Abstract
Free full text

Local sensory nerve control of skin blood flow during local warming in type 2 diabetes mellitus
Abstract
Cutaneous sensory nerve-mediated vasodilation is an important component of normal microvascular responsiveness to thermal and nonthermal stimuli. Since both neural and microvascular function can be impaired in type 2 diabetes mellitus (T2DM), we tested the hypothesis that local sensory nerve-mediated vasodilation during nonpainful local warming of the skin is less in T2DM compared with healthy controls (C) matched for age and body size. The rapid vasodilation during the first ~5 min of this local warming (“initial peak”) was previously shown to rely primarily on local sensory nerves. We measured skin blood flow in T2DM and C subjects (n = 7 in each group) at baseline and during 35 min of local warming of the skin to 42°C at two sites on the ventral forearm. One site was pretreated with 4% lidocaine (LIDO) to block local sensory innervation. During local warming, cutaneous vascular conductance (CVC) during the initial peak was not different between groups, either at the untreated site [T2DM 75 ± 2 vs. C 81 ± 6% of maximum CVC (%maxCVC); P > 0.05] or at the LIDO site (T2DM 63 ± 7 vs. C 64 ± 6%maxCVC; P > 0.05). The difference between untreated and LIDO sites (sensory nerve contribution) was also similar between groups (T2DM 13 ± 5 vs. C 18 ± 5%maxCVC; P > 0.05) and was smaller with LIDO than was previously shown with other local anesthetics. Our results suggest that relatively healthy individuals with T2DM do not exhibit impairments in local sensory nerve vasodilation during thermal stimulation compared with controls of similar age and body size.
in humans, nonpainful local warming of the skin elicits a biphasic vasodilation involving local sensory nerve mechanisms and nitric oxide (6, 8). Initial, rapid blood flow increases during the first 3–5 min (called the “initial peak” of the vasodilator response) are thought to be caused primarily by direct action of local sensory nerves, potentially through release of local vasodilators such as calcitonin gene-related peptide (CGRP) and substance P (5, 8). The second phase of the vasodilation is nitric oxide dependent and occurs over a longer period, typically 25–30 min, after which a plateau in blood flow is reached (6, 8).
Type 2 diabetes mellitus (T2DM) is strongly associated with neural and microvascular dysfunction. As the incidence and prevalence of T2DM are increasing, also increasing are the number of individuals who are otherwise relatively healthy and live with T2DM but manage it well so that the number of comorbid conditions is minimal. We have therefore focused our efforts on this growing population in our attempts to understand mechanisms of cutaneous microvascular dysfunction in T2DM (12–14). Recent studies in our laboratory have shown that relatively healthy individuals with T2DM exhibit less nitric oxide-dependent vasodilation during local and whole body thermal stimuli compared with age-matched controls (12, 13). Peripheral neuropathy is likely linked to microvascular dysfunction in T2DM, but specific mechanisms of diminished sensory nerve-mediated vasodilation during local warming of the skin are, as yet, undefined.
Impairments in sensory nerve function often contribute significantly to the morbidity associated with T2DM. In individuals with poorly managed T2DM, the progression of the diabetes is often associated with symptomatic neuropathy ranging from loss of sensation to chronically painful neuropathy (7, 10). Although we did not study individuals with significant comorbid conditions, we reasoned that a low level of sensory neural dysfunction might contribute to impaired sensory nerve-mediated vasodilation, even in individuals in whom clinically significant neuropathy is absent.
Therefore, our goal in the present study was to assess whether local sensory nerve-mediated vasodilation during local warming of the skin is impaired in individuals with T2DM who are otherwise healthy. We measured skin blood flow at the ventral forearm using laser-Doppler flowmetry (LDF), and local lidocaine application was used to block sensory innervation in a small area of skin. We tested the hypothesis that the local sensory nerve contribution to vasodilation during local warming is less in T2DM compared with healthy control subjects.
METHODS
Subjects
The study group included seven individuals with T2DM and seven control subjects of similar age and body size. All subjects were screened via telephone to rule out any comorbidities that could influence microvascular function. Subjects were excluded if they showed signs/symptoms of neuropathy or had a history of cardiovascular disease. In addition, any subjects taking thiazolidinediones or first-generation sulfonylureas were excluded from participation; other antidiabetic agents were accepted. As noted below, if a T2DM subject was taking an accepted agent, he or she stopped taking the medication for 2 wk before the study day. Of the five women participating in this study, two were premenopausal and three were postmenopausal but were not taking hormone replacement therapy. Subjects ranged from being sedentary to recreationally active; we did not include subjects with high levels of fitness or physical activity.
Subjects reported to the Mayo Clinical Research Unit (CRU) for a screening visit and again on the day of the study. Written informed consent was obtained before any procedures began. The protocol for this experiment was approved by the Institutional Review Board at the Mayo Clinic in Rochester, MN. Any T2DM subjects taking oral antidiabetic agents were instructed to temporarily withdraw their medication for 2 wk before the study visit. Subjects were requested to monitor their reflectance meter glucose values daily at home. If glucose values exceeded 300 mg/dl on more than one occasion during the 2-wk period, they were asked to resume their antidiabetic medications and were excluded from further participation.
Screening Visit
All subjects had a 12-lead ECG, and fasting blood samples were drawn and analyzed for glucose and hemoglobin A1c. Body composition was measured using dual-energy X-ray absorptiometry (DEXA). All individuals with T2DM, and all control subjects over 55 years of age, participated in a treadmill exercise test conducted at the Mayo Cardiovascular Health Clinic using a standard Bruce protocol, to identify and exclude subjects with occult cardiovascular disease.
Study Visit
Instrumentation and measurements.
Subjects arrived at the CRU at 7:00 A.M. after an overnight fast. Blood glucose was measured in all T2DM subjects before any procedures began. Two intradermal microdialysis fibers (MD-2000; Bioanalytical Systems) were placed in the skin on the ventral surface of the left forearm of each subject, as previously described (12, 13). Ice was placed on the skin surface for 2–3 min to numb the area. Then 25-gauge needles were inserted just under the surface of the skin with entry and exit points ~2.5 cm apart. Fibers were threaded through the lumen of each needle so that the 1-cm microdialysis membrane was completely within the dermis, and the needles were removed. The two fibers were placed at least 5 cm apart and taped in place. We then waited 60–90 min for the hyperemia related to needle insertion to resolve.
Our goal in placing microdialysis fibers was to allow us to use local microdialysis of sodium nitroprusside (SNP) for assessment of maximal cutaneous vascular conductance (CVC) at the end of each experiment. Before and during local warming, both sites received Ringer solution at 4 μl/min. One site served as a control site, and the other was used for topical lidocaine (LIDO) application for local inhibition of sensory nerves. Additional details about timing are described in Experimental Protocol.
Skin blood flow was measured as cutaneous red blood cell flux using Periflux laser-Doppler flowmetry (LDF) over the two microdialysis sites. Two integrating LDF probes (Periflux System 5000; Perimed, Stockholm, Sweden) were attached by adhesive to the left forearm to measure skin blood flow. The probes were attached to the skin in specialized holders that measured and controlled temperature over an area of 12 cm2 (Perimed). Each probe was placed directly over a microdialysis membrane. Subjects were instrumented with a three-lead ECG and blood pressure was measured beat-to-beat using a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands) placed on the left middle finger.
Experimental protocol.
Subjects remained seated for 30–45 min following fiber placement; they then moved to the supine position for the remaining instrumentation and experimental protocol. Topical LIDO cream (4%) was applied and was allowed to act for an additional 45 min before the cream was removed and the site was tested for sensation. At this point (~90 min after microdialysis fiber placement) sensory nerve blockade was tested by slight pinprick with a sterile needle; if sensation persisted, lidocaine was reapplied for an additional 15 min. No subject experienced persistent sensation after this additional application. Data recording was started at this point to determine the subsiding of any latent hyperemia due to trauma from microdialysis fiber placement. In this way, subjects had rested supine for at least 60 min before data recording began. We determined that a steady baseline had been reached when the LDF signal had decreased to a stable plateau for ≥10 min; at that time, we began data collection for the baseline period before local warming. Both sites were perfused with Ringer solution at a rate of 4 μl/min using a microperfusion pump (Harvard 22 syringe pump; Harvard Apparatus, South Natick, MA). Ringer infusion was continued at both sites throughout the subsequent local heating period.
Baseline data were recorded for 5 min while local temperature was held constant at 32°C. For local heating of the skin, local temperature was increased at both LDF sites to 42°C at a rate of ~1° every 7 s. A local temperature of 42°C was held constant for 35 min. Subjects were asked whether they felt any pain or discomfort during the local warming; no subject reported feeling any pain or discomfort. SNP (28 mM) was then infused at both LIDO and untreated sites for ~40 min to elicit maximum CVC (12, 13). Ambient temperature in the study room was held at 22–24°C during all experiments. Subjects were asked to report whole body thermal sensation, and all subjects reported feeling thermoneutral (not particularly warm or cold) throughout all experiments.
Data Recording and Analysis
LDF, ECG, and beat-to-beat blood pressure data were collected at 77 Hz using the Windaq data-acquisition system (Dataq Instruments, Akron OH) and stored for offline analysis. Skin blood flow (LDF) was divided by mean arterial pressure to calculate CVC. During local warming, initial peak CVC was assessed as a 30-s average during the highest point of the initial peak of the vasodilator response, and nadir CVC as a 30-s average during the short decline in blood flow after this initial peak. Plateau values were evaluated as the average of 2 min at the end of local warming before the onset of SNP microdialysis.
Maximum CVC was assessed as the average of the last 3 min during SNP microdialysis. CVC data are expressed as a percentage of this maximum (%maxCVC). To quantify the relative contribution of sensory nerves to the vasodilator responses observed in control subjects and T2DM subjects, we compared untreated sites with LIDO sites in both groups. The sensory nerve contribution was assessed by subtracting CVC at LIDO-treated sites from that at untreated sites during the initial peak of the vasodilator response during local warming. CVC data were compared between T2DM and control groups using Student's t-test; data were compared within groups by paired t-test. For all comparisons, P < 0.05 was accepted as statistically significant.
Power Analysis
Based on our previous work we estimated a priori a minimum difference to be detected of 13–18%maxCVC and an average SD of 8–11%maxCVC. Our sample size (n = 7 per group) gave us 80% power to detect these differences.
RESULTS
Subject Characteristics
As shown in Table 1, individuals in the T2DM group were similar to controls when comparing age, height, weight, body mass index (BMI), and percent body fat (P > 0.05 between groups). As expected, individuals with T2DM had higher fasting plasma glucose values and higher hemoglobin A1c levels (P < 0.02). Of the subjects in the T2DM group, five took metformin, one took metformin plus glimepiride, and one subject's diabetes was controlled by diet alone. As noted above all subjects stopped any antidiabetic medications for 2 wk before participation.
Table 1.
Descriptive characteristics of subjects in control and T2DM groups
| Control | T2DM | |||
|---|---|---|---|---|
| Mean | Range | Mean | Range | |
| n | 7 (3F) | 7 (2F) | ||
| Age, yr | 55 ± 5 | 40–70 | 56 ± 6 | 47–70 |
| Height, cm | 173 ± 4 | 162–187 | 175 ± 2 | 169–184 |
| Weight, kg | 76 ± 7 | 58–103 | 83 ± 3 | 76–95 |
| BMI, kg/m2 | 25.3 ± 2.1 | 19.7–31.8 | 27.1 ± 0.9 | 25.0–31.3 |
| %Body fat | 33.6 ± 5.9 | 12.2–45.6 | 33.0 ± 3.4 | 25.0–45.9 |
| Fasting glucose, mg/dl | 93 ± 4 | 82–107 | 149 ± 15* | 115–229 |
| HbA1c, % | 5.5 ± 0.1 | 5.1–5.7 | 6.6 ± 0.3* | 5.9–7.0 |
Values are means ± SE. F, female; T2DM, type 2 diabetes mellitus; BMI, body mass index; HbA1c, hemoglobin A1c.
CVC Responses
CVC at the untreated site during the initial peak of the vasodilator response was not different between groups (T2DM 75 ± 2 vs. control 81 ± 6% maxCVC; P > 0.05). In both groups, LIDO treatment caused a significant decrease in CVC during the initial peak (P < 0.05). The initial peak values for CVC at LIDO sites were also not different between groups (T2DM 63 ± 7 vs. control 64 ± 6% maxCVC; P > 0.05). Figure 1 shows a representative tracing of the initial peak and nadir of the CVC response during local warming at untreated and LIDO sites in a control subject, showing the decrease in initial peak CVC with LIDO treatment. Figure 2 shows average responses for initial peak CVC in both groups at untreated and LIDO sites.
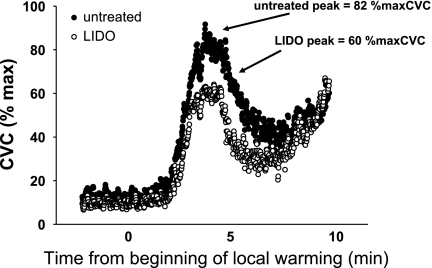
Individual record showing representative initial peak and nadir vasodilator responses during local warming of the skin in a control subject at an untreated site (filled symbols) and a lidocaine (LIDO)-treated site (open symbols). Note inhibition of initial peak vasodilator response at the LIDO site; this was consistent across all control and type 2 diabetes mellitus (T2DM) subjects. CVC, cutaneous vascular conductance; %maxCVC, percent of maximum CVC.
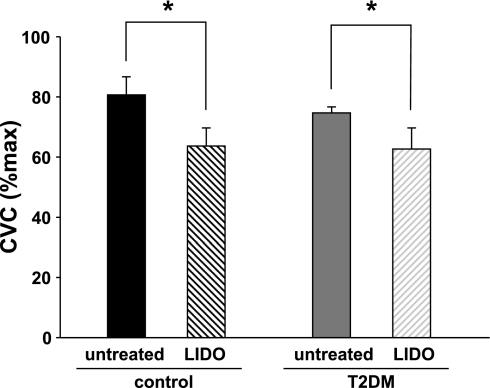
Average data from control and T2DM groups for initial peak vasodilation at untreated and LIDO-treated sites. Vasodilator response was significantly smaller at LIDO sites in both groups. However, the vasodilation was not different between groups at either site. *P < 0.05, untreated vs. LIDO.
The values for nadir CVC were also similar between groups, both at untreated sites (T2DM 64 ± 3 vs. control 57 ± 10%maxCVC; P > 0.10) and at LIDO sites (T2DM 46 ± 9 vs. control 36 ± 3%maxCVC; P > 0.10). The plateau values during local warming were not significantly affected by LIDO treatment, although there was a trend for a decrease in the control group (untreated 97 ± 3 vs. LIDO 92 ± 3%maxCVC, P = 0.08). In the T2DM group, plateau values were as follows: untreated 99 ± 5 vs. LIDO 91 ± 7%maxCVC, P > 0.10.
We used the difference between control and LIDO sites to evaluate the contribution of local sensory nerves to the initial peak vasodilation. This sensory nerve contribution was not different between groups (T2DM 13 ± 5 vs. control 18 ± 5% maxCVC; P > 0.05).
DISCUSSION
The main finding of the present study was that the initial rapid vasodilation (“initial peak”) during local warming of the skin was not different between a group of individuals with T2DM and healthy control subjects of similar age, body size, and adiposity. The sensory nerve contribution to this vasodilation, as assessed using local lidocaine application, was also not different between groups. Our findings suggest that individuals with relatively well-managed T2DM have the capacity to maintain local sensory nerve vasodilation in the skin in response to local thermal stimulation.
Nonpainful local warming (42°C) of the skin elicits a biphasic vasodilation consisting of two phases: an initial rapid peak during the first 3–5 min of warming, followed by a nadir, and then a slower nitric oxide-dependent phase that reaches a plateau after 25–30 min of warming (6, 8). The vascular response to locally warming the skin is not dependent on intact reflex innervation (8, 11), and thus appears to be an entirely local phenomenon. The initial peak of the vasodilation has been previously identified to rely on local sensory nerve mechanisms, as it was largely inhibited by local EMLA (topical 2.5% lidocaine, 2.5% prilocaine cream) (8). During rapid local warming, such as that performed in the present study, the initial peak was not greatly affected by local nitric oxide synthase inhibition with l-NAME microdialysis (8).
Our present data contrast somewhat with previous findings from our lab regarding other cutaneous vasodilator responses in T2DM. We previously reported that cutaneous vasodilation during whole body heating exhibited a delayed onset in T2DM subjects (14) and that overall vasodilator responses were lower at a given core temperature in T2DM compared with control subjects of similar age and body size (13). Additionally, we found that the prolonged local warming response (nitric oxide-dependent “plateau phase” vasodilation) was decreased in T2DM subjects (12, 14). We interpreted those previous findings to mean that decreased thermal and nitric oxide-dependent vasodilation may contribute to impaired thermoregulation in T2DM, even in relatively healthy individuals. The lack of difference between T2DM and control subjects in the present study regarding local sensory nerve vasodilation is likely due to different mechanisms of vasodilation between this and previous studies. Whereas the plateau phase of the local warming response, and the cutaneous vasodilation during whole body heating, both include substantial nitric oxide components, the initial peak during rapid local warming appears to include little or no role for nitric oxide (6, 8, 12, 13).
Interestingly, the pattern of cutaneous vasodilation during local warming can be variable depending on the rate of temperature increase. Using very slow local temperature increases (0.1°C per min), Houghton et al. were able to identify local temperature thresholds for local sensory nerve (axon reflex) vasodilation (5), which appeared as small, transient bursts of dilation during these slow temperature ramps. Those investigators used local pharmacological manipulation via microdialysis to show that these small bursts of vasodilation were inhibited (the temperature threshold was delayed) when either nitric oxide or the norepinephrine pathways were blocked (5). Also in support of roles for noradrenergic vasoconstrictor nerves and nitric oxide, Hodges et al. reported that presynaptic inhibition of vasoconstrictor nerves with bretylium and/or blockade of neuropeptide Y (a noradrenergic cotransmitter) inhibited axon reflex vasodilation in a similar slow local heating protocol (4). Interestingly, these authors showed in a subsequent study that bretylium also reduced the axon reflex vasodilation during rapid local heating (3). Clearly this vasodilator response is multifactorial, including potential roles for CGRP and substance P, as well as modulatory roles for nitric oxide and noradrenergic vasoconstrictor nerves. In the present work we have found that the initial peak response to rapid local warming, and the sensory nerve contribution as assessed using local lidocaine, did not appear to be affected in our T2DM subjects. However, we did not evaluate the pathways involved in slow local warming studied by Houghton et al. (5) and Hodges et al. (3, 4), so it remains unknown whether those may have been affected in our subjects.
Experimental Considerations
In our present work, we found that the contribution of local sensory nerves, as assessed by blockade with local lidocaine, was smaller than that which was reported using local application of EMLA cream in a previous study by Minson and colleagues (8). This was true in both groups of subjects, so it was likely not due to an effect of T2DM on the vasodilator process. There are a few methodological differences between our study and the previous work that might contribute to this difference between studies. First, EMLA contains 2.5% lidocaine and 2.5% prilocaine. In the present study, we chose to use a 4% lidocaine cream based on earlier reports of a vasoconstrictor effect of EMLA (1) and clinical observations of skin blanching with this medication. However, more recent work in the nail bed microcirculation indicated no vasoconstriction with EMLA (2), and another report showed no vasoconstriction with prilocaine in the small arteries of the rat tail (15). Therefore, it is possible that the mechanism of action of prilocaine (or some other difference between EMLA and lidocaine-only cream) could have contributed to greater inhibition of the initial peak vasodilation by Minson et al. (8). It is also worth noting that any potential generic vasoconstrictor effects of EMLA were minimal in the study of Minson et al. in terms of the second (plateau) phase of the vasodilator response, which was only slightly affected by EMLA (8). Another possible interpretation is that our lidocaine application provided incomplete local anesthesia. We do not believe this to be the case since our application was consistent with clinical guidelines, and local sensation was tested and found to be blocked in all subjects.
An additional consideration in this context is that, since diabetes is an age-related disease, our experimental design involved groups of subjects (both control and T2DM) who were significantly older than the group originally studied by Minson et al. (8). Since local sensory nerve-mediated vasodilation decreases with aging [as reported by Minson and colleagues in a subsequent study (9)], it is likely that the contributions of sensory nerves to local vasodilation were lower in our subjects compared with the younger group studied in the previous work (8).
It is not clear from the present data whether our findings could be extended to a more compromised population of individuals with diabetes. By design, our present group of subjects with T2DM did not have extensive comorbid conditions such as symptomatic neuropathy or retinopathy, nor were any included who had a history of cardiovascular disease. Thus these were clearly a relatively healthy group of individuals. In our view, these individuals are (unfortunately) representative of a growing group of individuals in the world today, in that they are not particularly “sick,” but they are chronically living with diabetes. However, had we studied a group of T2DM individuals who were less healthy, it is possible that we would have noted greater decrements in vasodilator responses than those we noted here. Similarly, it is important to note that even healthy individuals with T2DM represent a very heterogeneous population. Although we tried to limit our study subjects to relatively healthy individuals, we cannot tell from our present work whether an earlier history of poor glucose control could have caused neurovascular impairments that remain after good glucose control is achieved. For example, it is possible that a person who currently maintains very good glucose control may have impaired local neurovascular function due to poor glucose control during some unspecified period in the past. We are not able to address this possibility directly from our present data.
In summary, we present a comparison of cutaneous microvascular responses to local warming of the skin between individuals with T2DM and a control group of similar age, with specific focus on the sensory nerve-mediated vasodilation that occurs during the first few minutes of local warming. We report that the sensory nerve-mediated vasodilation did not appear to be impaired in our group of relatively healthy individuals with T2DM. We specifically chose this group of patients as they represent a large portion of the population with T2DM in the United States and across the world. In this context, we note that T2DM is a very heterogeneous condition, and that individuals with less well-managed disease, or more comorbid conditions, may have very different responses.
GRANTS
These studies were supported by National Institutes of Health Grant R01-HL-73884 and by Clinical and Translational Science Award (CTSA) Grant UL1-RR-024150 (to the Mayo Clinic).
ACKNOWLEDGMENTS
As always, we are grateful to our subjects for their patient and enthusiastic participation.
REFERENCES
Articles from Journal of Applied Physiology are provided here courtesy of American Physiological Society
Full text links
Read article at publisher's site: https://doi.org/10.1152/japplphysiol.01077.2009
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2822672
Free after 12 months at intl-jap.physiology.org
http://intl-jap.physiology.org/cgi/content/full/108/2/293
Free to read at intl-jap.physiology.org
http://intl-jap.physiology.org/cgi/content/abstract/108/2/293
Citations & impact
Impact metrics
Citations of article over time
Article citations
Relationships between transepidermal water loss, cutaneous microcirculatory function and autonomic nervous activity.
Int J Cosmet Sci, 39(3):275-283, 02 Nov 2016
Cited by: 7 articles | PMID: 27731900
Body temperature regulation in diabetes.
Temperature (Austin), 3(1):119-145, 04 Jan 2016
Cited by: 71 articles | PMID: 27227101 | PMCID: PMC4861190
Review Free full text in Europe PMC
The contribution of sensory nerves to cutaneous vasodilatation of the forearm and leg to local skin heating.
Eur J Appl Physiol, 115(10):2091-2098, 22 May 2015
Cited by: 13 articles | PMID: 25998144
Noninvasive examination of endothelial, sympathetic, and myogenic contributions to regional differences in the human cutaneous microcirculation.
Microvasc Res, 93:87-91, 15 Apr 2014
Cited by: 23 articles | PMID: 24742702
Comparison of the noradrenergic sympathetic nerve contribution during local skin heating at forearm and leg sites in humans.
Eur J Appl Physiol, 115(5):1155-1164, 09 Jan 2015
Cited by: 2 articles | PMID: 25572497
Go to all (15) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Sensory nerves and nitric oxide contribute to reflex cutaneous vasodilation in humans.
Am J Physiol Regul Integr Comp Physiol, 304(8):R651-6, 13 Feb 2013
Cited by: 26 articles | PMID: 23408029
Contribution of nitric oxide to cutaneous microvascular dilation in individuals with type 2 diabetes mellitus.
Am J Physiol Endocrinol Metab, 292(1):E314-8, 05 Sep 2006
Cited by: 52 articles | PMID: 16954331
Skin blood flow and nitric oxide during body heating in type 2 diabetes mellitus.
J Appl Physiol (1985), 106(2):566-570, 04 Dec 2008
Cited by: 40 articles | PMID: 19056994 | PMCID: PMC2644253
Topical anaesthesia does not affect cutaneous vasomotor or sudomotor responses in human skin.
Auton Autacoid Pharmacol, 33(3-4):25-33, 13 May 2013
Cited by: 5 articles | PMID: 23663206
Funding
Funders who supported this work.
NCRR NIH HHS (1)
Grant ID: UL1-RR-024150
NHLBI NIH HHS (1)
Grant ID: R01-HL-73884





