Abstract
Free full text

Hemin utilization is related to virulence of Streptococcus pneumoniae.
Abstract
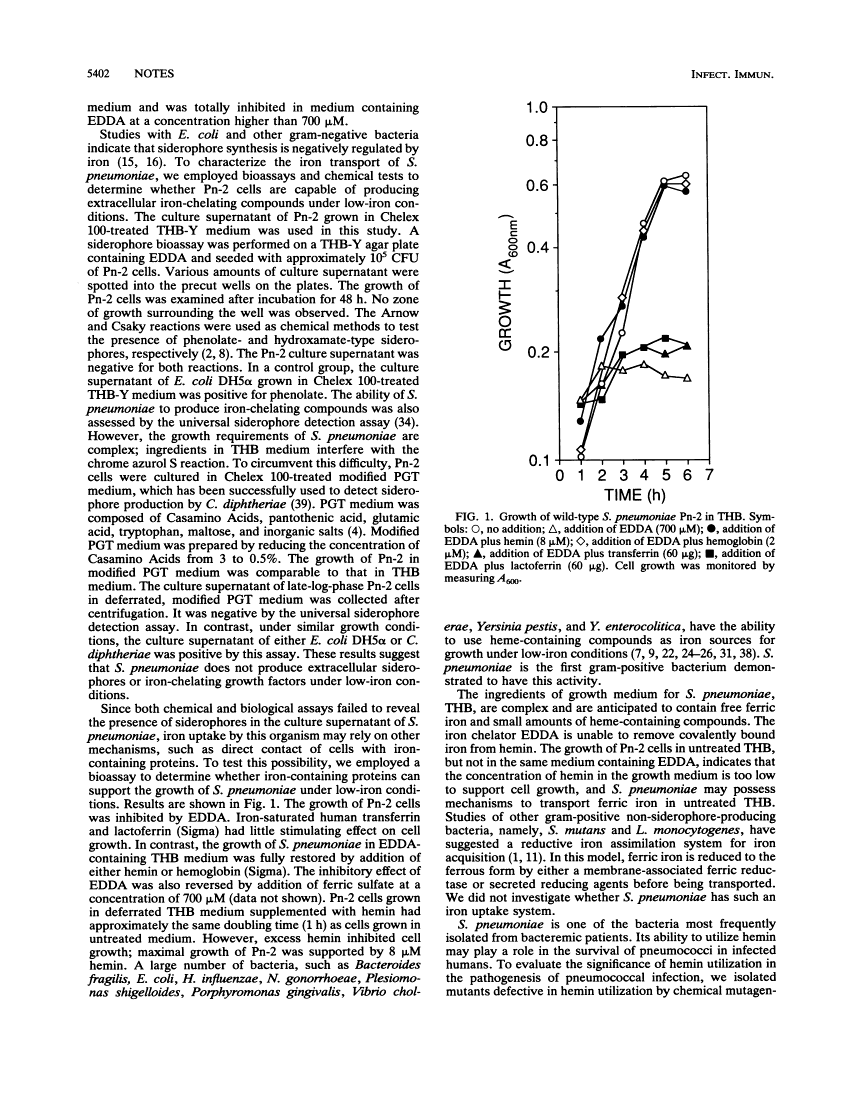
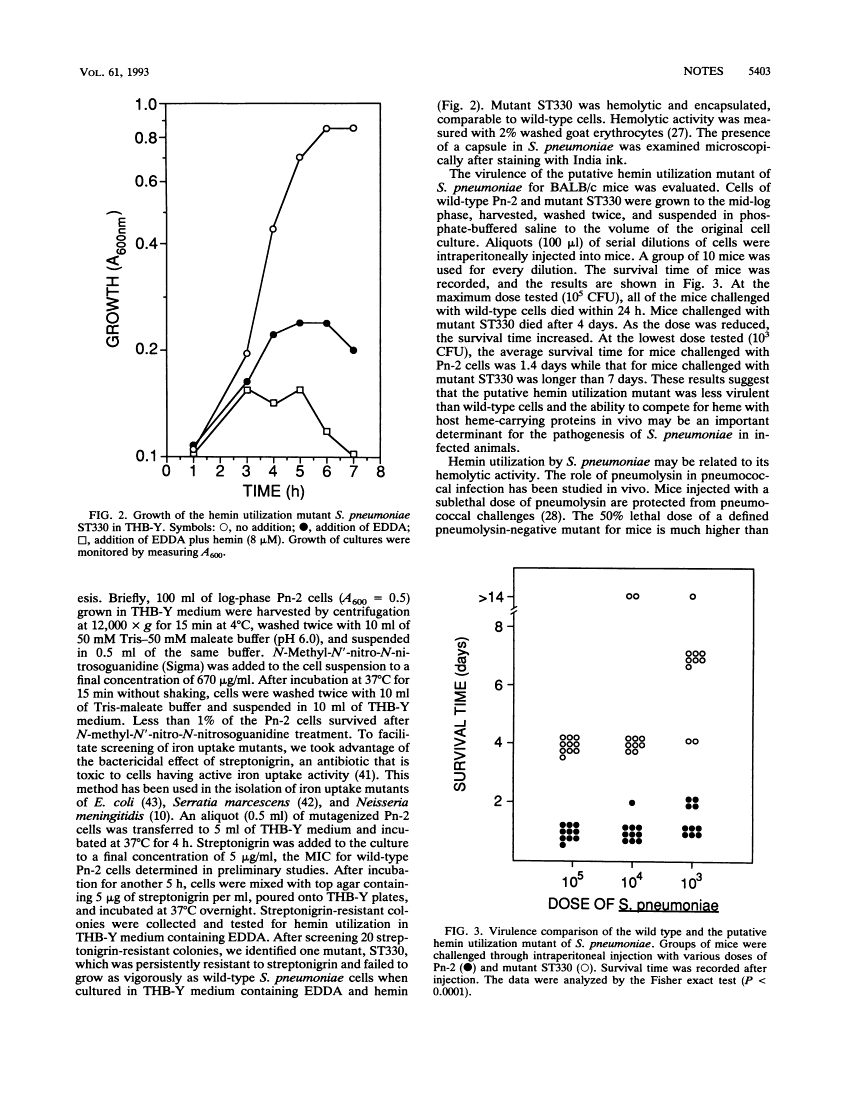
Streptococcus pneumoniae is a causative agent for bacterial pneumonia, otitis media, meningitis, and bacteremia. Mechanisms for acquisition of iron by this organism under low-iron conditions were investigated. Siderophore production was not detected by either chemical or biological methods. Its utilization of iron-containing compounds found in human hosts was tested. Both hemin and hemoglobin supported the full growth of S. pneumoniae in a culture lacking other iron sources, while lactoferrin and transferrin failed to do so. A mutant defective in hemin utilization was isolated and was less virulent than wild-type S. pneumoniae in experimental animals.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (853K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams TJ, Vartivarian S, Cowart RE. Iron acquisition systems of Listeria monocytogenes. Infect Immun. 1990 Aug;58(8):2715–2718. [Europe PMC free article] [Abstract] [Google Scholar]
- Bagg A, Neilands JB. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. [Europe PMC free article] [Abstract] [Google Scholar]
- BARDSDALE WL, PAPPENHEIMER AM., Jr Phage-host relationships in nontoxigenic and toxigenic diphtheria bacilli. J Bacteriol. 1954 Feb;67(2):220–232. [Europe PMC free article] [Abstract] [Google Scholar]
- Berry AM, Yother J, Briles DE, Hansman D, Paton JC. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989 Jul;57(7):2037–2042. [Europe PMC free article] [Abstract] [Google Scholar]
- Daskaleros PA, Stoebner JA, Payne SM. Iron uptake in Plesiomonas shigelloides: cloning of the genes for the heme-iron uptake system. Infect Immun. 1991 Aug;59(8):2706–2711. [Europe PMC free article] [Abstract] [Google Scholar]
- Dyer DW, McKenna W, Woods JP, Sparling PF. Isolation by streptonigrin enrichment and characterization of a transferrin-specific iron uptake mutant of Neisseria meningitidis. Microb Pathog. 1987 Nov;3(5):351–363. [Abstract] [Google Scholar]
- Evans SL, Arceneaux JE, Byers BR, Martin ME, Aranha H. Ferrous iron transport in Streptococcus mutans. J Bacteriol. 1986 Dec;168(3):1096–1099. [Europe PMC free article] [Abstract] [Google Scholar]
- Ferrante A, Rowan-Kelly B, Paton JC. Inhibition of in vitro human lymphocyte response by the pneumococcal toxin pneumolysin. Infect Immun. 1984 Nov;46(2):585–589. [Europe PMC free article] [Abstract] [Google Scholar]
- Gillespie SH. Aspects of pneumococcal infection including bacterial virulence, host response and vaccination. J Med Microbiol. 1989 Apr;28(4):237–248. [Abstract] [Google Scholar]
- Hanson MS, Hansen EJ. Molecular cloning, partial purification, and characterization of a haemin-binding lipoprotein from Haemophilus influenzae type b. Mol Microbiol. 1991 Feb;5(2):267–278. [Abstract] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. [Abstract] [Google Scholar]
- Herrington DA, Sparling PF. Haemophilus influenzae can use human transferrin as a sole source for required iron. Infect Immun. 1985 Apr;48(1):248–251. [Europe PMC free article] [Abstract] [Google Scholar]
- Kilian M, Mestecky J, Schrohenloher RE. Pathogenic species of the genus Haemophilus and Streptococcus pneumoniae produce immunoglobulin A1 protease. Infect Immun. 1979 Oct;26(1):143–149. [Europe PMC free article] [Abstract] [Google Scholar]
- Law D, Wilkie KM, Freeman R, Gould FK. The iron uptake mechanisms of enteropathogenic Escherichia coli: the use of haem and haemoglobin during growth in an iron-limited environment. J Med Microbiol. 1992 Jul;37(1):15–21. [Abstract] [Google Scholar]
- McKee AS, McDermid AS, Baskerville A, Dowsett AB, Ellwood DC, Marsh PD. Effect of hemin on the physiology and virulence of Bacteroides gingivalis W50. Infect Immun. 1986 May;52(2):349–355. [Europe PMC free article] [Abstract] [Google Scholar]
- Mickelsen PA, Sparling PF. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun. 1981 Aug;33(2):555–564. [Europe PMC free article] [Abstract] [Google Scholar]
- Otto BR, Sparrius M, Verweij-van Vught AM, MacLaren DM. Iron-regulated outer membrane protein of Bacteroides fragilis involved in heme uptake. Infect Immun. 1990 Dec;58(12):3954–3958. [Europe PMC free article] [Abstract] [Google Scholar]
- Paton JC, Ferrante A. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect Immun. 1983 Sep;41(3):1212–1216. [Europe PMC free article] [Abstract] [Google Scholar]
- Paton JC, Lock RA, Hansman DJ. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect Immun. 1983 May;40(2):548–552. [Europe PMC free article] [Abstract] [Google Scholar]
- Paton JC, Rowan-Kelly B, Ferrante A. Activation of human complement by the pneumococcal toxin pneumolysin. Infect Immun. 1984 Mar;43(3):1085–1087. [Europe PMC free article] [Abstract] [Google Scholar]
- Payne SM. Iron and virulence in the family Enterobacteriaceae. Crit Rev Microbiol. 1988;16(2):81–111. [Abstract] [Google Scholar]
- Perry RD, Brubaker RR. Accumulation of iron by yersiniae. J Bacteriol. 1979 Mar;137(3):1290–1298. [Europe PMC free article] [Abstract] [Google Scholar]
- Russell LM, Holmes RK. Initial characterization of the ferric iron transport system of Corynebacterium diphtheriae. J Bacteriol. 1983 Sep;155(3):1439–1442. [Europe PMC free article] [Abstract] [Google Scholar]
- Schneider R, Hantke K. Iron-hydroxamate uptake systems in Bacillus subtilis: identification of a lipoprotein as part of a binding protein-dependent transport system. Mol Microbiol. 1993 Apr;8(1):111–121. [Abstract] [Google Scholar]
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. [Abstract] [Google Scholar]
- Snow GA. Mycobactins: iron-chelating growth factors from mycobacteria. Bacteriol Rev. 1970 Jun;34(2):99–125. [Europe PMC free article] [Abstract] [Google Scholar]
- Stahl WL, O'Toole RD. Pneumococcal neuraminidase: purification and properties. Biochim Biophys Acta. 1972 May 12;268(2):480–487. [Abstract] [Google Scholar]
- Stoebner JA, Payne SM. Iron-regulated hemolysin production and utilization of heme and hemoglobin by Vibrio cholerae. Infect Immun. 1988 Nov;56(11):2891–2895. [Europe PMC free article] [Abstract] [Google Scholar]
- Tai SP, Krafft AE, Nootheti P, Holmes RK. Coordinate regulation of siderophore and diphtheria toxin production by iron in Corynebacterium diphtheriae. Microb Pathog. 1990 Oct;9(4):267–273. [Abstract] [Google Scholar]
- Yeowell HN, White JR. Iron requirement in the bactericidal mechanism of streptonigrin. Antimicrob Agents Chemother. 1982 Dec;22(6):961–968. [Europe PMC free article] [Abstract] [Google Scholar]
- Zimmermann L, Angerer A, Braun V. Mechanistically novel iron(III) transport system in Serratia marcescens. J Bacteriol. 1989 Jan;171(1):238–243. [Europe PMC free article] [Abstract] [Google Scholar]
- Zimmermann L, Hantke K, Braun V. Exogenous induction of the iron dicitrate transport system of Escherichia coli K-12. J Bacteriol. 1984 Jul;159(1):271–277. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.61.12.5401-5405.1993
Read article for free, from open access legal sources, via Unpaywall:
https://iai.asm.org/content/iai/61/12/5401.full.pdf
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/reprint/61/12/5401
Free to read at iai.asm.org
http://iai.asm.org/cgi/content/abstract/61/12/5401
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/iai.61.12.5401-5405.1993
Article citations
Media Matters, Examining Historical and Modern Streptococcus pneumoniae Growth Media and the Experiments They Affect.
Front Cell Infect Microbiol, 11:613623, 23 Mar 2021
Cited by: 5 articles | PMID: 33834003 | PMCID: PMC8021847
Review Free full text in Europe PMC
Iron Acquisition Systems of Gram-negative Bacterial Pathogens Define TonB-Dependent Pathways to Novel Antibiotics.
Chem Rev, 121(9):5193-5239, 16 Mar 2021
Cited by: 45 articles | PMID: 33724814 | PMCID: PMC8687107
Review Free full text in Europe PMC
Hemoglobin stimulates vigorous growth of Streptococcus pneumoniae and shapes the pathogen's global transcriptome.
Sci Rep, 10(1):15202, 16 Sep 2020
Cited by: 9 articles | PMID: 32938947 | PMCID: PMC7494912
The Pneumococcal Iron Uptake Protein A (PiuA) Specifically Recognizes Tetradentate FeIIIbis- and Mono-Catechol Complexes.
J Mol Biol, 432(19):5390-5410, 11 Aug 2020
Cited by: 7 articles | PMID: 32795535 | PMCID: PMC7502554
Iron Pathways and Iron Chelation Approaches in Viral, Microbial, and Fungal Infections.
Pharmaceuticals (Basel), 13(10):E275, 25 Sep 2020
Cited by: 19 articles | PMID: 32992923 | PMCID: PMC7601909
Review Free full text in Europe PMC
Go to all (71) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The pavA gene of Streptococcus pneumoniae encodes a fibronectin-binding protein that is essential for virulence.
Mol Microbiol, 41(6):1395-1408, 01 Sep 2001
Cited by: 142 articles | PMID: 11580843
Characterization of hemin binding activity of Streptococcus pneumoniae.
Infect Immun, 65(3):1083-1087, 01 Mar 1997
Cited by: 21 articles | PMID: 9038319 | PMCID: PMC175091
A solute binding protein of Streptococcus pneumoniae iron transport.
FEMS Microbiol Lett, 220(2):303-308, 01 Mar 2003
Cited by: 30 articles | PMID: 12670696
Sickly Sweet - How Sugar Utilization Impacts Pneumococcal Disease Progression.
Trends Microbiol, 29(9):768-771, 18 Feb 2021
Cited by: 5 articles | PMID: 33612397
Review