Abstract
Objective
To systematically review evidence bearing on the management of patients with amyotrophic lateral sclerosis (ALS).Methods
The authors analyzed studies from 1998 to 2007 to update the 1999 practice parameter. Topics covered in this section include slowing disease progression, nutrition, and respiratory management for patients with ALS.Results
The authors identified 8 Class I studies, 5 Class II studies, and 43 Class III studies in ALS. Important treatments are available for patients with ALS that are underutilized. Noninvasive ventilation (NIV), percutaneous endoscopic gastrostomy (PEG), and riluzole are particularly important and have the best evidence. More studies are needed to examine the best tests of respiratory function in ALS, as well as the optimal time for starting PEG, the impact of PEG on quality of life and survival, and the effect of vitamins and supplements on ALS.Recommendations
Riluzole should be offered to slow disease progression (Level A). PEG should be considered to stabilize weight and to prolong survival in patients with ALS (Level B). NIV should be considered to treat respiratory insufficiency in order to lengthen survival (Level B) and to slow the decline of forced vital capacity (Level B). NIV may be considered to improve quality of life (Level C) [corrected].Early initiation of NIV may increase compliance (Level C), and insufflation/exsufflation may be considered to help clear secretions (Level C).Free full text

Practice Parameter update: The care of the patient with amyotrophic lateral sclerosis: Drug, nutritional, and respiratory therapies (an evidence-based review)
Associated Data
Abstract
Objective:
To systematically review evidence bearing on the management of patients with amyotrophic lateral sclerosis (ALS).
Methods:
The authors analyzed studies from 1998 to 2007 to update the 1999 practice parameter. Topics covered in this section include slowing disease progression, nutrition, and respiratory management for patients with ALS.
Results:
The authors identified 8 Class I studies, 5 Class II studies, and 43 Class III studies in ALS. Important treatments are available for patients with ALS that are underutilized. Noninvasive ventilation (NIV), percutaneous endoscopic gastrostomy (PEG), and riluzole are particularly important and have the best evidence. More studies are needed to examine the best tests of respiratory function in ALS, as well as the optimal time for starting PEG, the impact of PEG on quality of life and survival, and the effect of vitamins and supplements on ALS.
Recommendations:
Riluzole should be offered to slow disease progression (Level A). PEG should be considered to stabilize weight and to prolong survival in patients with ALS (Level B). NIV should be considered to treat respiratory insufficiency in order to lengthen survival (Level B), and may be considered to slow the decline of forced vital capacity (Level C) and improve quality of life (Level C). Early initiation of NIV may increase compliance (Level C), and insufflation/exsufflation may be considered to help clear secretions (Level C).
GLOSSARY
- AAN
- = American Academy of Neurology;
- ALS
- = amyotrophic lateral sclerosis;
- FVC
- = forced vital capacity;
- HFCWO
- = high frequency chest wall oscillation;
- MIE
- = mechanical insufflation/exsufflation;
- MIP
- = maximal inspiratory pressure;
- NIV
- = noninvasive ventilation;
- PCEF
- = peak cough expiratory flow;
- Pdi
- = transdiaphragmatic pressure;
- PEG
- = percutaneous endoscopic gastrostomy;
- QOL
- = quality of life;
- RIG
- = radiologically inserted device;
- SNP
- = sniff nasal pressure;
- TIV
- = tracheostomy invasive ventilation.
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by loss of motor neurons in the spinal cord, brainstem, and motor cortex. The cause of the disease is still not known. ALS is not curable, but a number of important therapies are available. In 1999, the American Academy of Neurology (AAN) published an evidence-based practice parameter for managing patients with ALS.1 Since that publication, there have been some important new studies, including a randomized controlled trial of noninvasive ventilation in ALS.2 Although only 1 drug, riluzole, has shown modest benefit and received US Food and Drug Administration approval (see below), there have been advances in symptomatic treatment for patients with this disease. In this revision, we update the riluzole practice advisory and address other management issues for care of patients with ALS. This article addresses riluzole, lithium, nutrition, and respiratory care, while a companion article addresses breaking the news, symptom management, palliative care, cognitive and behavioral impairment, multidisciplinary clinics, and communication.
DESCRIPTION OF THE ANALYTIC PROCESS
We searched OVID, MEDLINE EMBASE, CINAHL, Science Citation Index, BIOETHICSLINE, International Pharmaceutical Abstracts (IPAB), OVID Current contents, Medline-ProQuest, EIFL, and INVEST from 1998 though September 2007, combining the words ALS, Lou Gehrig’s disease, and motor neuron disease with the following words using AND: respiratory, respiratory failure, respiratory insufficiency, nutrition, enteral nutrition, malnutrition, weight loss, gastrostomy, clinical trials, mechanical insufflation-exsufflation, high frequency chest wall oscillation, Vest, Bipap, tracheostomy ventilation, dysphagia, mechanical ventilation, noninvasive ventilation, hypoventilation, bronchial secretions, sleep-disordered breathing, and breath stacking. We reviewed the abstracts of these articles and examined 142 articles in their entirety. The diagnostic and therapeutic classification schemes used to grade the articles are summarized in appendices e-3a and e-3b on the Neurology® Web site at www.neurology.org. Recommendations were based on the levels of evidence as described in appendix e-4.
ANALYSIS OF THE EVIDENCE
Slowing the disease process.
What is the effect of riluzole on slowing the disease process or prolonging survival in ALS?
Riluzole is approved for slowing disease progress in ALS. This drug was the subject of a practice advisory published by the AAN in 1997.3 The practice advisory recommended riluzole 50 mg BID to prolong survival for those with definite or probable ALS less than 5 years duration, with forced vital capacity (FVC) >60%, and without tracheostomy (Level A). Expert opinion suggested potential benefit for those with suspected or possible ALS with symptoms longer than 5 years, FVC <60%, and tracheostomy for prevention of aspiration only. Since 1997, 2 other controlled clinical trials have been published (Class I)4,5 and all of the available evidence has been reviewed.6 Riluzole has a modest beneficial effect in slowing disease progression (prolonged survival of 2–3 months) based on 4 Class I trials. The number needed to treat to delay 1 death until after 12 months was 11. However, 5 studies using large databases spanning 5 to 10 years have suggested that treatment with riluzole might be associated with a prolonged survival of 6 months (Class II),7 10 months (Class III),8 12 months (Class III),9 14 months (Class III),10 or even 21 months (Class III).11 These cohort studies had longer-term follow-up than the clinical trials, but are subject to greater bias. After 10 years of patient experience, the drug appears to be safe but expensive. Fatigue and nausea are known side effects.
Conclusion.
Riluzole is safe and effective for slowing disease progression to a modest degree in ALS (4 Class I studies).
Recommendation.
Riluzole should be offered to slow disease progression in patients with ALS (Level A).
Does lithium carbonate prolong survival or slow disease progression in ALS?
A trial of lithium carbonate in ALS compared 16 patients treated with riluzole and lithium carbonate with 28 patients treated with riluzole alone (Class III).12 Mortality was lower and disease progression was slower in treated patients. The small sample size, lack of adequate blinding, and other design issues are of great concern.
Conclusion.
There are inadequate data on the effectiveness of lithium carbonate (1 Class III study).
Recommendation.
There are insufficient data at this time to support or refute treatment with lithium carbonate in patients with ALS (Level U).
Nutrition.
In ALS, factors that restrict adequate nutrition develop insidiously and progressively worsen. The functional consequences are choking, aspiration, weight loss, and dehydration. Dysphagia is a symptom experienced by the patient and is prima facie evidence of swallowing dysfunction. Videofluoroscopic evaluation of the swallowing mechanism may identify food textures that can be handled successfully. However, it is not a required test to establish the presence or absence of dysphagia.
Strategies to maintain oral nutritional intake consist of altering food consistency and using nutritional supplements. Ultimately, a percutaneous endoscopic gastrostomy (PEG) or equivalent device (e.g., radiologically inserted device [RIG]) may be needed as an alternative route for delivering nutrition (figure 1). It is important to emphasize to patients that PEG does not eliminate oral feeding but offers a convenient method for administering medication and fluid and stabilizing weight.13
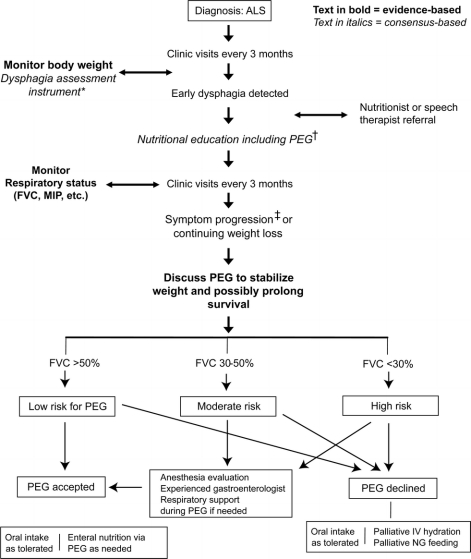
Figure 1 Nutrition management algorithm
*e.g., Bulbar questions in the Amyotrophic Lateral Sclerosis Functional Rating Scale, or other instrument. †Prolonged meal time; ending meal prematurely because of fatigue; accelerated weight loss due to poor caloric intake; family concern about feeding difficulties. ‡Percutaneous endoscopic gastrostomy: rule out contraindications.
What is the effect of enteral nutrition administered via PEG on weight stability?
In 9 studies, a total of 469 patients with ALS received enteral nutrition via PEG.14–22 Using patients as their own controls, 7 Class III studies demonstrated either weight stabilization or modest weight gain over 2–24 months.14–16,18,19,21,22 In 2 Class II studies17,20 in which PEG refusers served as controls, weight stabilization was demonstrated in the PEG group vs continued weight loss in controls (p = 0.03).
Conclusion.
Enteral nutrition administered via PEG is probably effective in stabilizing body weight/body mass index (2 Class II, 7 Class III studies).
Recommendation.
In patients with ALS with impaired oral food intake, enteral nutrition via PEG should be considered to stabilize body weight (Level B).
When is PEG indicated in ALS?
We found no studies that provide ALS-specific indications for PEG. The risk of PEG placement increased when the FVC declined below 50% of predicted (Class III).14 Risks of PEG placement include laryngeal spasm, localized infection, gastric hemorrhage, failure to place PEG due to technical difficulties, and death due to respiratory arrest.20,23
Conclusions.
There are no studies of ALS-specific indications for the timing of PEG insertion, although patients with dysphagia will possibly be exposed to less risk if PEG is placed when FVC is above 50% of predicted (1 Class III study).14
Recommendation.
There are insufficient data to support or refute specific timing of PEG insertion in patients with ALS (Level U).14
What is the efficacy of nutritional support via PEG in prolonging survival?
Two Class II and 7 Class III studies compared survival in patients receiving PEG (n = 585) vs those without PEG (n = 1619). One Class III study demonstrated a survival advantage vs control with multivariate analysis (p = 0.02) but not with univariate analysis (p = 0.09).16 A Class III population-based study from Italy found improved survival with PEG compared to patients with oral intake, also based on a multivariate analysis (3.89-fold; p = 0.0004).24 Two Class II studies demonstrated prolonged survival in the PEG group vs PEG refusers.17,20 Shaw et al.25 found similar results when patients with PEG were compared to nasogastric-fed controls (p = 0.03) (Class III). However, 4 Class III studies failed to find a significant survival benefit with PEG.19,21,23,26 All but one26 of the negative studies included patients not needing PEG as a control group. The positive studies used controls that refused PEG (Class II)17,20 or used a risk model and multivariate analysis based on factors that predicted survival (statistically controlling for confounders) (Class III).16,24
Conclusions.
Studies using appropriate controls or multivariate analysis demonstrated that PEG is probably effective in prolonging survival in ALS, although insufficient data exist to quantitate the survival advantage (2 Class II studies).
Recommendation.
PEG should be considered for prolonging survival in patients with ALS (Level B).
What is the effect of enteral nutrition delivered via PEG on quality of life?
There is no evidence regarding the effect of PEG on quality of life.
Conclusion.
No evidence exists regarding the effect of enteral nutrition on quality of life.
Recommendation.
There are insufficient data to support or refute PEG for improving quality of life in patients with ALS (Level U).
What is the efficacy of vitamin and nutritional supplements on prolonging survival or quality of life?
High-dose vitamins, minerals, and other nutriceuticals are used by more than 79% of patients with ALS (Class III).27 Only creatine and vitamin E have been examined for efficacy.
Creatine.
Creatine at 10 g/day and at 5 g/day failed to alter survival or the rate of functional decline of patients with ALS (Class I).28,29
Vitamin E.
Two studies failed to find benefit of vitamin E therapy in subjects treated concurrently with riluzole. Desnuelle et al.30 treated 144 participants with alpha-tocopherol (1,000 mg daily) and an equal number of controls. Vitamin E treatment did not slow the rate of functional deterioration (Class I); however, the progression to more severe states of ALS was slower (p = 0.045). Another study of 5,000 mg/day vitamin E plus riluzole vs riluzole alone found no change in survival or functional outcomes (Class I).31
Conclusions
Creatine, in doses of 5–10 g daily, is established as ineffective in slowing the rate of progression or in improving survival in ALS (2 Class I studies).
Vitamin E 5,000 mg/day plus riluzole is probably ineffective in improving survival or functional outcomes (1 Class I study). Vitamin E (1,000 mg/day plus riluzole) was marginally effective in slowing the progression of ALS from milder to more severe ALS health states using a single measure but is ineffective using multiple other measures (1 Class I study).
Recommendations.
Creatine, in doses of 5–10 g daily, should not be given as treatment for ALS because it is not effective in slowing disease progression (Level A). High-dose vitamin E should not be considered as treatment for ALS (Level B), while the equivocal evidence regarding low-dose vitamin E permits no recommendation (Level U).
Respiratory management.
The diagnosis and management of respiratory insufficiency is critical because most deaths from ALS are due to respiratory failure. Published guidelines for respiratory care were based on clinical experience, expert opinion, and observational research.1,32,33 While many questions remain unanswered, there have recently been several controlled studies that provide evidence to guide management (figure 2).
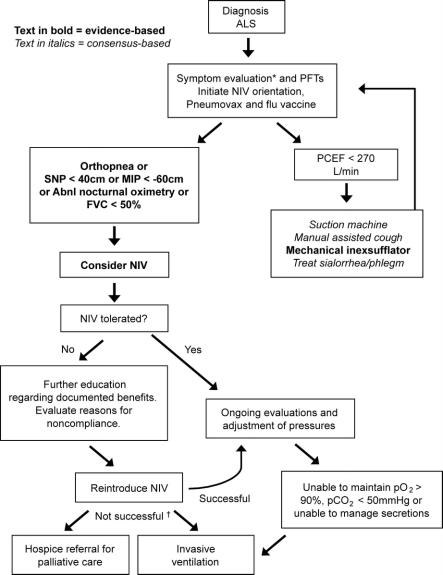
Figure 2 Respiratory management algorithm
PFT = pulmonary function tests; PCEF = peak cough expiratory flow; NIV = noninvasive ventilation; SNP = sniff nasal pressure; MIP = maximal inspiratory pressure; FVC = forced vital capacity (supine or erect); Abnl.nocturnal oximetry = pO2 <4% from baseline. *Symptoms suggestive of nocturnal hypoventilation: frequent arousals, morning headaches, excessive daytime sleepiness, vivid dreams. †If NIV is not tolerated or accepted in the setting of advancing respiratory compromise, consider invasive ventilation or referral to hospice.
What are the optimal pulmonary tests to detect respiratory insufficiency?
FVC is the most commonly used respiratory measurement in ALS,34 and it was a significant predictor of survival (Class III).35 FVC may be insensitive since 13/20 patients with an FVC >70% had abnormal maximal inspiratory pressure (MIP) <−60 cm (Class III).36
Nocturnal desaturations <90% for 1 cumulative minute was a more sensitive indicator of nocturnal hypoventilation than either FVC or MIP (Class III).36 FVC correlated poorly with symptoms of nocturnal hypoventilation and desaturation (Class III).37 Nocturnal oximetry correlated with survival (Class IV)38 (mean O2 saturations <93 mm Hg were associated with mean survival of 7 months vs 18 months when mean O2 saturation >93 mm Hg).
Supine FVC, although more difficult to perform, may be a better predictor of diaphragm weakness than erect FVC. FVC closely correlated with transdiaphragmatic pressure (Pdi), and a supine FVC <75% reliably predicted an abnormally low Pdi (Class III).39 Further, the difference between erect and supine FVC correlated with orthopnea (Class III).40
The sniff transdiaphragmatic pressure (sniff Pdi) detected hypercapnia (earlobe blood gas CO2 tension >6 kPa [normal <6]) with a sensitivity of 90% and a specificity of 87% (Class III).e1 Sniff nasal pressure (SNP) showed greater predictive power than either FVC or MIP. The sniff Pdi and the percent predicted SNP were both correlated with the apnea/hypopnea index on polysomnography. No test had predictive power in patients with bulbar weakness. SNP <40 cm H2O correlated with nocturnal hypoxemia (Class III).e2 When SNP was less than 30 cm, median survival was 3 months. In addition, SNP was more reliably recorded at later stages of ALS than either FVC or MIP.
Elevated bicarbonate and low serum chloride correlated with respiratory symptoms and were predictive of death within 5 months in 8/10 patients (Class III).e3 Bach et al. (Class III)e4 showed that tracheostomy or death was highly likely within 2 months of a decrease in daytime SpO2 <95% that could not be corrected by noninvasive ventilation (NIV).
The peak cough expiratory flow (PCEF) remains the most widely used measure of cough effectiveness. Patients with a mean PCEF above 337 L/min had a significantly greater chance of being alive at 18 months (Class III).e5
Conclusions
Nocturnal oximetry and MIP are possibly more effective in detecting early respiratory insufficiency than erect FVC (2 Class III studies).
Supine FVC is possibly more effective than erect FVC in detecting diaphragm weakness and correlates better with symptoms of nocturnal hypoventilation (2 Class III studies).
Sniff Pdi and SNP are possibly effective in detecting hypercapnia and nocturnal hypoxemia (2 Class III studies).
Recommendations
Nocturnal oximetry may be considered to detect hypoventilation (regardless of the FVC) (Level C).
Supine FVC and MIP may be considered useful in routine respiratory monitoring, in addition to the erect FVC (Level C).
SNP may be considered to detect hypercapnia and nocturnal hypoxemia (Level C).
Does NIV improve respiratory function or increase survival?
In a randomized controlled study, patients using NIV experienced a median survival benefit of 205 days (Class I).2 NIV was initiated based on orthopnea with an MIP <−60 cm or symptomatic hypercapnia. No survival benefit was seen in patients with poor bulbar function.
“Early” intervention with NIV (nocturnal oximetry demonstrating >15 desaturation events/hour) resulted in 11 months longer survival compared to controls, with some beneficial effect in bulbar patients (Class III).e6 Patients who used NIV >4 hours/day survived 7 months longer than patients using the device <4 hours/day (Class III).e7
FVC declined more slowly after introducing NIV (pre −2.2%/month compared to post −1.1%/month) (Class I/III)2 and the decline was slower in those who used NIV >4 hours/day (Class III).e7 A survival benefit of 20 months was observed in NIV-tolerant patients vs 5 months in NIV-intolerant patients (Class III).e8
Conclusion.
NIV is probably effective in prolonging survival (1 Class I, 3 Class III studies) and in slowing the rate of FVC decline (1 Class I, 1 Class III study).
Recommendation.
NIV should be considered to treat respiratory insufficiency in ALS, both to lengthen survival and to slow the rate of FVC decline (Level B).
How do invasive and noninvasive ventilation affect quality of life?
NIV had a positive impact on quality of life (QOL) in 4 Class III studies.36,e9-e11 There was improvement in energy, vitality,e9,e10 shortness of breath, daytime somnolence, depression, concentration problems, sleep quality, and physical fatigue for 10 months or more.e11 In one Class III study,2 patients using NIV had increased duration of QOL above 75% of baseline and increased time-weighted mean improvement in QOL.
There was no difference in QOL between those using NIV and patients with tracheostomy invasive ventilation (TIV) (Class III).e12 Most patients using either NIV (94%) or TIV (81%) would choose ventilation again. Caregivers of patients using TIV, however, rated their own QOL lower than that of their patient. Another series of 7 patients using TIV rated their general health as good based on the SF-12® Health Survey and none of the patients regretted his/her decision (Class III).e13
Conclusions
NIV is possibly effective in raising QOL for patients with ALS who have respiratory insufficiency (5 Class III studies).
TIV is possibly effective in preserving QOL for patients with ALS, but possibly with a greater burden for their caregivers (2 Class III studies).
Recommendations
NIV may be considered to enhance QOL in patients with ALS who have respiratory insufficiency (Level C).
TIV may be considered to preserve QOL in patients with ALS who want long-term ventilatory support (Level C).
What factors influence acceptance of invasive and noninvasive ventilation?
Compliance with NIV was improved when treatment was initiated early based on the presence of at least 15 desaturation events per hour (Class III).e6 A randomized pilot trial of early NIV intervention (2 desaturation events <90% for 1 cumulative minute, mean FVC = 77%) showed that 7 of 7 patients were compliant (Class III).36 This degree of compliance is much higher than prior reports (Class III)e14 of less than 50% when NIV was initiated based on prior recommendations.1
Noncompliance with NIV was seen in 75% of patients with ALS and frontotemporal dysfunction vs 38% in patients with classic ALS (relative risk 2.0) (Class III).e15 There was low compliance in bulbar patients (Class III)2,e16 but cognitive/executive function was not described.
Orthopnea was a strong predictor of benefit and also better compliance with NIV (Class III).e9 NIV use correlated with symptoms of orthopnea and dyspnea as well as with the use of PEG, speech devices, and riluzole (Class III).e17 Young age and preserved upper limb function also predicted better compliance.
Conclusions
Nocturnal oximetry is possibly effective in detecting early respiratory insufficiency and the early use of NIV possibly increases compliance (2 Class III studies).
Bulbar involvement and executive dysfunction possibly lower compliance with NIV (2 Class III studies).
Recommendation.
NIV may be considered at the earliest sign of nocturnal hypoventilation or respiratory insufficiency in order to improve compliance with NIV in patients with ALS (Level C).
What is the efficacy of targeted respiratory interventions for clearing secretions?
Expiratory respiratory muscle weakness can lead to ineffective cough, retained upper airway secretions, and pulmonary infection. PCEFs greater than 160 L/min are needed to clear secretions,e18 and clinicians recommend assistive devices when the PCEF falls below 270 L/min (Class III).e19
Mechanical insufflation/exsufflation (MIE) increased the PCEF by 17% in healthy controls, 26% in bulbar patients, and 28% in nonbulbar patients (Class III).e20 Manually assisted cough increased flow by 11% in bulbar and 13% in nonbulbar patients.
MIE via tracheostomy tube and an inflated cuff was more effective in eliminating airway secretions than ordinary suctioning (Class III).e21 SpO2, peak inspiratory pressure, mean airway pressure, and work of breathing all improved and patients reported that MIE was more comfortable and effective.
High frequency chest wall oscillation (HFCWO) is an alternative approach to clearing airway secretions that was effective in patients with cystic fibrosis.e22,e23 HFCWO in 9 patients with ALS showed no benefit in rate of decline of FVC or survival (Class III).e24 In a controlled study of 46 patients, HFCWO users had less breathlessness and fatigue but coughed more at night (Class III).e25
Conclusions
MIE is possibly effective for clearing upper airway secretions in patients with ALS who have reduced peak cough flow, although the clinically meaningful difference is unknown (4 Class III studies).
HFCWO is unproven for adjunctive airway secretion management (2 Class III studies with conflicting results).
Recommendations
MIE may be considered to clear secretions in patients with ALS who have reduced peak cough flow, particularly during an acute chest infection (Level C).
There are insufficient data to support or refute HFCWO for clearing airway secretions in patients with ALS (Level U).
Clinical context.
Medications with mucolytics like guaifenesin or N-acetylcysteine, a B-receptor antagonist (such as metoprolol or propanolol), nebulized saline, or an anticholinergic bronchodilator such as ipratropium are widely used; however, no controlled studies exist in ALS.
CLINICAL CONTEXT
This evidence-based review indicates some progress in evaluating new therapies for patients with ALS. More high-quality studies have been reported leading to more confident recommendations regarding the value of NIV and PEG.
It is one thing to publish an evidence-based practice parameter for the management of patients with ALS, and it is quite another to be able to track adherence in practice and to determine whether the publication of evidence-based guidelines has changed outcomes. The ALS patient CARE database was developed with the hope of standardizing new and effective therapies for patients with ALS and tracking outcomes to raise the standard of care.e26 Data obtained from the ALS CARE program have shown that the underutilization of many therapies (especially PEG and NIV) has persisted in the years since the original practice parameter on this topic, though there have been gains. These findings suggest that an evidence-based practice parameter may over time become more widely accepted and change practice. However, the persistent underutilization of therapies that improve survival and quality of life poses a challenge for ALS clinicians to continue to raise the standard of care for patients with ALS.
RECOMMENDATIONS FOR FUTURE RESEARCH
Lithium carbonate.
Study whether lithium slows disease progression or prolongs survival in ALS in larger clinical trials.
Nutrition
Develop ALS-specific indications for nutritional adequacy in ALS and for PEG and RIG.
Study the optimal timing of nutritional therapy administered via PEG or RIG.
Conduct clinical studies of novel antioxidants and supplements.
Respiratory management
Evaluate SNP as a criterion for NIV initiation.
Evaluate the impact of early NIV initiation on survival and quality of life.
Assess the impact of executive dysfunction on NIV compliance.
Evaluate the effect of hypoventilation on executive dysfunction.
Compare techniques for clearing upper airway secretions at various stages of respiratory and bulbar dysfunction.
Evaluate pulmonary tests, compliance with NIV, and outcomes in patients with bulbar dysfunction.
ACKNOWLEDGMENT
The authors thank additional members of the ALS Practice Parameter Task Force: Thomas Getchius, AAN staff; Gary Gronseth, MD; Dan H. Moore, PhD; Sharon Matland; Valerie Cwik; Larry Brower; and Sid Valo.
DISCLOSURE
Dr. Miller serves on the editorial board of the ALS Journal; received a speaker honorarium from the AANEM; served as a consultant to Celgene, Knopp Neurosciences Inc., Teva Pharmaceutical Industries Ltd., Taiji Biomedical, Inc., Sanofi-Aventis, Novartis, and Neuraltus; and receives research support from the NIH [R01 NS 44887 (PI)] and the Muscular Dystrophy Association (PI). Dr. Jackson serves as a consultant to Knopp Neurosciences Inc.; and receives research support from Knopp Neurosciences Inc., Insmed Inc., Solstice Neurosciences, Inc., the ALS Association, and the NIH NINDS [U01 NS042685-0 (Site PI), R01NS045087-01A2 (Site PI), and N01-AR-2250 (Site PI)]. Dr. Kasarskis serves as an Associate Editor for Amyotrophic Lateral Sclerosis; has received honoraria from the American Institute for Biological Studies (grant reviews); served as a consultant to Acceleron Pharma; holds equity in Amgen; and receives research support from the NIH/NINDS [R01-NS045087 (PI) and 1U01 NS049640 (Site PI). Dr. England serves as an Associate Editor for Current Treatment Options in Neurology; received a speaker honorarium from Teva Pharmaceutical Industries Ltd.; and serves as a consultant to Talecris. Ms. Forshew has served on a scientific advisory board for the ALS Association and receives research support from the Muscular Dystrophy Association. Dr. Johnston reports no disclosures. Dr. Kalra receives research support from the ALS Association of America and the ALS Society of Canada. Dr. Katz has received research support from Pfizer Inc. Dr. Mitsumoto served on scientific advisory boards for Avanir Pharmaceuticals, Knopp Neurosciences Inc., Neuralstem, Inc., Aisai Communication Technology Co., Ltd., and Otsuka Pharmaceutical Co., Ltd.; and receives research support from Avanir Pharmaceuticals, Teva Pharmaceutical Industries Ltd., Knopp Neurosciences Inc., Sanofi-Aventis, Athena Diagnostics, Inc., BioScrip, and the NIH/NINDS [DNA repository as a supplement and NIEHS Center grant]. Dr. Rosenfeld serves on the editorial board of Amyotrophic Lateral Sclerosis and has served as a consultant to Solstice Neurosciences, Inc. and Avicena Group, Inc. Dr. Shoesmith receives research support from the Muscular Dystrophy Association and her spouse is employed by Biovail Pharmaceuticals Canada. Dr. Strong serves on the editorial board of Amyotrophic Lateral Sclerosis. Dr. Woolley has received research support from Pfizer Inc., Eisai Inc., and the ALS Association (Co-I).
DISCLAIMER
This statement is provided as an educational service of the American Academy of Neurology. It is based on an assessment of current scientific and clinical information. It is not intended to include all possible proper methods of care for a particular neurologic problem or all legitimate criteria for choosing to use a specific procedure. Neither is it intended to exclude any reasonable alternative methodologies. The AAN recognizes that specific patient care decisions are the prerogative of the patient and the physician caring for the patient, based on all of the circumstances involved. The clinical context section is made available in order to place the evidence-based guideline(s) into perspective with current practice habits and challenges. No formal practice recommendations should be inferred.
CONFLICT OF INTEREST
The American Academy of Neurology is committed to producing independent, critical and truthful clinical practice guidelines (CPGs). Significant efforts are made to minimize the potential for conflicts of interest to influence the recommendations of this CPG. To the extent possible, the AAN keeps separate those who have a financial stake in the success or failure of the products appraised in the CPGs and the developers of the guidelines. Conflict of interest forms were obtained from all authors and reviewed by an oversight committee prior to project initiation. AAN limits the participation of authors with substantial conflicts of interest. The AAN forbids commercial participation in, or funding of, guideline projects. Drafts of the guideline have been reviewed by at least three AAN committees, a network of neurologists, Neurology® peer reviewers and representatives from related fields. The AAN Guideline Author Conflict of Interest Policy can be viewed at www.aan.com.
Notes
Address correspondence and reprint requests to the American Academy of Neurology, 1080 Montreal Avenue, St. Paul, MN 55116 moc.naa@senilediug
See also page 1227
Supplemental data at www.neurology.org
Appendices e-1–e-4, tables e-1 and e-2, and references e1– e26 are available on the Neurology® Web site at www.neurology.org.
Approved by the Quality Standards Subcommittee on November 5, 2008; by the Practice Committee on February 19, 2009; and by the AAN Board of Directors on July 30, 2009.
Disclosure: Author disclosures are provided at the end of the article.
Received February 19, 2009. Accepted in final form July 29, 2009.
REFERENCES
Articles from Neurology are provided here courtesy of American Academy of Neurology
Full text links
Read article at publisher's site: https://doi.org/10.1212/wnl.0b013e3181bc0141
Read article for free, from open access legal sources, via Unpaywall:
https://n.neurology.org/content/neurology/73/15/1218.full.pdf
Free to read at www.neurology.org
http://intl.neurology.org/cgi/content/abstract/73/15/1218
Subscription required at www.neurology.org
http://intl.neurology.org/cgi/content/full/73/15/1218
Subscription required at www.neurology.org
http://intl.neurology.org/cgi/reprint/73/15/1218.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/102177239
Article citations
Health care resource utilization and costs across stages of amyotrophic lateral sclerosis in the United States.
J Manag Care Spec Pharm, 30(11):1239-1247, 01 Nov 2024
Cited by: 0 articles | PMID: 39471269 | PMCID: PMC11522455
Update on Inherited Pediatric Motor Neuron Diseases: Clinical Features and Outcome.
Genes (Basel), 15(10):1346, 21 Oct 2024
Cited by: 0 articles | PMID: 39457470 | PMCID: PMC11507535
Review Free full text in Europe PMC
Perceived Pain in People Living with Amyotrophic Lateral Sclerosis-A Scoping Review.
Nurs Rep, 14(4):3023-3039, 17 Oct 2024
Cited by: 0 articles | PMID: 39449457 | PMCID: PMC11503277
Review Free full text in Europe PMC
The Seattle Amyotrophic Lateral Sclerosis (ALS) Patient Project Database: observational, longitudinal, dyadic characterization of people with ALS and their partners.
Health Psychol Behav Med, 12(1):2396137, 04 Sep 2024
Cited by: 0 articles | PMID: 39239358 | PMCID: PMC11376292
Physical therapy for the management of global function, fatigue and quality of life in amyotrophic lateral sclerosis: systematic review and meta-analyses.
BMJ Open, 14(8):e076541, 25 Aug 2024
Cited by: 0 articles | PMID: 39182937 | PMCID: PMC11404137
Review Free full text in Europe PMC
Go to all (334) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology.
Neurology, 73(15):1227-1233, 01 Oct 2009
Cited by: 304 articles | PMID: 19822873 | PMCID: PMC2764728
Review Free full text in Europe PMC
Respiratory therapies for amyotrophic lateral sclerosis: a primer.
Muscle Nerve, 46(3):313-331, 01 Sep 2012
Cited by: 19 articles | PMID: 22907221
Review
[Proposals from a French expert panel for respiratory care in ALS patients].
Rev Mal Respir, 41(8):620-637, 16 Jul 2024
Cited by: 0 articles | PMID: 39019674
Review
Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease.
Cochrane Database Syst Rev, 10:CD004427, 06 Oct 2017
Cited by: 43 articles | PMID: 28982219 | PMCID: PMC6485636
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIAMS NIH HHS (1)
Grant ID: N01-AR-2250
NINDS NIH HHS (5)
Grant ID: R01 NS 44887
Grant ID: R01-NS045087
Grant ID: U01 NS042685-0
Grant ID: 1U01 NS049640
Grant ID: R01NS045087-01A2




