Abstract
Free full text

Translocation of double stranded DNA through membrane adapted phi29 motor protein nanopore
Abstract
Biological pores have been used to study the transport of DNA and other molecules but most pores have channels that allow only the movement of small molecules and single-stranded DNA and RNA. The bacteriophage phi29 DNA-packaging motor, which allows double-stranded DNA to enter and exit during a viral infection, contains a connector protein that has a 3.6 – 6.0 nm wide channel. Here we show that a modified version of the connector protein, when reconstituted into liposomes and inserted into planar lipid bilayers, can act as conductive channels to allow the translocation of double-stranded DNA. Single-channel conductance assays and quantitative PCR confirmed the translocation through the pore. The measured conductance of a single connector channel was 4.8 nS in 1 M KCl. This engineered and membrane-adapted phage connector is expected to have interesting applications in nanotechnology and nanomedicine, such as MEMS sensing, microreactors, gene delivery, drug loading, and DNA sequencing.
The genome of linear dsDNA viruses is packaged into a preformed procapsid1–4. This entropically unfavorable process is accomplished by an ATP-driven motor5–8. In bacteriophage phi29, the motor uses one ATP to package 2 bp5 or 2.5 bp of DNA9. The protein hub of this motor is a truncated cone structure, termed a connector (Fig. 1a), that allows dsDNA to enter during maturation and exit during infection 10–14. The connector has a central channel (Fig. 1b) formed by twelve GP10 protein subunits. While the connector proteins of viruses share little sequence homology and vary in molecular weight, there is significant underlying structural similarity15. By demonstrating viral DNA packaging and procapsid conversion to infectious virions, phi29 DNA packaging motor was the first to be assembled in vitro in a defined system and remains one of the most well studied16. The motor utilizes six pRNA (packaging RNA)17–20 to gear the machine (Fig. 1c). Engineering such a nanomotor outside its natural environment has tremendous potential to impact biology, engineering, medicine, and various other fields of nanobiotechnology.

a, Side view of the phi29 connector showing the acidic (red), basic (blue), and other (white) amino acids. 12,13,38 b, Top view of the connector showing the diameter of the narrow part and wide part of the channel. c, Illustration of the entire phi29 DNA packaging motor showing DNA translocation through the connector. d-e, A TEM image of purified connectors with C-terminal modification before (c) and after (d) the array formation. f, Coomasie-blue stained SDS-gel showing connectors.
Electrophysiological measurements of individual membrane channels have been used to study a variety of processes including the transport of DNA, RNA, pharmaceutical agents, peptides, proteins, and polymers21–25. The transient blockade of ionic current through the alpha-hemolysin (α-HL) pore has been used to measure the length of single-stranded DNA or RNA26. Subsequently, DNA hairpin molecules have been used to decelerate the DNA translocation rate through the α-HL pore to demonstrate the discrimination between single nucleotide polymorphisms27. Detection of base pair stacking and strand orientation within the pore have also been investigated28,29. Most studies involving nucleic acid transport through nanopores have focused on α-HL. However, the limiting lumen diameter (1.5 nm) has restricted the DNA and RNA applications to single-strands30. A similar limitation was also reported for the MspA nanopore31.
A few studies of channels have shown evidence of dsDNA transport32–34, but their voltage gating properties have limited their biomedical applications. For this reason some researchers have switched to fabricating synthetic metal or silicon nanopores for potential DNA sequencing35,36. Conversely, synthetic nanopores are less reproducible and not as readily engineered for specific pore modification or conjugation. As a result, the search for alternate protein nanopores is still ongoing. The portal nature of the phi29 connector has inspired us to examine whether it could be utilized to explore membrane-based nanopore applications.
An artificial membrane architecture could allow detailed investigations into discrete motor mechanisms, as well as open future avenues for the study of sensing, drug delivery, and therapeutic dsDNA packaging. The phi29 connector is ideally suited for this endeavor because its available crystal structure allows for explicit engineering12,13,37,38. Furthermore, the procedures for large scale production and purification of phi29 connector have been developed38–41.
In this study, the connector protein was redesigned to include distinct regions of hydrophilicity. The modified connector was inserted into liposomes and a lipid bilayer. The presence of the channel across the lipid bilayer was confirmed by single channel conductance measurements and translocation of dsDNA.
Modifying the phi29 connector
In general, membrane pores and ion channels contain a hydrophobic domain which anchors the protein to the membrane. Analysis of the surface charge of the connector revealed that its central surface region exhibits slight hydrophobicity compared with the two flanking layers at the wide and narrow ends, respectively (Fig. 1a, b)12,13. To facilitate connector purification, a C-terminus his or strep tag was inserted just downstream of a six glycine linker for improved affinity tag flexibility. Six glycine linkers were included to provide end-flexibility (Supplementary Fig. 1). After purification to homogeneity, it was found that the modified GP10 self-assembled into the dodecameric structure with similar morphology to the 12-fold symmetric wild type connector (Fig. 1f), as observed by TEM (Fig. 1d and e). The existence of a native and authentic motor configuration was verified through its competency to package the double-stranded DNA after incorporation into the procapsid (Supplementary Fig. 2) and to assemble the resulting DNA-filled capsid into the infectious phi29 virion (Supplementary Fig. 3).
Reconstituting the connector into liposomes
A procedure for reconstituting the connector into liposomes was developed by co-incubation of the connector with the lipid in the presence of sucrose. Such incubation provided an opportunity for the hydrophobic layer of the connector to interact with the hydrophobic domain of the lipid molecules. The dehydration–rehydration method42 led to the production of giant liposomes up to 50 µm (Fig. 2 a-c). The insertion of the connector protein into the lipid membrane was confirmed by fluorescence microscopy, filtration assay, and sedimentation analysis (Fig. 2 a-g). The presence of the connector in the membrane was visible with fluorescence microscopy, showing a clear fluorescent ring around the liposome (Fig. 2b). The fluorescent ring was very similar to the liposome generated with fluorescent lipids NBD-PE (Fig. 2a). No fluorescent ring was observed when the fluorescently-tagged connector was mixed non-specifically with the non-connector inserted liposome (Fig. 2c). The free connectors were removed by filtration using a membrane with a pore size of 0.45 µm or by 5–20% sucrose gradient ultracentrifugation (Fig. 2 d-f and 2g).
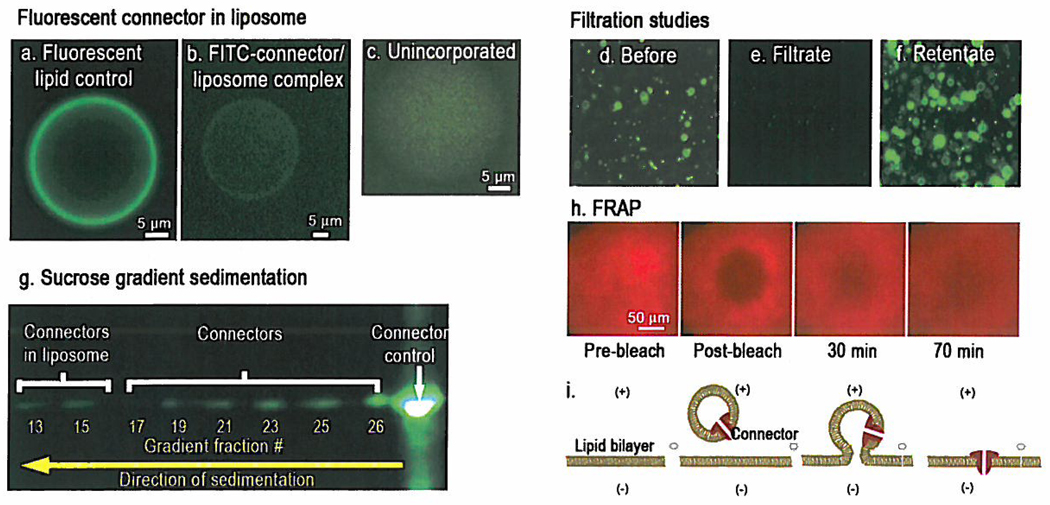
a-c, Epifluorescence images of liposome: lipid labeled with NBD-PE without connector (a); a proteoliposomes reconstituted by FITC labeled connectors (b); FITC-connector mixed non-specifically with liposomes (c). d-f, Membrane filtration isolated most of the free connectors. g, Separation of liposome/FITC-connector complexes by sucrose gradient sedimentation. Free connectors appeared in the top fractions while proteoliposomes remained in the lower fractions. Fractions #1–12 are not shown. h, Fluidity of fluorescent (red) lipid bilayer demonstrated by FRAP (Fluorescence Recovery After Photobleaching) showing that the fluorescence intensity of photobleached area (black) was gradually increased over time due to lipid diffusion. i, Schematic showing the insertion of the connector into a planar lipid bilayer.
Incorporating the connector into planar lipid membranes
Since none of the above experiments could distinguish between loose attachment of the connector to the bilayer surface and tight incorporation of it into the bilayer membrane to form a channel, a single channel conductance assay was performed. Results showed that direct incubation of the connector with liposomes or a planar lipid bilayer did not lead to channel formation in the bilayer membrane (Fig. 3a). Connector insertion into the bilayer only occurred when the connector reconstituted proteoliposomes were fused into the bilayer (Fig. 3b and 3c). The channel insertion was observed through a discrete step-wise increase in conductance as shown in a continuous current trace (Fig. 3b), under either positive or negative transmembrane voltage (Fig. 3c).

a, BLM with connector only (top) or liposomes only (bottom). b, Addition of connector-containing proteoliposomes resulted in multiple insertions. Reproduced in over 200 experiments. Inserts: Insertion of one (top) and two connectors simultaneously (bottom). c, One connector insertion at positive voltage (top) and at negative voltage (bottom). d, Distribution of current jump after multiple connector insertions. e, Current-voltage relationship of single connector channels. Error bars represent 3–5 insertions under each applied voltage from a total of 38 inserted connectors in 4 individual experiments. f-g, Slopes of current traces with 1, 2 or 3 connectors(f) and 1 connector in the presence of dsDNA (cis- chamber) (g). d-f, 5mM Tris buffer, pH 7.9 with 0.5M NaCl. g, TMS with 1M NaCl.
Normally, the insertion of one connector into the bilayer results in an increase in the current of approximately 65 pA (equivalent to 1.6 nS) at a potential of −40 mV in a 5 mM Tris (pH 7.9)/0.5 M NaCl. Occasionally a 130.9 pA jump, attributed to insertion of two connectors, was also observed (Fig. 3d). Similar results were obtained when the channel conductance was measured in TMS/1 M NaCl (Supplementary Fig. 4). In this case, the occurrence of simultaneous insertion of 2 connectors and 3 connectors was 4.7% and 1.9%, respectively.
For conductance measurements, a plot of the I-V curve was obtained under different voltages (Fig. 3e). The average conductance per single pore was 1.57±0.16 nS/pore (a total of 38 inserted connectors) in a 5 mM Tris/0.5 M NaCl (Fig. 3e) and 3.21±0.51 nS/pore (a total of 213 inserted connectors) in a TMS/1 M NaCl buffer (Supplementary Fig. 4). As a comparison, the conductance measurements were also performed for connector channels under a ramp voltage (Fig. 3f and 3g). The slopes from the fitted curves representing the conductance of formed channels in the 5 mM Tris/0.5 M NaCl were 1.59 nS/single pore, 3.40 nS/two pores, and 4.98 nS/three pores, respectively. When NaCl concentration increased to 1 M (TMS buffer with 1 M NaCl), the slope of the curve was 3.12 nS/ single pore. A buffer of 5 mM HEPES /1 M KCl was also used for conductance measurements (Table 1).
Table 1
Comparison of Single Channel Conductance from the GP-10 Connector and α-Hemolysin
| Conductance (nS/pore)a | ||||
|---|---|---|---|---|
| Proteins | Pore diameter (nm) | Cross section area (nm2) | ||
| at 0.5 M NaCl | at 1 M KCl | |||
| Connector | 3.6 | 10.2 | 1.57±0.16 | 4.84±0.15 |
| α-HL | 1.530 | 1.8 | 0.31±0.05b | 0.94±0.01c |
| Ratio (Connector/α-HL) | 2.4 | 5.7 | 5.1 | 5.1 |
The channel conductance of the connector was compared with that of α-HL using solutions of different ionic strength (Table 1). It has been reported that the diameter of the narrow end of the connector channel is 3.6 nm, while the channel formed by α-HL has a diameter of only 1.5 nm30. Therefore, the ratio of the cross-sectional area of the channels between the connector and α-HL is 5.7. The ratio of measured conductance of the connector to α-HL is 5.1 (Table 1). Since the conductance of a channel is proportional to its cross-sectional area, it can be concluded that the cross-sectional area of the connector in the buffer solutions was approximately 5.1 fold greater than that of α-HL, which compares well with the ratio of cross-sectional areas from the crystal data of both proteins. Moreover, compared with other transmembrane proteins or ion channel proteins with larger channels, e.g., Streptolysin43, Kir44, VDAC45 and bacterial porins32, the connector channel has additional advantages. For example, the connector channels were stable and did not exhibit voltage gating under the reported conditions. The channel conductance was uniform, demonstrating a linear response to applied voltages between −100 mV and 100 mV (Fig. 3f and 3g).
Translocation of double-stranded DNA
Both linear and circular plasmid Cx43 DNA (5.5 kb) were used to examine the translocation of dsDNA through the connector channel. In the case of the linear DNA plasmid, DNA translocation induced numerous current blockades which led to the current jump of single connector insertion transiently reduced by 25–45% (Fig.4c). Similar results were also found in translocation experiments of a 35-bp dsDNA (Supplementary Fig. 5). However, when the linear Cx43 was added to the cis-chamber, no such blockades were observed until the voltage was switched to positive potential (Fig. 3g). The short-lived blockades could be attributed to the occurrence of DNA translocations. In contrast, in the absence of DNA, the current trace was quiescent (Fig. 4a and Supplementary Fig. 5b). Occasionally, unspecific blockades were observed with a minimum detectable time. These unspecific blockades rarely occurred compared to DNA translocation events. They were usually characterized with detectable time very close to the limit of sampling frequency. (Fig. 4b and Fig. 3g). When circular plasmid dsDNA Cx43 was used, no translocation of the circular plasmid was observed (Fig. 4b upper left). Interestingly, when the same amount of circular plasmid digested by DNase I was added to the chamber, a burst of transient blockades occurred (Fig. 4b lower left). The same results were also observed when the linear Cx43 digested by DNase I was used (Fig. 4b lower right). All the above results confirmed that only the linear dsDNA passed through the connector channels.
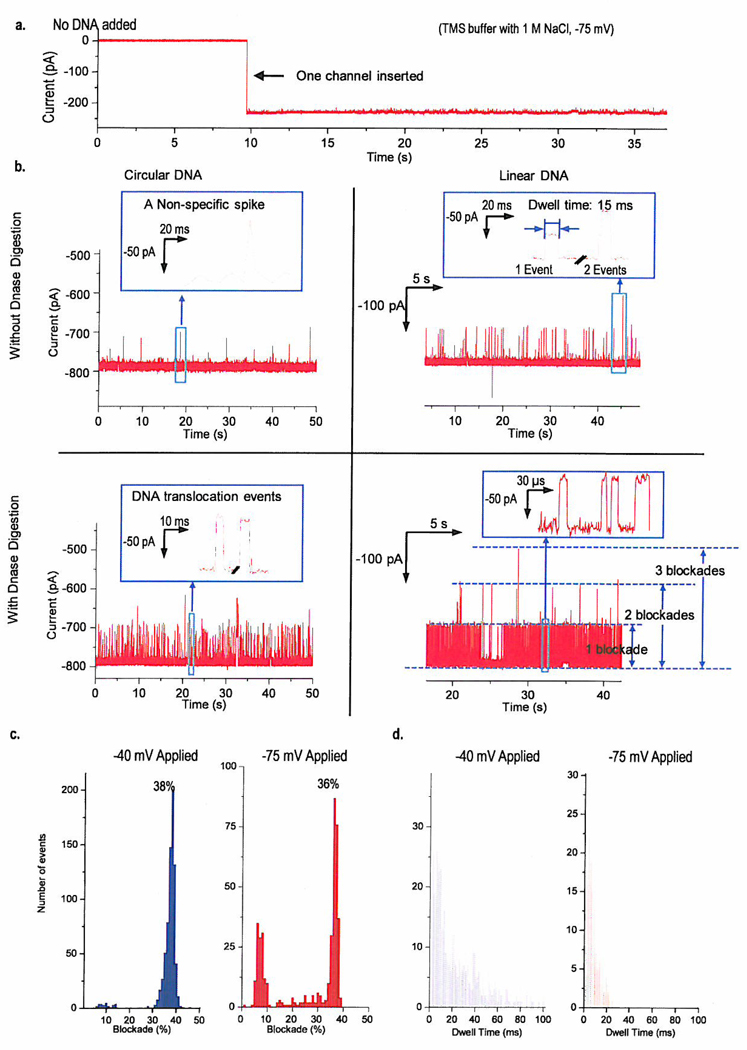
a, A typical current trace when BLM contains a connector but no DNA (Control). b, Representative blockades caused by 45pM double-stranded circular and linear plasmid DNA without and with DNase digestion in BLM containing 3 connectors. c, d, Histogram showing the percentage of current blockade (c) and dwell time (d) caused by linear plasmid dsDNA under −40 mV and −75 mV. DNA translocation experiment was repeated 45 times.
Occasionally, we also witnessed blockade events in the range of 5–15% (Fig. 4c right) with a dwell time from several to hundreds of milliseconds. The events were attributed to non-specific blockades other than DNA translocation because they were found to occur in the absence of DNA (Supplementary Fig. 5b). The non-specific blockade events could be due to interactions of connector pores with lipid or lipid micelles because their occurrence increased after the addition of more liposomes to the chamber (data not shown). The occurrence of the non-specific blockades can be minimized when the diluted connector reconstituted liposomes are used and/or lower transmembrane voltage is applied (Fig. 4c left). Interestingly, we occasionally observed simultaneous blockade events (insert in Fig. 4b upper right and Supplementary Fig. 5a). These events were recorded under multiple pore conditions. A continuous current trace recording events before and after addition of DNA was also shown (Supplementary Fig. 5b). On one occasion, a burst of DNA blockades was observed after the insertion of a third connector. In comparison, when DNA was premixed with buffer before connector insertion, the DNA blockades were observed immediately after the first insertion occurred (Supplementary Fig. 5a). This result indicates a lack of a stirring facility in the DNA chamber leading to a delay in DNA translocation.
The blockade rates were affected by two factors: DNA concentration and transmembrane voltage. In the presence of 45 pM DNA under 3 connector insertions, the blockade rate was approximately 0.8–1 blockades/s (Fig. 4b upper right). Under the same number of insertions, when 4 µM of DNA was placed in the chamber, the blockade rate was approximately 5–5.8 blockades/s (Supplementary Fig. 5a and 5b). For the linear Cx43 DNA, the blockade rate increased as the ramping voltage was applied (Fig. 3g).
To calculate the dwell time (τp) for DNA translocation events, we grouped blockade episodes greater than 32% since this percentage of blockade seemed consistent with the ratio of cross sectional area between dsDNA and the pore. A histogram of these events can be seen in Fig. 4d. It should also be noted that 6 (under −75 mV) and 20 (under −40 mV) individual outlying events scattered between 120 ms to 9800 ms were not included in the graph for clarity. The dwell time distribution under −40 mV seemed broader than that under −75 mV. The average dwell time for DNA blockades under the −75 mV and under −40 mV was 9.2 ms and 22.1 ms respectively (Note: only the events less than 50 ms under −40 mV were used for the calculation). As a comparison, the distribution of dwell time for the 35-bp dsDNA are also included (Supplementary Fig. 6b and 6c). The average of the dwell times in this case was 0.53 ms. Therefore, it can be concluded that the dwell time of DNA translocation was affected by applied voltage and the size of DNA.
To verify the passage of dsDNA through the connector channels, quantitative PCR (Q-PCR) was used to quantify the translocation of 141-bp DNA under a constant voltage. DNA was added to the trans- side and samples were taken from the cis- side for quantification at 30 minute intervals. For comparison, control experiments were performed in the absence of connectors (Fig. 5c). Experiments with connector insertions in BLMs showed an increase in the number of DNA molecules in the cis-chamber over time (N=9 experiments). In contrast, in the absence of connectors the number of DNA molecules in cis-chamber remained undetectable over the 90-minute time course (N=4 experiments). Moreover, the DNA translocation rate was affected by the number of inserted connectors (Supplementary Fig. 7a).
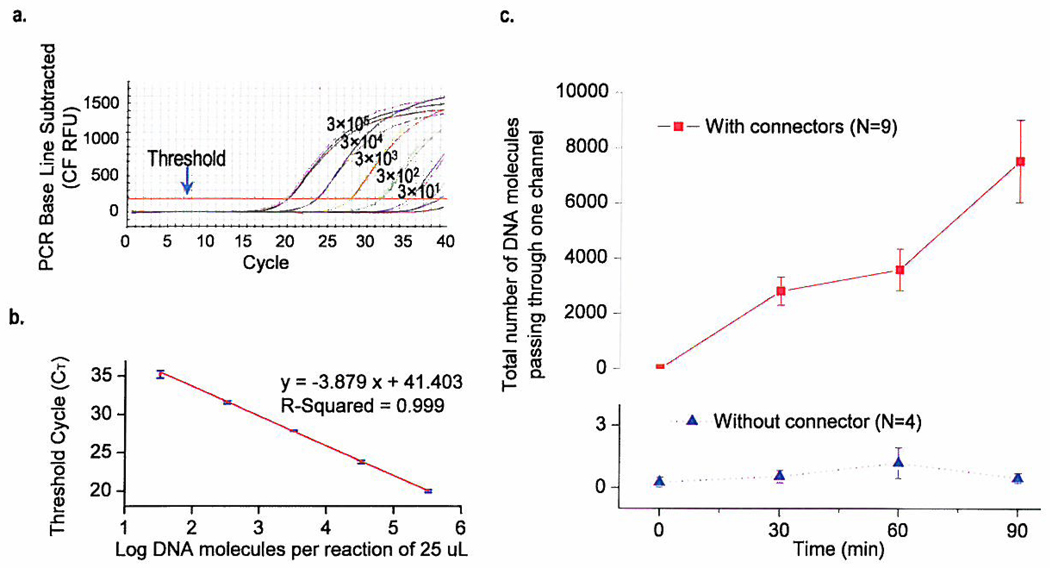
a, Q-PCR amplification curves of the dilution series run in triplicate. b, A standard curve with the CT plotted against the log of the starting quantity of template for each dilution. c, Quantitative analysis of the total number of DNA passing through one of the connectors in the lipid membrane from the trans- chamber to the cis- chamber (top). Negative controls (bottom) were carried out under the same condition but without connectors. The error bars represent standard deviations of the mean from 9 independent experiments and 4 negative control experiments.
To verify that the increase of the DNA copy number in the cis-chamber was due to DNA translocation through the channels rather than membrane leakage, three additional experiments were carried out under the conditions of known leakage of BLM or partitions. When leaking occurred, the copy number of DNA per ul of solution in the cis-chamber was approximately 104 – 105 fold higher than those experiments without leakage (Supplementary Fig. 7b).
Conclusion
Herein, an engineered form of the GP10 connector has been incorporated into a lipid bilayer, forming a highly conductive nanopore. By evaluating the changes of the conductance and performing Q-PCR analysis, the translocation of the linear 5.5-kb plasmid DNA, 141-bp DNA and 35-bp DNA through the channel was confirmed. This work provides a system or a tool for future electrophysiology studies of the phi29 DNA packaging motor. Also, the connector is a biological nanopore that is extremely reproducible and easily engineered, making it ideal and suitable for future biomedical applications.
METHODS
Preparation of giant lipid vesicles containing the reengineered connector
To prepare the fluorescent giant lipid vesicles, 1 mL of 1 mg/mL DOPC or DPhPC and 1% (molar ratio) NBD-PE were mixed in a vial. Chloroform was evaporated by a gentle stream of nitrogen gas, and the lipid vial was further dried in a desiccator overnight. To rehydrate the lipid film, 2 mL of 200 mM – 300 mM sucrose was used to bud vesicles off the glass and into the solution. The vial was then covered with parafilm and stored overnight. An aliquot was taken from the middle of the solution and then transferred into a Petri dish. After settling, the vesicles were observed with epi-fluorescence microscopy (Fig. 2 a–c).
Incorporation of the connector into giant vesicles was accomplished as described above, except the NBD-PE was omitted. A volume of 100 µL of FITC-labeled reengineered connectors was added to the above dehydrated lipid with a final lipid:connector mole ratio of 75:1 (or as low as 4000:1 to 16000:1 for BLM experiments) (Fig. 2b).
Insertion of the connector into planar bilayer lipid membrane
A two-step approach was used to incorporate the connector into the planar bilayer lipid membrane (BLM). The first step was the preparation of unilamellar lipid vesicles containing the reengineered connector as described above. The next step was to fuse the extruded liposome into a planar BLM (Fig. 2i). The fluidity of the lipid bilayer was demonstrated by FRAP (Fluorescence Recovery After Photobleaching) (Fig. 2h). An excitation light was focused continuously on the bilayer to bleach the dye. The photobleached area appeared dark. But after the light was off, it gradually recovered due to the diffusion of the fluorescent lipid.
A standard BLM chamber (BCH-1A from Eastern Sci LLC) was utilized to form horizontal BLMs. A thin Teflon film with an aperture of 70–120 µm (TP-01 from Easter Sci LLC) or 180–250 µm (TP-02 from Easter Sci LLC) in diameter was used as a partition to separate the chamber into cis- (working volume 250 µL) and trans- (working volume 2.5 mL) compartments. After the aperture was pre-painted with 0.5 µL 3% (w/v) DPhPC n-decane solution twice to ensure the complete coating of the entire edge of the aperture, these compartments were filled with conducting buffers (5 mM Tris/pH 7.9, TMS, or 5 mM HEPES/pH 7.9, with varying concentration of NaCl or KCl).
Formation of the bilayer membrane on the partition is a key step for connector insertion into the bilayer (Fig. 2i). Considering all experiments, the occurrence of successful connector insertions was about 47–83%, which varied from person to person based on BLM experience and the quality of prepared proteoliposomes. So far, we have carried out a total of 280 separate BLM experiments in which successful connector insertions were found.
For single conductance measurements, the giant liposome/connector complex prepared earlier must be extruded using a polycarbonate membrane with pore size of 200 nm or 400 nm to generate small unilamellar liposomes. This liposome stock solution was further diluted by 10–20 fold for the BLM experiments before use. For insertion of connectors, 0.5–2 µL of the diluted liposome solution was loaded into the cis-chamber.
Conductance was measured in two ways: the first was derived at specific but constant holding potentials, and the second from the slope of the current trace induced by a scanning potential starting at −100 mV and ramping to 100 mV after incorporation of GP10 connector into the lipid membrane (Fig. 3f and 3g).
Q-PCR analysis
For Q-PCR analysis, the connector/liposome complexes were added to the cis-side (working volume 500 µL). 141-bp DNA was added to the trans-side with a final concentration of 25 nM. As a negative control, the DNA was added without the addition of connector/liposome complexes. A potential of −95 mV was applied and samples were collected from the cis- side at 30 min intervals for Q-PCR analysis. DNA concentration was determined by a DU530 UV/Vis spectrometer (Beckman Coulter). Absolute quantification was used to determine the copy number of DNA in samples collected. Standard curves were constructed using the 141-bp DNA with 10 fold dilution of known concentration (Fig. 5a and 5b). Each dilution was assayed in triplicate. iQTM SYBR Green Supermix (Bio-Rad) was used for the Q-PCR reaction. Q-PCR was carried out in the iCycler iQTM multicolor real-time PCR detection system (Bio-Rad). The sequences for forward and reverse primers corresponding to the DNA template were 5’-TAA TAC GAC TCA CTA TTA GAA CGG CAT CAA GGT GAA CTC AAG ATT TTG TAT GTT GGG GAT TA-3’ and 5’-AAG AAC GGC ATC AAG GTG AAC TTC AAG ATA ATT GAC AGC AGG CAA TCA AC-3’ respectively (purchased from IDT).
See also Supplementary Materials and Methods.
Supplementary Material
supp figs
supp material
ACKNOWLEDGEMENTS
We thank Rong Zhang for Q-PCR analysis, Jacob Schmidt and Liqun Gu for α-hemolysin proteins; Farzin Haque for BLM experiments; Yi Shu for DNA preparation, Cole Brokamp for advice on BLM, Nicola Stonehouse, Ying Cai and Feng Xiao for recombinant connectors; Annapoorna Butti for FRAP, Jarek Meller and Andrew Herr for connector amphiphilicity analysis; Anne Vonderheide for manuscript modification. The research was supported by NIH Nanomedicine Development Center: Phi29 DNA Packaging Motor for Nanomedicine, through the NIH Roadmap for Medical Research (PN2 EY 018230) (PG) and NIH Grant GM59944 (PG). PG is a co-founder of Kylin Therapeutics, Inc.
Footnotes
AUTHOR CONTRIBUTIONS
P.G. designed and led the project in collaboration with C.M. in single pore measurements. V.S. (when she was a postdoc with P.G. at Purdue University), J.G. and P.J. developed protocols for incorporating the connector into the membrane of liposome for image characterization and BLM experiments. P.J contributed the data for conductance measurements and Q-PCR. P.J. and J.G. contributed the data for double-stranded DNA translocation experiment. P.J. and J.G. performed all the data analysis. D.W. provided fusion procedure and technical training for BLM experiment. T.L. contributed materials and participated in PCR analysis. P.G. prepared the first draft of the manuscript with the input by C.M.. P.J., J.G. D.W. and T.L. assisted the manuscript revision. D.W. and P.J. contributed equally to this work.ADDITIONAL INFORMATION
Supplementary information accompanies this paper at www.nature.com/naturenanotechnology. Reprints and permission information is available online at http://npg.nature.com/reprintsandpermissions/.Correspondence and requests for materials should be addressed to P.G.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1038/nnano.2009.259
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2777743
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/nnano.2009.259
Article citations
Detection of Biomolecules Using Solid-State Nanopores Fabricated by Controlled Dielectric Breakdown.
Sensors (Basel), 24(8):2420, 10 Apr 2024
Cited by: 0 articles | PMID: 38676038 | PMCID: PMC11053845
Characterization and genomic analysis of a broad-spectrum lytic phage HZ2201 and its antibiofilm efficacy against Pseudomonas aeruginosa.
Virus Res, 335:199184, 02 Aug 2023
Cited by: 3 articles | PMID: 37532140 | PMCID: PMC10407953
Unlocking the Power of Nanopores: Recent Advances in Biosensing Applications and Analog Front-End.
Biosensors (Basel), 13(6):598, 31 May 2023
Cited by: 0 articles | PMID: 37366963 | PMCID: PMC10296294
Review Free full text in Europe PMC
Revolving hexameric ATPases as asymmetric motors to translocate double-stranded DNA genome along one strand.
iScience, 26(6):106922, 19 May 2023
Cited by: 0 articles | PMID: 37305704 | PMCID: PMC10250835
Review Free full text in Europe PMC
Localized Nanopore Fabrication via Controlled Breakdown.
Nanomaterials (Basel), 12(14):2384, 12 Jul 2022
Cited by: 2 articles | PMID: 35889608 | PMCID: PMC9323289
Review Free full text in Europe PMC
Go to all (126) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Forces from the Portal Govern the Late-Stage DNA Transport in a Viral DNA Packaging Nanomotor.
Biophys J, 111(1):162-177, 01 Jul 2016
Cited by: 8 articles | PMID: 27410744 | PMCID: PMC4945582
Single pore translocation of folded, double-stranded, and tetra-stranded DNA through channel of bacteriophage phi29 DNA packaging motor.
Biomaterials, 53:744-752, 27 Mar 2015
Cited by: 16 articles | PMID: 25890769 | PMCID: PMC4405662
Methods for Single-Molecule Sensing and Detection Using Bacteriophage Phi29 DNA Packaging Motor.
Methods Mol Biol, 1805:423-450, 01 Jan 2018
Cited by: 2 articles | PMID: 29971730
Single-molecule studies of viral DNA packaging.
Adv Exp Med Biol, 726:549-584, 01 Jan 2012
Cited by: 21 articles | PMID: 22297530 | PMCID: PMC5933933
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NEI NIH HHS (5)
Grant ID: PN2 EY018230-01
Grant ID: PN2 EY018230
Grant ID: PN2 EY018230-02
Grant ID: PN2 EY 018230
Grant ID: PN2 EY018230-03
NIGMS NIH HHS (3)
Grant ID: GM59944
Grant ID: R01 GM059944-04
Grant ID: R01 GM059944





