Abstract
Free full text

Use of a mutant cell line to study the kinetics and function of O-linked glycosylation of low density lipoprotein receptors.
Abstract
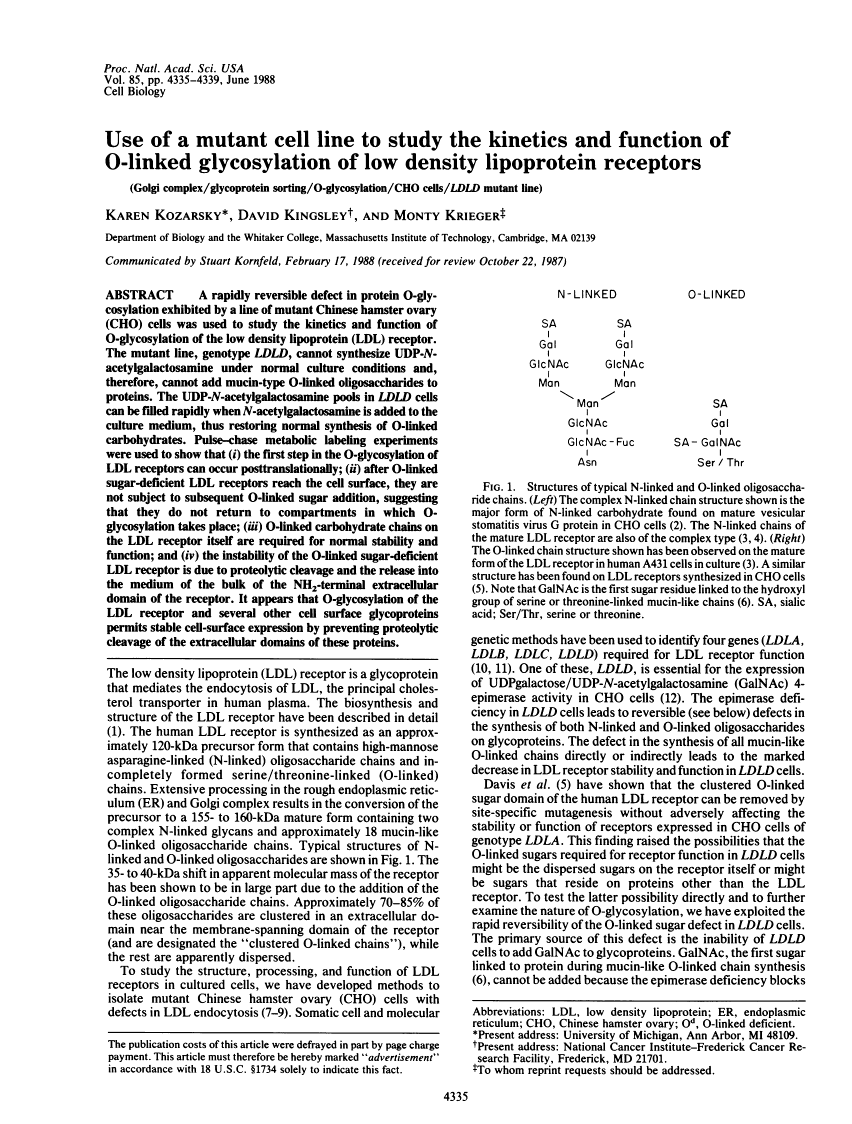
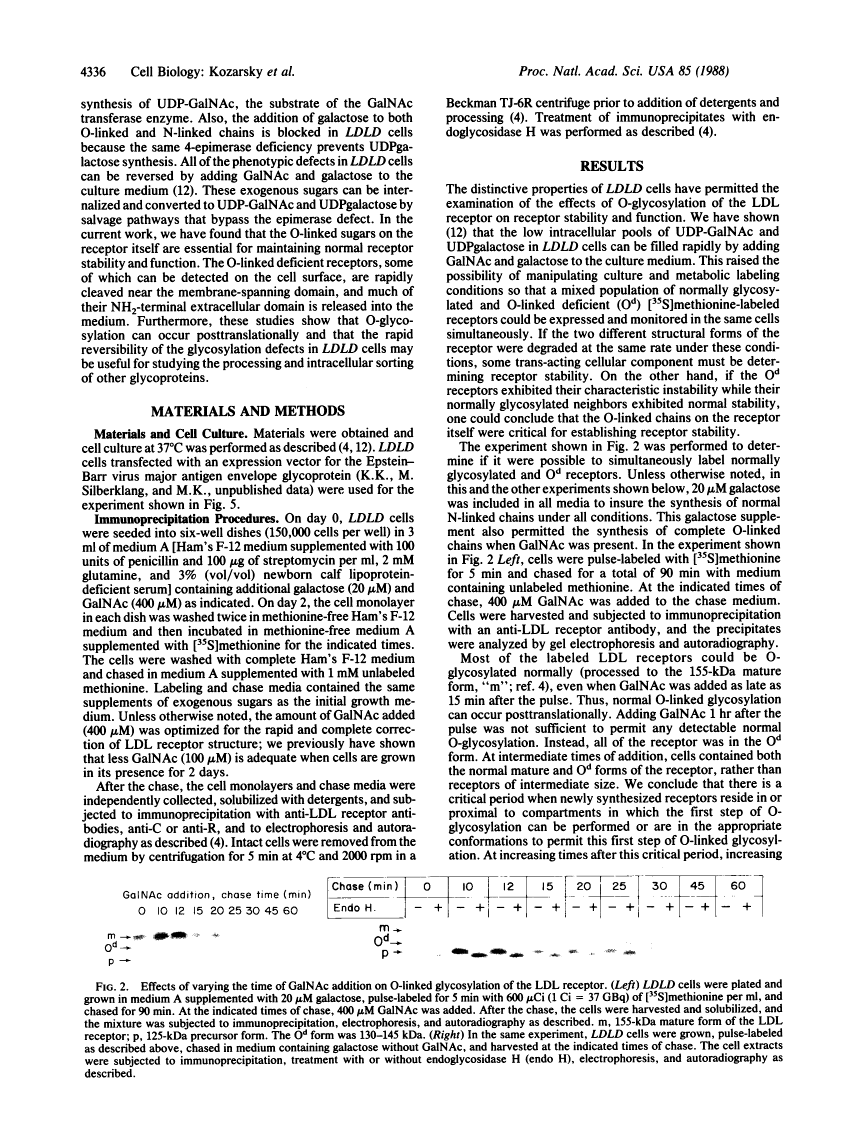
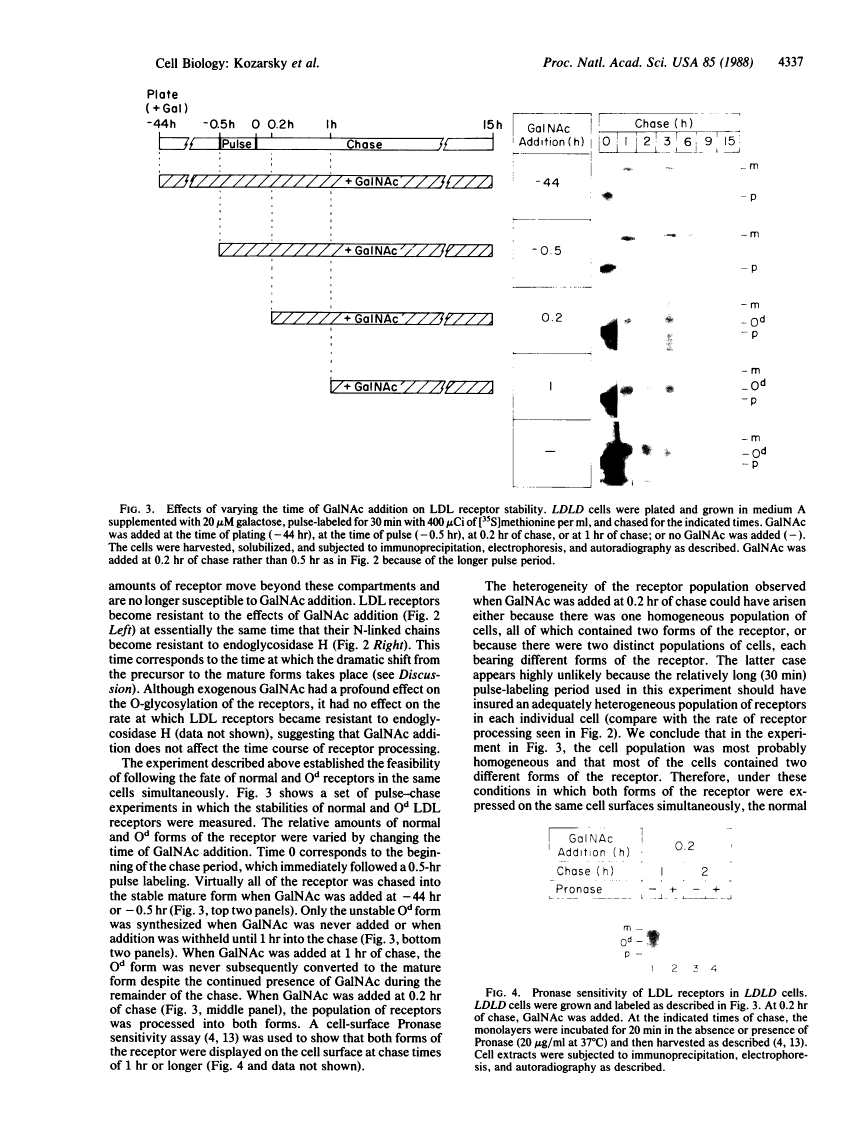
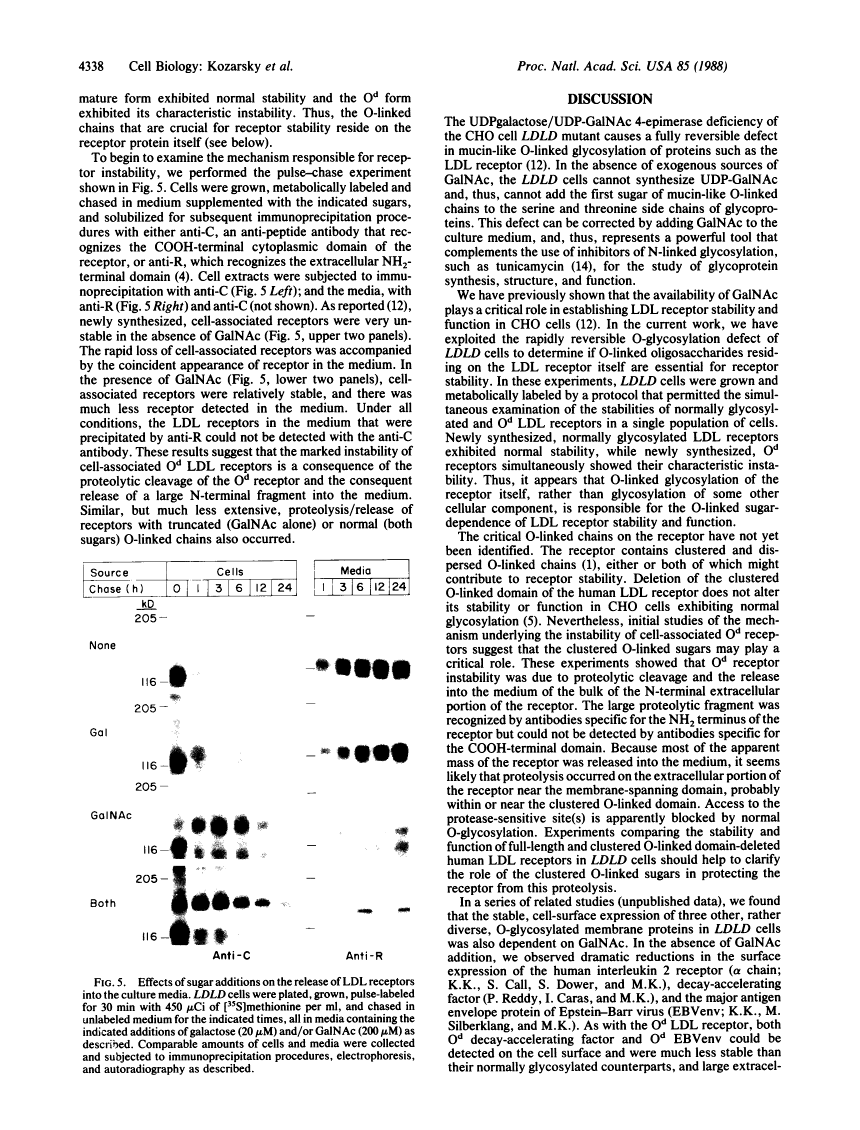
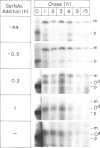
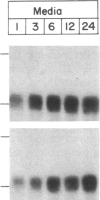
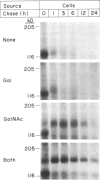
A rapidly reversible defect in protein O-glycosylation exhibited by a line of mutant Chinese hamster ovary (CHO) cells was used to study the kinetics and function of O-glycosylation of the low density lipoprotein (LDL) receptor. The mutant line, genotype LDLD, cannot synthesize UDP-N-acetylgalactosamine under normal culture conditions and, therefore, cannot add mucin-type O-linked oligosaccharides to proteins. The UDP-N-acetylgalactosamine pools in LDLD cells can be filled rapidly when N-acetylgalactosamine is added to the culture medium, thus restoring normal synthesis of O-linked carbohydrates. Pulse-chase metabolic labeling experiments were used to show that (i) the first step in the O-glycosylation of LDL receptors can occur posttranslationally; (ii) after O-linked sugar-deficient LDL receptors reach the cell surface, they are not subject to subsequent O-linked sugar addition, suggesting that they do not return to compartments in which O-glycosylation takes place; (iii) O-linked carbohydrate chains on the LDL receptor itself are required for normal stability and function; and (iv) the instability of the O-linked sugar-deficient LDL receptor is due to proteolytic cleavage and the release into the medium of the bulk of the NH2-terminal extracellular domain of the receptor. It appears that O-glycosylation of the LDL receptor and several other cell surface glycoproteins permits stable cell-surface expression by preventing proteolytic cleavage of the extracellular domains of these proteins.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.4M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goldstein JL, Brown MS, Anderson RG, Russell DW, Schneider WJ. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. [Abstract] [Google Scholar]
- Cummings RD, Kornfeld S, Schneider WJ, Hobgood KK, Tolleshaug H, Brown MS, Goldstein JL. Biosynthesis of N- and O-linked oligosaccharides of the low density lipoprotein receptor. J Biol Chem. 1983 Dec 25;258(24):15261–15273. [Abstract] [Google Scholar]
- Kozarsky KF, Brush HA, Krieger M. Unusual forms of low density lipoprotein receptors in hamster cell mutants with defects in the receptor structural gene. J Cell Biol. 1986 May;102(5):1567–1575. [Europe PMC free article] [Abstract] [Google Scholar]
- Davis CG, Elhammer A, Russell DW, Schneider WJ, Kornfeld S, Brown MS, Goldstein JL. Deletion of clustered O-linked carbohydrates does not impair function of low density lipoprotein receptor in transfected fibroblasts. J Biol Chem. 1986 Feb 25;261(6):2828–2838. [Abstract] [Google Scholar]
- Krieger M, Brown MS, Goldstein JL. Isolation of Chinese hamster cell mutants defective in the receptor-mediated endocytosis of low density lipoprotein. J Mol Biol. 1981 Aug 5;150(2):167–184. [Abstract] [Google Scholar]
- Krieger M, Martin J, Segal M, Kingsley D. Amphotericin B selection of mutant Chinese hamster cells with defects in the receptor-mediated endocytosis of low density lipoprotein and cholesterol biosynthesis. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5607–5611. [Europe PMC free article] [Abstract] [Google Scholar]
- Krieger M. Complementation of mutations in the LDL pathway of receptor-mediated endocytosis by cocultivation of LDL receptor-defective hamster cell mutants. Cell. 1983 Jun;33(2):413–422. [Abstract] [Google Scholar]
- Kingsley DM, Krieger M. Receptor-mediated endocytosis of low density lipoprotein: somatic cell mutants define multiple genes required for expression of surface-receptor activity. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5454–5458. [Europe PMC free article] [Abstract] [Google Scholar]
- Kingsley DM, Kozarsky KF, Hobbie L, Krieger M. Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/UDP-GalNAc 4-epimerase deficient mutant. Cell. 1986 Mar 14;44(5):749–759. [Abstract] [Google Scholar]
- Tolleshaug H, Hobgood KK, Brown MS, Goldstein JL. The LDL receptor locus in familial hypercholesterolemia: multiple mutations disrupt transport and processing of a membrane receptor. Cell. 1983 Mar;32(3):941–951. [Abstract] [Google Scholar]
- Strous GJ. Initial glycosylation of proteins with acetylgalactosaminylserine linkages. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2694–2698. [Europe PMC free article] [Abstract] [Google Scholar]
- Patzelt C, Weber B. Early O-glycosidic glycosylation of proglucagon in pancreatic islets: an unusual type of prohormonal modification. EMBO J. 1986 Sep;5(9):2103–2108. [Europe PMC free article] [Abstract] [Google Scholar]
- Abeijon C, Hirschberg CB. Subcellular site of synthesis of the N-acetylgalactosamine (alpha 1-0) serine (or threonine) linkage in rat liver. J Biol Chem. 1987 Mar 25;262(9):4153–4159. [Abstract] [Google Scholar]
- Elhammer A, Kornfeld S. Two enzymes involved in the synthesis of O-linked oligosaccharides are localized on membranes of different densities in mouse lymphoma BW5147 cells. J Cell Biol. 1984 Jul;99(1 Pt 1):327–331. [Europe PMC free article] [Abstract] [Google Scholar]
- White DA, Speake BK. The effect of cycloheximide on the glycosylation of lactating-rabbit mammary glycoproteins. Biochem J. 1980 Oct 15;192(1):297–301. [Europe PMC free article] [Abstract] [Google Scholar]
- Hanover JA, Elting J, Mintz GR, Lennarz WJ. Temporal aspects of the N- and O-glycosylation of human chorionic gonadotropin. J Biol Chem. 1982 Sep 10;257(17):10172–10177. [Abstract] [Google Scholar]
- Kimura JH, Lohmander LS, Hascall VC. Studies on the biosynthesis of cartilage proteoglycan in a model system of cultured chondrocytes from the Swarm rat chondrosarcoma. J Cell Biochem. 1984;26(4):261–278. [Abstract] [Google Scholar]
- Lohmander LS, Hascall VC, Yanagishita M, Kuettner KE, Kimura JH. Post-translational events in proteoglycan synthesis: kinetics of synthesis of chondroitin sulfate and oligosaccharides on the core protein. Arch Biochem Biophys. 1986 Oct;250(1):211–227. [Abstract] [Google Scholar]
- Hanover JA, Lennarz WJ, Young JD. Synthesis of N- and O-linked glycopeptides in oviduct membrane preparations. J Biol Chem. 1980 Jul 25;255(14):6713–6716. [Abstract] [Google Scholar]
- Niemann H, Boschek B, Evans D, Rosing M, Tamura T, Klenk HD. Post-translational glycosylation of coronavirus glycoprotein E1: inhibition by monensin. EMBO J. 1982;1(12):1499–1504. [Europe PMC free article] [Abstract] [Google Scholar]
- Shida H, Matsumoto S. Analysis of the hemagglutinin glycoprotein from mutants of vaccinia virus that accumulates on the nuclear envelope. Cell. 1983 Jun;33(2):423–434. [Abstract] [Google Scholar]
- Roth J. Cytochemical localization of terminal N-acetyl-D-galactosamine residues in cellular compartments of intestinal goblet cells: implications for the topology of O-glycosylation. J Cell Biol. 1984 Feb;98(2):399–406. [Europe PMC free article] [Abstract] [Google Scholar]
- Spielman J, Rockley NL, Carraway KL. Temporal aspects of O-glycosylation and cell surface expression of ascites sialoglycoprotein-1, the major cell surface sialomucin of 13762 mammary ascites tumor cells. J Biol Chem. 1987 Jan 5;262(1):269–275. [Abstract] [Google Scholar]
- Dunphy WG, Brands R, Rothman JE. Attachment of terminal N-acetylglucosamine to asparagine-linked oligosaccharides occurs in central cisternae of the Golgi stack. Cell. 1985 Feb;40(2):463–472. [Abstract] [Google Scholar]
- Snider MD, Rogers OC. Intracellular movement of cell surface receptors after endocytosis: resialylation of asialo-transferrin receptor in human erythroleukemia cells. J Cell Biol. 1985 Mar;100(3):826–834. [Europe PMC free article] [Abstract] [Google Scholar]
- Snider MD, Rogers OC. Membrane traffic in animal cells: cellular glycoproteins return to the site of Golgi mannosidase I. J Cell Biol. 1986 Jul;103(1):265–275. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.85.12.4335
Read article for free, from open access legal sources, via Unpaywall:
https://www.pnas.org/content/pnas/85/12/4335.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.85.12.4335
Article citations
Substrate O-glycosylation actively regulates extracellular proteolysis.
Protein Sci, 33(8):e5128, 01 Aug 2024
Cited by: 0 articles | PMID: 39074261 | PMCID: PMC11285871
Cosmc regulates O-glycan extension in murine hepatocytes.
Glycobiology, 34(10):cwae069, 01 Aug 2024
Cited by: 0 articles | PMID: 39216105 | PMCID: PMC11398974
Severe kidney dysfunction in sialidosis mice reveals an essential role for neuraminidase 1 in reabsorption.
JCI Insight, 8(20):e166470, 23 Oct 2023
Cited by: 2 articles | PMID: 37698928 | PMCID: PMC10619504
Truncation of GalNAc-type O-glycans Suppresses CD44-mediated Osteoclastogenesis and Bone Metastasis in Breast Cancer.
Mol Cancer Res, 21(7):664-674, 01 Jul 2023
Cited by: 3 articles | PMID: 37040171 | PMCID: PMC10320474
Deciphering Protein O-GalNAcylation: Method Development and Disease Implication.
ACS Omega, 8(22):19223-19236, 24 May 2023
Cited by: 2 articles | PMID: 37305274 | PMCID: PMC10249083
Review Free full text in Europe PMC
Go to all (88) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The role of O-linked sugars in determining the very low density lipoprotein receptor stability or release from the cell.
FEBS Lett, 451(1):56-62, 01 May 1999
Cited by: 47 articles | PMID: 10356983
Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/UDP-GalNAc 4-epimerase deficient mutant.
Cell, 44(5):749-759, 01 Mar 1986
Cited by: 179 articles | PMID: 3948246
Expression of ApoE gene in Chinese hamster cells with a reversible defect in O-glycosylation. Glycosylation is not required for apoE secretion.
J Biol Chem, 264(16):9137-9140, 01 Jun 1989
Cited by: 22 articles | PMID: 2498327
Glycosylation mutations of serine/threonine-linked oligosaccharides in low-density lipoprotein receptor: indispensable roles of O-glycosylation.
J Cell Sci, 98 ( Pt 2):131-134, 01 Feb 1991
Cited by: 9 articles | PMID: 1905298
Review