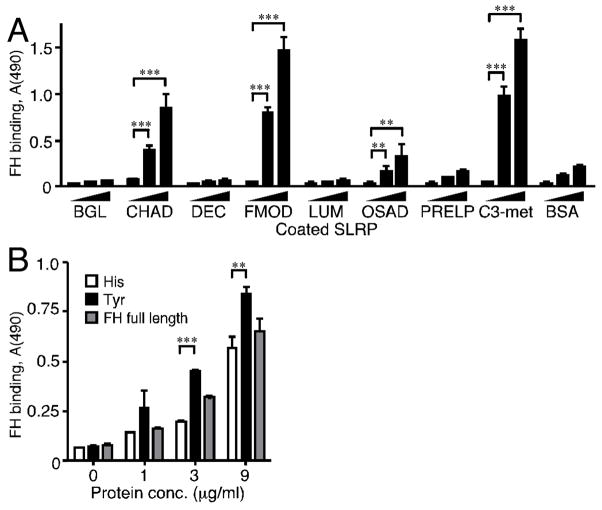
A The indicated SLRPs were immobilized in microtiter plates, the wells were blocked and incubated with two concentrations of full-length human FH. The amount of bound FH was assessed with a specific antibody. B CHAD was coated onto microtiter plates, and after blocking, either full length FH or one of two constructs representing polymorphic variants of CCP domains 6–8 of FH was allowed to bind. Bound protein was detected with a specific polyclonal antibody. In A the data were collected from two representative duplicate experiments (n=4), while B shows data from a representative experiment done in triplicate (n=3). Error bars denote standard deviation. Asterisks indicate significance accordingly: **, p < 0.01; ***, p < 0.001.
3.5. Binding of CHAD to polymorphic variants of FH
Since CHAD appeared to bind FH strongly we examined the nature of this interaction in more detail. Recently, attention has been drawn to a possible connection between a polymorphic variant of FH and the common eye disease age-related macular degeneration (AMD). Individuals with a histidine instead of a tyrosine in the 384 position of the amino acid sequence of FH (Day et al., 1988) run a higher risk of developing AMD than those who display the more common tyrosine variant (Hageman et al., 2005; Haines et al., 2005; Klein et al., 2005). Using a direct binding assay, we evaluated the binding of CHAD to recombinant constructs of the two polymorphic variants of FH. The AMD-associated variant showed significantly less binding to immobilized CHAD than the non-disease associated variant (Fig. 7B).
4. Discussion
The present study describes how SLRPs may modulate the activation of complement. We show that OSAD, like FMOD, is a potent activator of the classical pathway of complement in vitro. Furthermore, both these SLRPs trigger the activation of the alternative pathway, albeit at a low level. FMOD and OSAD bind both C1q and FH, which results in a prominent deposition of early complement components but a relatively lower terminal pathway activation (see Table I). Although our data clearly show that a number of SLRPs bind to C1q and/or FH, implying that these interactions modulate physiological functions, their consequences in health and disease require further investigation.
Based on sequence similarity, the SLRP-family can be divided into four sub-families (Fig. 1 and (Svensson et al., 2001)). Decorin and biglycan belong to one subfamily, as both have chondroitin/dermatan-sulfate chains in their N-terminal domains and show more than 50 % sequence identity. FMOD, OSAD, PRELP and lumican represent a second subfamily, while CHAD belongs to a third subfamily, with a different type of disulphide loop structure in its C-terminus. FMOD and lumican both contain N-terminal domains with sulfated tyrosine residues (Antonsson et al., 1991). This structural similarity is particularly interesting in light of the data presented here. FMOD binds C1q and is a strong activator of complement. However, its close structural relative lumican, with fewer tyrosine sulfate residues, binds C1q only weakly and shows no complement activation. This suggests that the relatively minor differences between the structures of FMOD, OSAD and lumican suffice for weaker C1q binding and lack of complement activation, in the case of the latter. PRELP, another member of the same family, with a basic N-terminal portion, shows negligible C1q binding. Most members of this second SLRP subfamily are expressed in many tissues and show similar expression patterns (Grover et al., 1995). OSAD however has thus far mainly been found in bone and is known to bind to osteoblasts (Rehn et al., 2006; Sommarin et al., 1998; Wendel et al., 1998). Thus, it is intriguing that we in the present study see such strong activity on OSAD, both in binding and complement activation assays. The higher bone turnover in osteoporosis and OA may be coupled to the inflammation present in these conditions, albeit not a prominent feature. As shown in the present study, both FMOD and OSAD interact mainly with C1q heads and trigger the complement cascade. This property may prove deleterious in inflammatory diseases where the integrity of the ECM is compromised by proteolytic degradation leading to increased release of the intact proteins, or fragments thereof. These then become exposed to complement factors and may promote complement activation and, hence, further opsonization of self-tissues, anaphylaxis and general inflammation.
Virtually all complement components can be found in healthy cartilage. For example, chondrocytes express several components of the classical pathway in vivo and their production is increased by cytokines (Bradley et al., 1996). This indicates that complement activity might be enhanced via expression of complement proteins within cartilage itself. Inflammatory mediators, which are abundant in disease may exacerbate the situation further. Elevated levels of complement activation products and increased consumption of C3 and C4 can be detected in synovial fluids of patients suffering from RA (Moxley and Ruddy, 1985; Okroj et al., 2007). However, it is unclear what triggers complement in arthritic joints and we propose that exposed or liberated FMOD and OSAD or fragments thereof may fuel the complement cascade in addition to other activators such as dying cells and their debris.
The EM images provide a clearer picture of how C1q binds SLRPs, compared with the binding assay results obtained using purified heads and tails. This may be due to the fact that proteolysis reactions used to generate the C1q head and tail species leave a small stretch of tail residues in the cleaved head fragment, and vice versa (Reid and Porter, 1976; Thielens et al., 1993). Furthermore, cryptic and even non-specific binding sites may be exposed when the C1q molecule is fragmented. These would not be exposed in the intact molecule used for electron microscopy. Taken together we propose that the C1-activating SLRPs bind mainly to C1q heads, as many other classical activators of C1q, while non-activators bind predominantly to the stalks, although part of the head may be involved in this interaction as well. The stalks bind C1s/C1r, which undergo conformational changes when ligands bind the globular head domains of C1q. The binding of biglycan and decorin in this region may perturb this process. Accordingly, we have confirmed previously published observation (Groeneveld et al., 2005) that decorin and biglycan bind more readily to C1q than to the whole C1 complex (Fig 2 vs Fig. 4). Furthermore, binding of biglycan and decorin to C1 inhibits activation of the classical pathway by antibodies (Groeneveld et al., 2005).
In the present study we show that FH not only binds FMOD but also OSAD and CHAD (Fig. 7A). We propose that binding of FH is the main factor responsible for the low deposition of C9 observed when complement is initiated by FM and OSAD in contrast to IgG. In agreement with this hypothesis we have previously shown that the low deposition of C9 upon activation by FM is accompanied by low deposition of all components of MAC and a decreased release of C5a (Sjöberg et al., 2005). This implies that the complement cascade is affected as early as the level of C5-convertase and possibly C3-convertase consistent with action of FH. The most plausible reason for the fact that we do not observe decrease in deposition of C3b is that the antibody used detects not only C3b but also iC3b that would occur as the result of FH binding. However, we can not exclude that additional mechanisms are responsible for the observed relative inefficiency of MAC assembly. Our previous study (Sjöberg et al., 2005) demonstrated that FH and C1q bind to different sites on FMOD. Whether these sites are exposed on the intact protein simultaneously, or whether fragments containing only one of these sites are released is not clear, but this pattern could be important in understanding the mechanisms and regulation of inflammation in diseases involving cartilage. The simultaneous interactions between SLRPs, FH and C1q are most likely not accidental. The three most potent activators of C1q all bind the inhibitor FH, while those that bind C1q but do not activate show no binding to FH. These two binding phenomena may coincide to avoid excessive complement activation. Furthermore, we have recently found that several SLRPs, including FMOD and OSAD, interact strongly and directly with C4BP, the other main soluble complement inhibitor (manuscript in preparation).
A noteworthy parallel in this context is the interaction between CRP and C1q. CRP readily activates the classical pathway of complement and is also one of the most abundant proteins in the circulation during acute-phase inflammation. CRP interacts both with FH (Aronen et al., 1992) and C4BP (Sjöberg et al., 2006) and is thus a prime example of a potent complement activator interacting directly with complement inhibitors. It is important to remember that even though the terminal pathway may be activated to a lesser extent than would be expected from the early events, the complement system still operates with considerable potency. For instance: release of C3a promotes anaphylaxis while C3b is a major opsonin promoting phagocytosis.
A number of studies have shown a clear association between AMD, a common eye disease, and the Y384H polymorphism in FH (Hageman et al., 2005; Haines et al., 2005). The AMD-associated variant of FH (His) binds CRP less strongly than the more common Tyr-variant (Laine et al., 2007; Sjöberg et al., 2007; Skerka et al., 2007). CHAD is expressed in the human eye (Johnson et al., 2006) and therefore the interaction between FH and CHAD was examined in more detail in the present study. We found that CHAD binds the disease-associated variant poorly compared to the Tyr-variant. This is consistent with what was observed previously for FMOD (Sjöberg et al., 2007). If CHAD and FMOD do indeed bind FH in the eye, we propose that the AMD-associated variant of FH has lower affinity for these SLRPs and therefore exerts a smaller effect on complement regulation compared with the non-disease-associated Tyr-variant.
Aberrant and misguided complement activation is the culprit in many acute and chronic diseases. For instance, complement activation contributes significantly to the development of ischemia injury, sepsis, AMD, SLE, multiple sclerosis and RA. In RA, articular cartilage is destroyed and during this process there is widespread release of cartilage constituents, such as SLRPs and their conventional binding partners. This, in turn, leads to the sudden generation, and release into synovial fluid and blood, of immune-reactive epitopes, the formation of immune complexes, and ultimately activation of complement. It is also acknowledged that, at the same time, other fragments may act as inhibitors of the cascade. Further understanding of the dual roles of these molecules in the complex activation of complement in disease should pave the way for new therapeutic endeavors. We believe that we have gone some way in unraveling the nature and effects of interactions between complement and extracellular matrix components, taking us a step closer to developing novel therapeutics aimed at combating the aberrant complement activation that contributes to debilitating disease in joints and cartilage.
Acknowledgments
This study was supported by grants from the Swedish Research Council, Swedish Foundation for Strategic research (INGVAR), National Institutes of Health (NIAMS), Foundations of Österlund, Kock, King Gustav V’s 80th Anniversary Foundation, Knut and Alice Wallenberg, Inga-Britt and Arne Lundberg and research grants from the University Hospitals in Malmö and Lund.
We acknowledge Viveka Tillgren for preparing recombinant osteoadherin and Jonatan Sjölander for excellent technical assistance.
The abbreviations used
- C4BP
- C4b-binding protein
- CCP
- complement control protein
- CRP
- C-reactive protein
- CHAD
- chondroadherin
- ECM
- extracellular matrix
- FH
- factor H
- FMOD
- fibromodulin
- KS
- keratan sulfate
- LPS
- lipopolysaccharide
- LRR
- leucine-rich repeat (protein family)
- MAC
- membrane attack complex
- NGF
- N-glycosidase F
- NHS
- normal human serum
- OA
- osteoarthritis
- OSAD
- osteoadherin
- PRELP
- proline-arginine-rich-end-leucine-rich repeat protein
- SLRP
- small leucine rich repeat protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonsson P, Heinegård D, Oldberg A. Posttranslational modifications of fibromodulin. J Biol Chem. 1991;266:16859–61. [Abstract] [Google Scholar]
- Aronen M, Lehto T, Meri S. Regulation of alternative pathway complement activation by an interaction of C-reactive protein with factor H. Immunology and Infectious Diseases. 1992;3:83–87. [Google Scholar]
- Baschong W, Lucocq JM, Roth J. “Thiocyanate gold”: small (2–3 nm) colloidal gold for affinity cytochemical labeling in electron microscopy. Histochemistry. 1985;83:409–11. [Abstract] [Google Scholar]
- Bing DH, Almeda S, Isliker H, Lahav J, Hynes RO. Fibronectin binds to the C1q component of complement. PNAS. 1982;79:4198–4201. [Europe PMC free article] [Abstract] [Google Scholar]
- Blom AM, Kask L, Dahlbäck B. CCP1–4 of the C4b-binding protein α-chain are required for Factor I mediated cleavage of C3b. Mol Immunol. 2003a;39:547–56. [Abstract] [Google Scholar]
- Blom AM, Villoutreix BO, Dahlbäck B. Mutations in α-chain of C4BP that selectively affect its Factor I cofactor function. J Biol Chem. 2003b;278:43437–43442. [Abstract] [Google Scholar]
- Bohnsack JF, Tenner AJ, Laurie GWKKH, Martin GR, Brown EJ. The C1q subunit of the first complement component binds to laminin: a mechanism for the deposition and retention of immune complexes in basement membrane. PNAS. 1985;82:3824–3828. [Europe PMC free article] [Abstract] [Google Scholar]
- Bradley K, North J, Saunders D, Schwaeble W, Jeziorska M, Woolley DE, Whaley K. Synthesis of classical pathway complement components by chondrocytes. Immunology. 1996;88:648–56. [Abstract] [Google Scholar]
- Clark SJ, Higman VA, Mulloy B, Perkins SJ, Lea SM, Sim RB, Day AJ. H384 allotypic variant of factor H associated with age-related macular degeneration has different heparin-binding properties from the non-disease-associated form. J Biol Chem. 2006;281:24713–20. [Abstract] [Google Scholar]
- Day AJ, Willis AC, Ripoche J, Sim RB. Sequence polymorphism of human complement factor H. Immunogenetics. 1988;27:211–4. [Abstract] [Google Scholar]
- Engel J, Furthmayr H. Electron microscopy and other physical methods for the characterization of extracellular matrix components: laminin, fibronectin, collagen IV, collagen VI, and proteoglycans. Methods Enzymol. 1987;145:3–78. [Abstract] [Google Scholar]
- Gaboriaud C, Thielens NM, Gregory LA, Rossi V, Fontecilla-Camps JC, Arlaud GJ. Structure and activation of the C1 complex of complement: unraveling the puzzle. Trends Immunol. 2004;25:368–73. [Abstract] [Google Scholar]
- Groeneveld TW, Oroszlan M, Owens RT, Faber-Krol MC, Bakker AC, Arlaud GJ, McQuillan DJ, Kishore U, Daha MR, Roos A. Interactions of the extracellular matrix proteoglycans decorin and biglycan with C1q and collectins. J Immunol. 2005;175:4715–23. [Abstract] [Google Scholar]
- Grover J, Chen XN, Korenberg JR, Roughley PJ. The human lumican gene. Organization, chromosomal location, and expression in articular cartilage. J Biol Chem. 1995;270:21942–9. [Abstract] [Google Scholar]
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–32. [Abstract] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. [Abstract] [Google Scholar]
- Heinegård D, Aspberg A, Franzén A, Lorenzo P. Non-collagenous glycoproteins in the extracellular matrix, with particular reference to cartilage and bone. In: Royce P, Steinmann B, editors. Connective tissue and its heritable disorders: molecular, genetic, and medical aspects. Wiley-Liss Inc; New York: 2002. [Google Scholar]
- Heinegård D, Larsson T, Sommarin Y, Franzen A, Paulsson M, Hedbom E. Two novel matrix proteins isolated from articular cartilage show wide distributions among connective tissues. J Biol Chem. 1986;261:13866–13872. [Abstract] [Google Scholar]
- Hildebrand A, Romaris M, Rasmussen LM, Heinegård D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302:527–34. [Europe PMC free article] [Abstract] [Google Scholar]
- Johnson JM, Young TL, Rada JA. Small leucine rich repeat proteoglycans (SLRPs) in the human sclera: identification of abundant levels of PRELP. Mol Vis. 2006;12:1057–66. [Abstract] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. [Europe PMC free article] [Abstract] [Google Scholar]
- Krumdieck R, Höök M, Rosenberg LC, Volanakis JE. The proteoglycan decorin binds C1q and inhibits the activity of C1 complex. J Immunol. 1992;149:3695–3701. [Abstract] [Google Scholar]
- Laine M, Jarva H, Seitsonen S, Haapasalo K, Lehtinen MJ, Lindeman N, Anderson DH, Johnson PT, Jarvela I, Jokiranta TS, Hageman GS, Immonen I, Meri S. Y402H polymorphism of complement factor H affects binding affinity to C-reactive protein. J Immunol. 2007;178:3831–6. [Abstract] [Google Scholar]
- Lucchini M, Couble ML, Romeas A, Staquet MJ, Bleicher F, Magloire H, Farges JC. Alpha v beta 3 integrin expression in human odontoblasts and co-localization with osteoadherin. J Dent Res. 2004;83:552–6. [Abstract] [Google Scholar]
- Mansson B, Wenglen C, Mörgelin M, Saxne T, Heinegård D. Association of chondroadherin with collagen type II. J Biol Chem. 2001;276:32883–8. [Abstract] [Google Scholar]
- Morgan BP, Walport MJ. Complement deficiency and disease. Immunol Today. 1991;12:301–6. [Abstract] [Google Scholar]
- Moxley G, Ruddy S. Elevated C3 anaphylatoxin levels in synovial fluids from patients with rheumatoid arthritis. Arthritis Rheum. 1985;28:1089–95. [Abstract] [Google Scholar]
- Okroj M, Heinegård D, Holmdahl R, Blom AM. Rheumatoid arthritis and the complement system. Ann Med. 2007;39:517–30. [Abstract] [Google Scholar]
- Önnerfjord P, Heathfield TF, Heinegård D. Identification of tyrosine sulfation in extracellular leucine-rich repeat proteins using mass spectrometry. J Biol Chem. 2004;279:26–33. [Abstract] [Google Scholar]
- Paques EP, Huber R, Preiss H, Wright JK. Isolation of the globular region of the subcomponent q of the C1 component of complement. Hoppe Seylers Z Physiol Chem. 1979;360:177–183. [Abstract] [Google Scholar]
- Rehn AP, Chalk AM, Wendel M. Differential regulation of osteoadherin (OSAD) by TGF-beta1 and BMP-2. Biochem Biophys Res Commun. 2006;349:1057–64. [Abstract] [Google Scholar]
- Reid KB, Porter RR. Subunit composition and structure of subcomponent Clq of the first component of human complement. Biochem J. 1976;155:19–23. [Europe PMC free article] [Abstract] [Google Scholar]
- Schedel J, Wenglen C, Distler O, Muller-Ladner U, Scholmerich J, Heinegard D, Krenn V. Differential adherence of osteoarthritis and rheumatoid arthritis synovial fibroblasts to cartilage and bone matrix proteins and its implication for osteoarthritis pathogenesis. Scand J Immunol. 2004;60:514–23. [Abstract] [Google Scholar]
- Shen Z, Gantcheva S, Sommarin Y, Heinegard D. Tissue distribution of a novel cell binding protein, osteoadherin, in the rat. Matrix Biol. 1999;18:533–42. [Abstract] [Google Scholar]
- Sjöberg A, Önnerfjord P, Mörgelin M, Heinegård D, Blom AM. The extracellular matrix and inflammation: fibromodulin activates the classical pathway of complement by directly binding C1q. J Biol Chem. 2005;280:32301–8. [Abstract] [Google Scholar]
- Sjöberg AP, Trouw LA, Clark SJ, Sjölander J, Heinegård D, Sim RB, Day AJ, Blom AM. The factor H variant associated with age-related macular degeneration (His-384) and the non-disease-associated form bind differentially to C-reactive protein, fibromodulin, DNA, and necrotic cells. J Biol Chem. 2007;282:10894–900. [Abstract] [Google Scholar]
- Sjöberg AP, Trouw LA, McGrath FD, Hack CE, Blom AM. Regulation of complement activation by C-reactive protein: targeting of the inhibitory activity of C4b-binding protein. J Immunol. 2006;176:7612–20. [Abstract] [Google Scholar]
- Skerka C, Lauer N, Weinberger AA, Keilhauer CN, Suhnel J, Smith R, Schlotzer-Schrehardt U, Fritsche L, Heinen S, Hartmann A, Weber BH, Zipfel PF. Defective complement control of Factor H (Y402H) and FHL-1 in age-related macular degeneration. Mol Immunol. 2007;44:3398–406. [Abstract] [Google Scholar]
- Sommarin Y, Wendel M, Shen Z, Hellman U, Heinegård D. Osteoadherin, a cell-binding keratan sulfate proteoglycan in bone, belongs to the family of leucine-rich repeat proteins of the extracellular matrix. J Biol Chem. 1998;273:16723–9. [Abstract] [Google Scholar]
- Soo C, Hu FY, Zhang X, Wang Y, Beanes SR, Lorenz HP, Hedrick MH, Mackool RJ, Plaas A, Kim SJ, Longaker MT, Freymiller E, Ting K. Differential expression of fibromodulin, a transforming growth factor-beta modulator, in fetal skin development and scarless repair. Am J Pathol. 2000;157:423–33. [Europe PMC free article] [Abstract] [Google Scholar]
- Sorvillo J, Gigli I, Pearlstein E. Fibronectin binding to complement subcomponent C1q. Biochem J. 1985:207–215. [Europe PMC free article] [Abstract] [Google Scholar]
- Svensson L, Oldberg A, Heinegård D. Collagen binding proteins. Osteoarthritis Cartilage. 2001;9 Suppl A:S23–8. [Abstract] [Google Scholar]
- Tenner AJ, Lesavre PH, Cooper NR. Purification and radiolabeling of human C1q. J Immunol. 1981;27:648–53. [Abstract] [Google Scholar]
- Thielens NM, Bally IM, Ebenbichler CF, Dierich MP, Arlaud GJ. Further characterization of the interaction between the C1q subcomponent of human C1 and the transmembrane envelope glycoprotein gp41 of HIV-1. J Immunol. 1993;151:6583–92. [Abstract] [Google Scholar]
- Walport MJ. Complement. First of two parts. N Engl J Med. 2001a;344:1058–1066. [Abstract] [Google Scholar]
- Walport MJ. Complement. Second of two parts. N Engl J Med. 2001b;344:1140–1144. [Abstract] [Google Scholar]
- Wendel M, Sommarin Y, Heinegård D. Bone matrix proteins: isolation and characterization of a novel cell-binding keratan sulfate proteoglycan (osteoadherin) from bovine bone. J Cell Biol. 1998;141:839–47. [Europe PMC free article] [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.molimm.2008.09.018
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2760063?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.molimm.2008.09.018
Article citations
Metformin prevents the onset and progression of intervertebral disc degeneration: New insights and potential mechanisms (Review).
Int J Mol Med, 54(2):71, 04 Jul 2024
Cited by: 0 articles | PMID: 38963023 | PMCID: PMC11232665
Review Free full text in Europe PMC
The role of the complement system in disc degeneration and Modic changes.
JOR Spine, 7(1):e1312, 02 Feb 2024
Cited by: 4 articles | PMID: 38312949 | PMCID: PMC10835744
Review Free full text in Europe PMC
Molecular cues for immune cells from small leucine-rich repeat proteoglycans in their extracellular matrix-associated and free forms.
Matrix Biol, 123:48-58, 02 Oct 2023
Cited by: 6 articles | PMID: 37793508 | PMCID: PMC10841460
Review Free full text in Europe PMC
Human theca arises from ovarian stroma and is comprised of three discrete subtypes.
Commun Biol, 6(1):7, 04 Jan 2023
Cited by: 4 articles | PMID: 36599970 | PMCID: PMC9812973
Contribution of animal models to the mechanistic understanding of Alternative Pathway and Amplification Loop (AP/AL)-driven Complement-mediated Diseases.
Immunol Rev, 313(1):194-216, 06 Oct 2022
Cited by: 2 articles | PMID: 36203396 | PMCID: PMC10092198
Review Free full text in Europe PMC
Go to all (80) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Complement inhibitor C4b-binding protein interacts directly with small glycoproteins of the extracellular matrix.
J Immunol, 182(3):1518-1525, 01 Feb 2009
Cited by: 34 articles | PMID: 19155499
The extracellular matrix and inflammation: fibromodulin activates the classical pathway of complement by directly binding C1q.
J Biol Chem, 280(37):32301-32308, 26 Jul 2005
Cited by: 102 articles | PMID: 16046396
Degradation of small leucine-rich repeat proteoglycans by matrix metalloprotease-13: identification of a new biglycan cleavage site.
Arthritis Res Ther, 8(1):R26, 03 Jan 2006
Cited by: 64 articles | PMID: 16507124 | PMCID: PMC1526547
Structural correlations in the family of small leucine-rich repeat proteins and proteoglycans.
J Struct Biol, 155(2):294-305, 19 May 2006
Cited by: 126 articles | PMID: 16884925
Review
Funding
Funders who supported this work.
NIAMS NIH HHS (2)
Grant ID: U01 AR050926-04
Grant ID: U01 AR050926-05




