Abstract
Free full text

Local IL-21 Promotes the Therapeutic Activity of Effector T cells by Decreasing Regulatory T Cells Within the Tumor Microenvironment
Abstract
The eradication of tumors by the immune system depends on the generation of antigen-specific T cells which can migrate to sites of tumor growth and maintain their effector functions despite local tumor-derived T-cell inhibitory factors. Interleukin-21 (IL-21) is an IL-2-related cytokine that has shown limited evidence of antitumor activity in murine models and early phase clinical trials. Effect of local IL-21 on T-cell responses within the tumor microenvironment, however, has not been extensively evaluated. Thus, we developed a stably transfected IL-21-secreting B16 melanoma cell line to test the effects of local IL-21 on endogenous and adoptively transferred T-cell responses. Tumors expressing IL-21 exhibited delayed growth in vivo, which was associated with an increase in activated systemic effector and memory CD8+ T–cell responses. Local IL-21 also enhanced the therapeutic effects of adoptively transferred gp100-specific T cells and was synergistic with IL-2. The effect was also associated with an increased proliferation of local CD8+ T cells and decreased accumulation of regulatory CD4+FOXP3+ T cells within the tumor microenvironment. These data suggest that local IL-21 enhances endogenous and adoptively transferred T-cell immunity through increased effector CD8+ T cells and decreased CD4+ regulatory T cells in the tumor microenvironment.
Introduction
The role of the immune system in the rejection of solid tumors has been well demonstrated in both carcinogen-induced and spontaneous experimental tumor models.1,2 In these models, immune-mediated eradication of established tumors depends on the presence of tumor antigen-primed CD8+ effector T cells, the ability of such T cells to traffic to sites of tumor growth, the persistence of cells in sufficient concentrations at the tumor site and the capacity of the T cells to maintain cytotoxic effector functions in the face of local immunosuppressive mechanisms.3 The identification of targeted T-cell antigens and considerable early phase clinical testing of vaccines designed to elicit tumor-specific T-cell immunity supported the concept that T cells could be primed, at least in the peripheral blood, in cancer patients but therapeutic responses were disappointing.4 More recently, reports have suggested better results with adoptive transfer of large numbers of antigen-specific CD8+ or CD4+ T cells in patients with metastatic melanoma.5,6 While these clinical studies support the concept that a significant concentration of effector T cells at sites of tumor growth can mediate tumor rejection, the loss of therapeutic activity has been associated with changes in the tumor or local environment, such as loss of tumor antigen and/or MHC expression, increased concentrations of inhibitory cytokines (i.e., IL-10 and TGF-β), accumulation of regulatory and suppressor immune cells (i.e., CD4+CD25+FOXP3+ regulatory T cells (Tregs) and CD68+ macrophages) and presence of other anti-inflammatory mediators (i.e., arginase, VEGF, PGE2) in the tumor microenvironment.7,8,9,10 Thus, strategies aimed at maintaining effector T cells within the tumor microenvironment following vaccination or adoptive transfer are a high priority.
Interleukin-2 (IL-2) is a four-bundle α-helical cytokine produced primarily by activated T cells and functions to regulate T-cell homeostasis. Recombinant human IL-2 has been used as single-agent therapy for the treatment of metastatic melanoma and renal cell carcinoma, as an adjuvant to tumor-antigen vaccination and to support adoptively transferred T cells in vivo.11,12,13 IL-2, however, also functions to promote CD4+CD25+FOXP3+ Tregs in vitro and in vivo, suggesting a potential negative influence on T-cell immunity.14,15,16 This effect on Tregs may have important implications as shown in a recent murine tumor model where regression was directly related to the ratio of proliferating CD8+ T cells to CD4+ Tregs within the tumor microenvironment.17 Thus, while IL-2 may promote effector CD8+ T cells, the effect on Tregs may inhibit potential therapeutic activity of effector T cells, especially if the tumor microenvironment is permissive to the accumulation of Tregs.
IL-21 is a type I cytokine that shares a common cytokine receptor γ chain with other members of the IL-2 family, including IL-4, IL-7, and IL-15 (ref. 18). IL-21 is produced by Th1 cells and exerts regulatory effects on virtually all cells of the immune system, including B, T, and NK cells. In T cells, IL-21 can block IL-2-induced apoptosis and promotes the differentiation and long-term survival of CD8+ T cells.19 IL-21 has also shown limited clinical benefit as a single agent against melanoma and renal cell carcinoma in a Phase I clinical trial.20 Furthermore, in an experimental tumor model, depletion of Tregs before IL-21 exposure resulted in optimal expansion of antigen-specific CTL in vitro and in vivo.21,22 While the dose, schedule and route of administration significantly influence the effects of IL-2, the net effect of these parameters on IL-21 biological activity is not known. Furthermore, the role of IL-21 in the tumor microenvironment has not been previously evaluated.
Thus, we hypothesized that local IL-21 would significantly enhance the therapeutic activity of antigen-specific T-cell responses against an established murine melanoma. To test this, we first established a stably transfected B16 cell line that secretes functional IL-21 and found that mice did exhibit delayed tumor growth after challenge with the tumor. The delayed tumor growth was associated with induction of endogenous activated and memory T-cell responses. Local IL-21 also improved the therapeutic activity of adoptively transferred tumor-reactive CD8+ T cells. Analysis of the tumor microenvironment showed that these effects were due, in part, to a decrease in the accumulation of Tregs shifting the balance of effector CD8+ T cells and CD4+ Tregs. These data suggest that local expression of IL-21 may be an approach for improving the therapeutic effects of both endogenous vaccine-primed T cells and adoptively transferred T cells.
Results
B16 cells secreting functional IL-21 display delayed growth in vivo
B16 melanoma cells transfected with pcDNA containing murine IL-21 transcript were selected in G418-containing medium to obtain stably transfected clones. Stable clones demonstrating IL-21 secretion by enzyme-linked immunosorbent assay (ELISA) (data not shown) were tested for their growth kinetics in vitro. The growth rate of one such B16 clone expressing IL-21 (B16/mIL-21) was similar to that of B16 expressing an empty vector pcDNA (B16/pcDNA) or the parental B16 cell line (Figure 1a), and this clone was selected for further study. To document the functional property of secreted IL-21, naive T cells (0.1 × 106) derived from splenocytes were plated in an anti-CD3 precoated plate and increasing doses of IL-21 (0 to 1,000 pg/ml) in supernatant from B16/mIL-21 clones grown on minimal growth medium were added in the absence or presence of anti-CD28 antibodies. Supernatant from B16/mIL-21 cultures, but not from B16/pcDNA cultures, augmented T-cell proliferation in a dose-dependent manner (Figure 1b). Subcutaneous (SC) injection of the B16/mIL-21 in syngeneic C56BL/6 animals resulted in delayed tumor growth compared to B16/pcDNA or parental B16 tumors (Figure 2). B16/mIL-21 tumors were significantly smaller than B16/pcDNA or B16 at the indicated times (*P < 0.05, **P < 0.003).
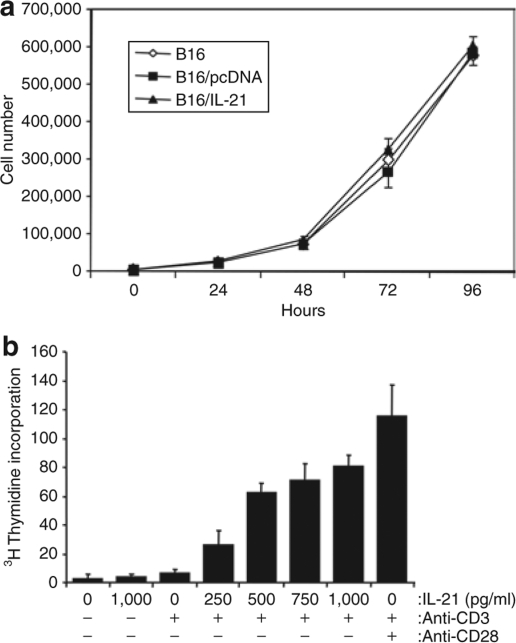
B16/mIL-21 melanoma cells exhibit normal growth kinetics and produce functional IL-21. (a) Growth curve of stably transfected B16 melanoma secreting IL-21 in vitro. (b) IL-21 dose-dependent proliferation of mouse naive splenocytes. Proliferation assay was performed triplicates in an anti CD3 precoated plate, increasing doses of supernatant containing mIL-21 (conditioned medium) were added at the time of stimulation as described in Materials and Methods. Anti CD3 and CD28 antibodies are used to stimulate cells. Bars, means ± SD.
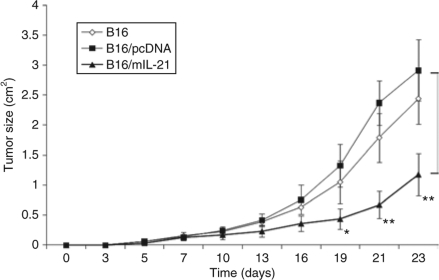
Local IL-21 results in delayed tumor growth. Tumor growth of B16, B16/pcDNA, and B16/mIL-21 in syngeneic mice is represented as average tumor size (mean ± SD) of each experimental group (n = 5), as detailed in Materials and Methods. Tumor growth data shown are representative pattern of tumor growth in one of three experiments. Statistical analysis with t-test showed that the difference in tumor size between the control group and B16/mIL-21 was significant. *P < 0.05, **P < 0.003.
Local IL-21 induces systemic CD137+hiCD8+ T-cell expansion and T-cell memory
To examine the effect of IL-21 on tumor microenvironment, tumors were isolated at day 23 and TIL were analyzed by flow cytometry. IL-21 secreting tumors showed a significant increase in frequency of CD3+ T cells (Figure 3a, P < 0.0149). The percentage (41.5%) and absolute number (14,000 cells) of tumor infiltrating CD3+ T cells were significantly higher in IL-21-secreting tumors. A notable increase was seen in CD8+ cells in B16/mIL-21 tumor (13.5% of TIL and 4,554 cells versus 4% of TIL and 1,349 cells), while there was little change in the number of CD4+ T cells (24% and 8,096 cells) compared to control group B16/pcDNA (20% and 6,746 cells, Figure 3b, P < 0.0212). The ratio of CD4+ T cells to CD8+ T cells was 0.7 for the B16/mIL21 while they were 1.40 and 1.19 for B16/pcDNA and B16, respectively. The frequency of NK population in the spleen and tumor by surface phenotype CD49b+NK1.1+CD3− staining revealed similar pattern across the animal groups. (≤2% of CD45+ TIL). Phenotypic characterization of CD4+ and CD8+ T cells in B16/mIL-21 tumor showed up-regulation of CD137 expression and a CD62LlowCCR7low phenotype suggesting that IL-21 enhanced generation of activated effector memory T cells (Figure 3c). Analysis of activation markers CD25 and CD69 also revealed that T cells isolated from IL-21 producing tumors showed an increase compared to those isolated from control groups (data not shown).
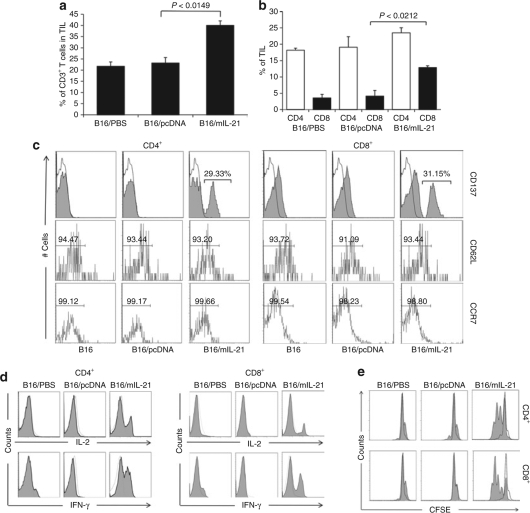
Local IL-21 results in an increase in the frequency of effector and memory CD8+ T cells. (a) IL-21 strongly enhances infiltration of CD3+ T cells in tumor. The percentage of CD4+, CD8+ T cells in tumor tissue was determined by flow cytometry and based on CD45+ cells. (b) Specific increases in the frequency of CD8+ T cells in TIL. (c) Single-cell suspension of tumor isolated from each group at day 23 was stained with CD137, CD62L, and CCR7 for activated memory T cells phenotypes followed by flow cytometric analysis. (d) Splenocytes isolated from each group at day 23 were stimulated for 24 hours with phorbol myristate acetate/Ionomycin A followed by intracellular staining of IL-2 and IFN-γ and flow cytometric analysis in CD4+ T cells and CD8+ from the lymphocyte populations. The unshaded histogram in the plot indicates the isotype antibody control. (e) Splenocytes were carboxyfluorescein succinimidyl ester (CFSE) labeled and stimulated with suboptimal dose of anti-CD3 mAb (0.02 µg/ml) for 72 hours followed by surface staining of CD4 and CD8 and flow cytometry analysis of CFSE dilution. CFSE profiles gated on CD4+ and CD8+ in the plot are shaded histogram and the dotted line indicates the unstimulated cells.
To evaluate the effect of local IL-21 secretion on the induction of systemic T cells in vivo, the functional capacity of spleen-derived CD8+ T cells was evaluated for cytokine secretion in vitro. Both CD4+ and CD8+ T cells enriched from splenocytes of mice bearing B16/mIL-21 tumors (23 days) produced significantly higher level of IL-2 and IFN-γ determined by intracellular cytokine staining (Figure 3d). Next, to determine whether CD8+ T cells were functionally related to a memory cell phenotype, splenocytes were labeled with carboxyfluorescein succinimidyl ester (CFSE) and stimulated with suboptimal anti-CD3 antibody (0.02 µg/ml) for 72 hours followed by CFSE dilution analysis. Both CD4+ and CD8+ T cells of B16/mIL-21 injected mice initiated proliferation at 0.02 µg/ml while cells from control B16/pcDNA showed no proliferation (Figure 3e). Both CD4+ and CD8+ T cells underwent robust proliferation with a minimum of three divisions. The initial basal level of proliferation without anti-CD3 stimulation was similar among the animal groups suggesting that the higher proliferative rate of T cells from B16/mIL-21 tumor–bearing mice is because of induction of systemic memory T cells. This suggests that local IL-21 secretion enhances proliferation and accumulation of activated memory CD8+ T cells.
Local IL-21 secretion prevents accumulation of CD4+CD25+FOXP3+ Tregs within the tumor microenvironment
Because IL-21 belongs to the IL-2 family and IL-2 treatment has been shown to increase Tregs in melanoma and renal cell carcinoma patients, we analyzed the effects of local IL-21 on the frequency of Tregs. First, we evaluated the frequency of Tregs, defined by expression of CD4, CD25, and FOXP3, in the spleen and found no difference between mice bearing B16/m-IL-21, B16/pcDNA, or parental B16 SC tumors (data not shown) at day 23. However, when cells were analyzed from single-cell suspensions from the tumors, B16/mIL-21 tumors showed a statistically significant decrease in Treg frequency within day 23 tumors compared to mice with non-IL-21-secreting tumors (Figure 4a,b). Representative Tregs cells defined as Foxp3+CD4+ and Foxp3+CD25+ T cells in CD4+CD3+ T–cell populations are shown in Figure 4a. The frequency of Tregs in TIL of B16/mIL-21 tumor was significantly lower than control tumors (Figure 4b, P < 0.0225). Further the absolute number of Treg in the TIL population of B16/mIL-21 decreased to 236 cells from control group with 337 cells. Because of the limited number of Tregs in the TIL for the functional assay, Tregs were isolated from the spleen for suppression function in mixed coculture assays using CD4+CD25− T cells stimulated by anti-CD3 and -CD28 antibodies with or without Tregs derived from mice harboring different tumors. The level of suppression by Tregs was similar among all groups (Figure 4c). Thus, fewer Tregs accumulated in the tumor microenvironment of mice with established tumor secreting IL-21 without affecting the Treg suppressive capacity on a per cell basis.
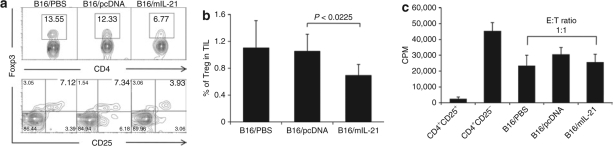
Local IL-21 prevents the accumulation of Tregs in the tumor microenvironment. (a) Representative staining for CD4+FOXP3+ and CD25+FOXP3+ cells in B16/mIL-21 tumor infiltrating cells. Gated CD4+ T cells stained with CD25 and FOXP3. Percentage of CD25+FOXP3+ cells is indicated in the upper right quadrant. (b) Graph shows the proportion of CD4+CD25+FOXP3+ Tregs in tumor infiltrating CD4+ T cells of each animal group. *P < 0.05. (c) Whole splenocytes were isolated and Treg cells were enriched by microbead cell isolation method and purity of 92–95% was determined by flow cytometry. CD4+CD25− T cells were cocultured with CD4+CD25+ Treg cells at 1:1 ratio for 3 days in anti-CD3 mAb precoated plate with anti-CD28 mAb stimulation.
Local IL-21 enhances the therapeutic response of adoptively transferred T cells
We next sought to determine whether local IL-21 secretion could also augment adoptively transferred antigen-specific T cells in the murine melanoma model. This was accomplished using the well-established Pmel-1 experimental model with minor modifications.23 Tumors (B16/mIL-21, B16/pcDNA or parental B16) were established by SC injection of 0.3 × 106 cells at day 0. When tumors reached about 50 mm2 (day 7–10), the mice were sublethally irradiated with 5 Gy followed by adoptive transfer of in vitro preactivated Pmel-1 cells (5 × 106 CD8+Vβ13+ T cells) by tail vein injection. This was followed by vaccination with 100 µg of hgp10025–33 peptide SC alone or with 6 × 106 IU of recombinant human IL-2 intraperitoneally (IP) as described in Materials and Methods. First, we validated previous reports showing that mice harboring B16 melanoma treated with adoptively transferred activated Pmel-1 T cells followed by gp100 vaccination requires the addition of IL-2 to reduce tumor growth (Figure 5a). When the B16/mIL-21 tumors, which secrete local IL-21, were treated with adoptively transferred activated pmel-1 T cells and gp100 vaccination they exhibited a similar degree of delayed growth as mock-transfected B16 tumors treated by adoptive transfer, vaccination and IL-2 (Figure 5b). The most significant therapeutic response, however, was seen in mice treated with local IL-21, Pmel-1 T cells, vaccination and IL-2 (Figure 5b). Thus, local IL-21 may substitute for systemic IL-2 in adoptive T-cell transfer therapy and also appears to act synergistically with IL-2 to induce tumor regression following adoptive T-cell transfer.
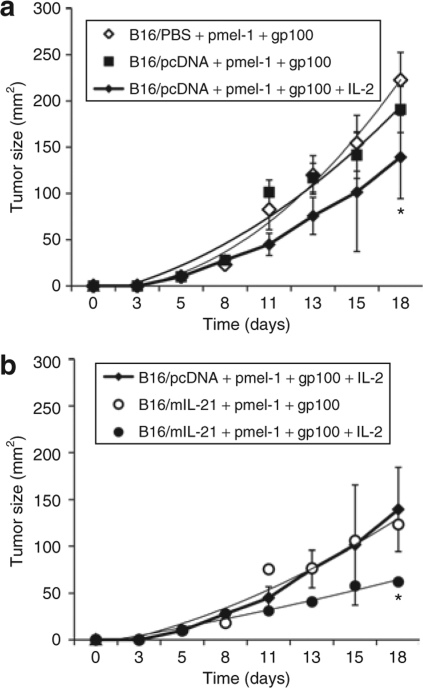
Local IL-21 enhances therapeutic responses of adoptively transferred T cells and acts synergistically with IL-2. (a) Tumor response to adoptive transfer of 5 × 106 activated gp100-specific T cells (pmel-1), gp100 peptide vaccination and systemic IL-2 treatment as described in Materials and Methods (n = 5). (b) Enhanced tumor response to the adoptive transfer of pmel-1 cell and gp100 peptide vaccination in mice bearing IL-21 secreting tumor (n = 5). Error bars represents ± SDs.
Local IL-21 stimulates adoptively transferred tumor-specific T cells in vivo
In order to correlate the in vivo therapeutic response with tumor antigen-specific T-cell responses, the effect of local IL-21 on the gp100-specific TCRvβ13+Thy1.1+ T cells (Pmel-1) in the tumor site were analyzed by flow cytometry. While tumors received Pmel-1 T-cell transfer, vaccination and IL-2 injections demonstrated an increase in Thy1.1+ T cells within the tumors, the number of Thy1.1+ T cells was greatest in those tumors producing local IL-21 (Figure 6a). We next tested gp100-specific T-cell function by in vitro stimulation with gp100 peptide and used IFN-γ production by intracellular cytokine staining as the readout in spleen. The level of IFN-γ producing Thy1.1 T cells were similar in animal groups treated with IL-2, bearing IL-21 secreting tumor, and a group bearing mIL-21 tumor treated with IL-2. All these three groups showed increase in Thy1.1+CD8+ T cells an average of tenfold increase in IFN-γ positive T cells compared to control groups upon peptide stimulation in vitro (Figure 6b, all P < 0.05). This suggested but did not prove that local IL-21 promoted the proliferation of T cells within the tumor microenvironment and systemic compartment.
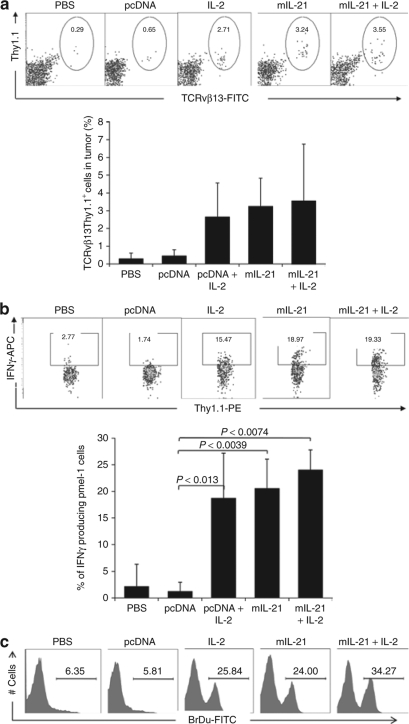
Local IL-21 enhances therapeutic responses of adoptively transferred T cells through expansion of CD8+ T cells. (a) IL-21 induced accumulation of Thy1.1+TCRvβ13+T cells in tumor. The percentage of Thy1.1+TCRvβ13+ T cells is indicated in the oval gate of each dot plot. The plots shown were gated on small lymphocytes infiltrating tumor. Bar graph on the right shows the average percentages of Thy1.1+TCRvβ13+ T cells and the error bar represents ± SDs. The bar graph shows the % of average IFN-γ producing cells. (b) Effector cell function was analyzed by production of IFN-γ by whole splenocytes by stimulation with gp100 peptide in vitro. The percentage of IFN-γ+ Thy1.1+ T cells is indicated in the box gate of each plot determined by intracellular cytokine staining analyzed by flow cytometry. Bar graph on the right shows the average percentages of Thy1.1+IFN-γ+ T cells and the error bar represents ± SDs. (c) IL-21 and IL-2 induced tumor antigen specific-T cells proliferation in vivo. All Vβ13+ T cells were analyzed for BrdU staining in lymphocyte populations of whole splenocytes.
To confirm whether IL-21 induced expansion of gp100-specific TCRvβ13+Thy1.1+ T cells in vivo, mice were injected IP with BrDu for 24 hours. Splenocyte-derived T cells were analyzed by flow cytometry for BrDu incorporation with antibodies against CD8, Vβ13 and BrdU. T cells from mice with local IL-21-secreting tumors induced gp100-specific T-cell proliferation in vivo and a further increase in BrdU positive T cells was observed by additional IL-2 treatment (Figure 6c). Thus, local IL-21 induces expansion of activated Pmel-1 cells in vivo.
Local IL-21 reduced Treg accumulation following adoptive T-cell transfer
Because peptide vaccination and systemic IL-2 could increase Treg cells, we carefully analyzed the frequency of Tregs in the spleen and tumor at different times after adoptive T-cell transfer in our model (Figure 7). The frequency of Tregs in the spleen of B16/mIL-21 mice was significantly lower than control groups at day 5 (P < 0.0457; absolute number of cells, 0.603 × 106 versus 0.942 × 106), but this difference was no longer apparent at day 10 (Figure 7a). In fact, the frequency of Treg was higher in IL-2-treated group at day 10 compared to control groups consisted with studies of IL-2 mediated upregulation of IL-2 (absolute number of cells, 1.09 × 106 versus 0.75 × 106). B16/mIL-21 tumor–bearing mice treated with additional IL-2 showed significant decrease in Tregs at day 5 (P < 0.0052) but not at day 10 (P < 0.0677) spleen. In contrast, Tregs within the tumor microenvironment of B16/mIL-21 tumor–bearing mice showed a sustained reduction on days 5 and 10 (Figure 7b, P < 0.0248 for day 5, 0.0453 for day 10). Further, the frequency of Tregs in B16/mIL-21 tumor–bearing mice treated with additional IL-2 showed significant decrease at days 5 and 10. The absolute number of Tregs also decreased to 472 cells in mIL-21 tumor compared to pcDNA tumor with 971 cells at day 5. Similarly, absolute number of Treg was maintained low in the mIL-21 tumor at day 10 to control pcDNA tumor (440 versus 890 cells). Consistent with untreated B16/mIL-21-bearing mouse data (Figure 4c), the suppressive capacity of the Tregs was similar among all animal groups (data not shown). These data support the conclusion that peptide vaccination and systemic IL-2 did not increase the Treg frequency in vivo of mice harboring tumors engineered to secrete IL-21 and also suggest that local IL-21 may block accumulation of Tregs within the tumor microenvironment altering the ratio of adoptively transferred T cells and Tregs.
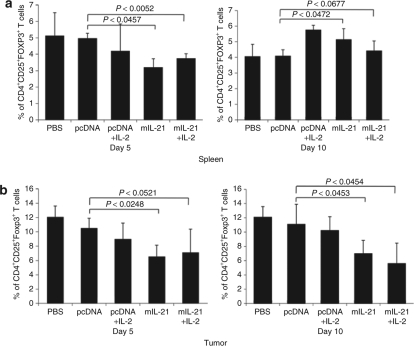
Local IL-21 prevents the accumulation of Tregs in the tumor microenvironment following adoptive T-cell transfer. (a) Treatment of IL-21 and combination with IL-2 induced decrease in the frequency of Treg cells in spleen at day 5 while no difference was observed at day 10. The percentage of Treg cells were from CD4+ T cells. (b) In contrast, Treg cells in the tumor displayed low frequency at day 5 and maintained low at day 10. The percentage of Treg is based on CD4+ T cells of the small lymphocytes in tumor single-cell suspension.
Discussion
Immune recognition of tumors occurs in cancer patients is documented by the frequent observation of T-cell infiltration into tumors and has the impact on prognosis.24,25,26,27,28,29 The presence of tumor-infiltrating T cells alone, however, does not guarantee rejection of established tumors. Thus, the induction of tumor-reactive T cells and their migration to sites of tumor growth is not sufficient for tumor rejection. Effector T cells must also overcome factors in the tumor microenvironment that prevent immunological destruction of antigenic tumors.30,31,32,33 These factors include the accumulation of regulatory cells (i.e., CD4+ Tregs, myeloid-derived suppressor cells and CD68+ tumor-associated macrophages), high levels of suppressive cytokines and growth factors (i.e., TGF-β, IL-10, and VEGF) and aberrant expression of tumor antigens, MHC complexes, costimulatory (i.e., B7.1) and coinhibitory (i.e., B7-H4) molecules by tumor and/or stroma cells.9,34,35,36,37,38 The consequence of these factors is that infiltrating T cells may not be able to maintain effector functions, as evidenced by recent reports showing loss of the T-cell receptor (TCR)-zeta signal-transducing chain in tumor-infiltrating T cells compared to peripheral blood T cells in cancer patients.39,40 Collectively, these studies imply that tumors harbor the ability to neutralize effector T cells through diverse cellular and molecular mechanisms even if such T cells enter the tumor microenvironment.
In this study, we examined the antitumor effects of local IL-21 using a stably transfected B16 clone after showing that the tumor cells secrete functional IL-21 but does not affect tumor growth kinetics in vitro. In fact, these tumor cells grew in vivo as well, but local production of IL-21 did result in a significant delay in tumor growth of the otherwise relatively nonimmunogenic B16 melanoma (see Figure 2). Although we do not know which antigens are responsible for the delayed growth, the fact that these responses were T cell mediated is supported by the phenotypic characterization of the tumor-infiltrating T cells, which revealed an increase in CD137+CD8+ T cells. These cells were also considered true effector cells based on intracellular cytokine staining for IL-2 and IFN-γ (Figure 3). We also observed an increase in memory CD4+ and CD8+ T cells in the spleen of mice harboring IL-21 secreting tumors, which further supports the notion that tumor growth was arrested through a tumor-reactive T-cell response. Similar effects on adoptively transferred T cells was seen with mice expressing local IL-21 having the greatest delay in tumor growth and highest number of tumor infiltrating effector CD8+ T cells. Significant interest has recently been also focused on the potential of IL-21 to activate NK cells and dendritic cells thereby promoting antitumor activity.41,42,43,44,45 In contrast IL-21 downregulates NKG2D expression in primary human NK cells.46 Further studies focused on effect of local production of IL-21 on NK and NKT cell populations and their contribution to tumor rejection is in progress. The effect of IL-21 on dendritic cells is also somewhat controversial that its opposing effects on dendritic cell maturation and function have been reported both in human and murine models.44,45
A prior Phase I clinical trial suggested that IL-21 could induce objective clinical responses in a small subset of patients but also reported that the optimal dosing, schedule, and routes of IL-21 administration was unknown. Our data would support local delivery or expression of IL-21 as a possible strategy for promoting CD8+ T–cell immunity while minimizing toxicity and the suppressive influence of the tumor microenvironment.
A major obstacle to successful tumor immunotherapy is the presence of large numbers of CD4+CD25+FOXP3+ Treg cells, especially within the tumor microenvironment where such cells may directly and indirectly inhibit tumor-specific CD8+ effector T cells. In this study, we found that local IL-21 secretion resulted in a decreased accumulation of Tregs by as much as 50% compared to tumors in control mice (Figure 4). We also observed a decrease in Tregs within the tumor but not the spleen of mice harboring IL-21 secreting tumors treated by adoptive transfer of activated Pmel T cells suggesting this may be a more general phenomenon of local IL-21 secretion. An important observation was that the lowest number of Tregs was found in mice with tumors secreting IL-21 and also receiving systemic IL-2 after T-cell transfer because IL-2 is known to promote Treg activity in patients with melanoma and renal cell carcinoma,9,10 local delivery of IL-21 may be a strategy for blocking the inhibitory effects of these cells on effector CD8+ T cells within the tumor microenvironment. In fact, mice with local IL-21 secreting tumors treated with adoptively transferred T cells and IL-2 had the highest number of IFN-γ-producing TCRvβ13+Thy1.1+ CD8+ T cells within the tumors. A recent in vitro study showing IL-21 selectively antagonizes suppression of human Tregs on CD4+CD25− T cells but not on CD8+ T cells suggest an additional mechanism by which IL-21 could have a direct effect on responding CD4+CD25− T cells rather than on Tregs themselves.22
Another important observation in our data is the differential effect of locally produced IL-21 on Tregs in the spleen and tumor microenvironment. Tregs were decreased early (day 5) after T-cell transfer in both the spleen and tumor of mice with B16 tumors secreting IL-21. The frequency of Tregs returned to baseline levels in the spleen by day 10 but re-accumulation in the tumor was blocked in mice whose tumors secreted IL-21 (Figure 7). Thus, it appears that Tregs are systemically repopulated while locally produced IL-21 prevents accumulation within the tumor microenvironment for longer periods of time, which may allow the expansion of CD8+ effector T cells and subsequently altering the effector to Treg ratio in the tumor microenvironment. In fact, the ratio of effector-to-Tregs in the tumor of metastatic melanoma patients is linearly correlated with tumor necrosis underscoring the importance of this ratio in determining therapeutic outcomes with immunotherapy.47,48 Because IL-2 may promote Treg expansion and given recent evidence showing that IL-2 and IL-21 confer opposing effects on CD8+ T cells in adoptive immunotherapy, it is noteworthy that our data suggest a potential synergistic effect between local IL-21 and systemic IL-2 (ref. 49). This may not be surprising given that IL-2 promotes early CD8+ T–cell activation and IL-21 supports secondary expansion T cells and prevents IL-2-induced apoptosis.50 Thus, local IL-21 secretion, through both expansion of effector CD8+ T cells and reduced accumulation of CD4+ Tregs, may be useful for overcoming the limitations of IL-2 as single-agent therapy or as adjuvant in adoptive T-cell transfer regimens.
In summary, we have shown that local IL-21 secretion enhances the therapeutic response of B16 melanoma through promoting both endogenous and adoptively transferred T-cell immunity. This likely occurs through proliferation and expansion of effector and memory CD8+ T cells and by blocking accumulation of CD4+ Tregs within the tumor microenvironment. The delivery of IL-21 into established tumors may be a novel strategy for improving responses to IL-2, vaccination and adoptive T-cell transfer approaches in the treatment of patients with cancer.
Materials And Methods
Animals. Pmel-1 TCR transgenic mice were obtained from the Jackson Laboratory (strain 5023, Bar Harbor, ME) and C57BL/6 mice were obtained from Charles River Laboratory (Wilmington, MA). These mice were housed in pathogen-free conditions at the Institute for Comparative Medicine of Columbia University according to approved institutional protocols. The B16 murine melanoma cell line was kindly provided by Dr Raphael Clynes, Columbia University.
Cloning of Murine IL-21. The murine IL-21 gene was cloned from PHA-activated splenocytes by RT-PCR and integrated into the mammalian expression vector pcDNA3 (Invitrogen, Carlsbad, CA) by using primers for the PCR; forward primer 3′-ATGGAGAGGACCCTTGTCT-5′ and backward primer 3′-CTAGGAGAGATGCTGATGAATC-5′. PCR conditions were as follows: 5 min 94 °C; 30 cycles (30 s 94 °C, 1 min 68 °C, 1 min 72 °C); 7 min 72 °C. The PCR product was purified using a PCR purification kit (Qiagen, Valencia, CA) and cloned into the KpnI and XhoI sites of the expression vector pcDNA3 and was verified by DNA sequencing. Expression vector pcDNA3/mIL-21 was linearized using BglII for transfection into B16 cells. A stable transfected B16 cell line expressing IL-21 was generated by transfecting 3 × 105 cells with 5 µg of linear plasmid by Lipofectamine 2000. Transfected cells were selected by antibiotic geneticin at the concentration of 1,500 µg/ml (Invitrogen) and screened for expression of IL-21 by ELISA and selected line was denoted as B16/mIL-21 and control B16 cell line containing only pcDNA vector is denoted as B16/pcDNA.
Detection of IL-21 by ELISA. ELISA was performed to detect and quantify the concentration of soluble IL-21 produced by B16/mIL-21 following the manufacturer's recommendation (Mouse IL-21 DuoSet, DY594, R&D system, Minneapolis, MN). Briefly, micro 96-well plates were coated with highly purified capture anti-IL-21 and incubated overnight at 4 °C. Plates were washed and filtered supernatant of B16/mIL-21 cells was applied and incubated for 2 hours. Bound IL-21 was detected with biotin-conjugated detection anti-IL-21 antibodies followed by an enzyme-labeled a streptavidin, and color development was read by ELISA-plate reader at OD450 nm. Homogenous cell populations were obtained by limiting dilution and clones with high expression of IL-21 were selected. The stable mIL-21-transfected cell line is referred to hereafter as B16/mIL-21, and control cell line B16/pcDNA3.
Proliferation assay by 3[H]-thymidine incorporation and CFSE labeling. Primary 3-day proliferation assay was performed with mouse splenocytes in a 96-well anti-CD3 precoated plate (Biocoat; BD Biosciences, San Jose, CA). Antimouse CD28 antibody was added at concentration of 10 µg/ml to the appropriate wells. 3[H]Thymidine was added to the cultures 18 hours before harvesting and incorporation was determined by scintillation spectrometry using a LKB 1250 Betaplate counter (Perkin-Elmer, Boston, MA). Mean cpm of triplicate cultures and the SD from the mean were calculated. In some experiments, supernatants of B16/mIL-21 cells were added to responding cells at various concentrations (mIL-21 from 0 to 1,000 pg/ml). Supernatant from control B16/pcDNA3 was used at the same volume in parallel control cultures. For CFSE labeling, cells at a density of 1 × 107 cells per ml were stained for 10 min at 37 °C with 5 µmol/l CFSE (carboxyfluorescein diacetate succinimidyl; Molecular Probes, Carlsbad, CA) in phosphate buffered saline with 0.1% (vol/vol) bovine serum albumin. Reactions were “quenched” by the addition of an equal volume of 100% fetal calf serum followed by three washes in phosphate buffered saline with 0.5% (vol/vol) bovine serum albumin to ensure complete removal of free CFSE.
Mixed lymphocyte proliferation assay. Regulatory CD4+CD25+FOXP3+ T cells were enriched with Treg isolation kit (Miltenyi Biotec, Auburn, CA) according to the manufactures' recommendations. The purity of enriched Treg cells was 85–90%. All assays were performed in 96-well U-bottom plates (BD Biosciences), precoated with antihuman CD3 (10 µg/ml) and stimulated with soluble antihuman CD28 (10 µg/ml) for 72 hours at 37 °C and 5% CO2. Tregs were cocultured with CD4+CD25− T cells at 5.0 × 103 cells/well at a 1:1 ratio. Cultures were then pulsed with 3[H]Thymidine (Perkin-Elmer) for the last 16–18 hours of culture, harvested on glass fiber filters, and incorporated radioactivity measured with a liquid scintillation counter (Wallac, Waltham, MA). All experiments were done in triplicate.
In vitro activation of Pmel-1 cells. Pmel-1 splenocytes from transgenic mice were depleted of erythrocytes by hypotonic lysis and cultured in RPMI supplemented with 10% heat-inactivated FBS (complete medium) with 30 IU/ml rhIL-2 (R&D systems, Minneapolis, MN) in the presence of 1 µmol/l hgp10025–33 peptide, and used on days 5–10 after start of the culture. The synthetic, H-2Db-restricted peptides hgp10025–33 were synthesized by New England peptide LLG (Gardner, MA) to a purity >99% by HPLC and amino acid analysis.
Adoptive cell transfer, vaccination, and cytokine administration. C57BL/6 mice at 6–8 week of age were implanted SC with 3 × 105 B16 melanoma cells secreting functional IL-21. To deplete endogenous T-cell populations, C57BL/6 tumor–bearing mice were sublethally irradiated with 5 Gy before adoptive transfer. When tumor size reaches 50 mm2, in vitro-activated Pmel-1 splenocytes (5 × 106 CD8+Vβ13+ T cells) were transferred by tail vein injection. Following adoptive transfer of Pmel-1 T cells, mice (n = 5 for each group) were vaccinated by SC injection with 100 µl phosphate buffered saline/IFA emulsion containing 100 µg of hgp10025–33 peptide and injected IP with 6 × 106 IU of recombinant human IL-2 in phosphate buffered saline twice daily for a total of five doses. Tumor sizes were measured every second day with a caliper as the product of two perpendicular diameters.
BrdU labeling in vivo and detection. Mice were injected IP with 1 mg BrdU (5-bromodeoxyuridine). Mice were killed after 24 hours and BrdU incorporation was analyzed according to the manufacturer's instructions (BD Biosciences). Cell surface antigens were stained with appropriate antibodies, followed by fixation and permeabilization. BrdU epitopes were exposed by digestion for 1 hour at 37 °C with DNAse I and were detected with monoclonal antibody to BrdU.
Flow cytometry and intracellular IFN-γ detection. On the days indicated after vaccination, mice were killed, and spleen and tumor were harvested and homogenized into a single-cell suspension. Cells were labeled with the following mAbs (BD Biosciences): FITC-conjugated anti-Vβ13 (MR12-3), PE-conjugated anti-Thy1.1 (HIS51), PE-conjugated anti-CD44 (IM7), PE-conjugated anti-CD62L (MEL-14), and PerCP-conjugated anti-CD8a (53-6.7). Samples were analyzed using a FACSCalibur flow cytometry and CellQuest software. For intracellular staining, tumors were homogenized and pulsed with 1 µmol/l human gp10025–33 and Golgi-Stop (BD Biosciences) for 3.5 hours. Cells were then first stained for surface markers followed by permeabilization using the Cytofix/Cytoperm kit (BD Biosciences), and stained with allophycocyanin-conjugated anti-IFN-γ mAbs (XMG1.2).
Statistical analysis. Student's t-test (two-tailed, assuming equal variance) was used for statistical analyses of differences between treated and control groups. Data are shown as mean ± SD. Differences were recognized as significant at P < 0.05.
REFERENCES
- Atkins MB. Interleukin-2: clinical applications. Semin Oncol. 2002;29:12–17. [Abstract] [Google Scholar]
- Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–3132. [Europe PMC free article] [Abstract] [Google Scholar]
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. [Europe PMC free article] [Abstract] [Google Scholar]
- Gattinoni L, Powell DJ Jr, Rosenberg SA., and Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. [Europe PMC free article] [Abstract] [Google Scholar]
- Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Vetto JT, et al. A new approach to the therapy of cancer based on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2. Surgery. 1986;100:262–272. [Abstract] [Google Scholar]
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. [Europe PMC free article] [Abstract] [Google Scholar]
- Restifo NP, Surman DR, Zheng H, Palese P, Rosenberg SA., and García-Sastre A. Transfectant influenza A viruses are effective recombinant immunogens in the treatment of experimental cancer. Virology. 1998;249:89–97. [Europe PMC free article] [Abstract] [Google Scholar]
- Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L, et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci USA. 2000;97:11445–11450. [Europe PMC free article] [Abstract] [Google Scholar]
- Cesana GC, DeRaffele G, Cohen S, Moroziewicz D, Mitcham J, Stoutenburg J, et al. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol. 2006;24:1169–1177. [Abstract] [Google Scholar]
- Ahmadzadeh M., and Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. [Abstract] [Google Scholar]
- Walker M, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003;112:1437–1443. [Europe PMC free article] [Abstract] [Google Scholar]
- Shen X, Zhou J, Hathcock KS, Robbins P, Powell DJ Jr, Rosenberg SA, et al. Persistence of tumor infiltrating lymphocytes in adoptive immunotherapy correlates with telomere length. J Immunother. 2007;30:123–129. [Europe PMC free article] [Abstract] [Google Scholar]
- Powell DJ Jr, Dudley ME, Robbins PF., and Rosenberg SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–250. [Europe PMC free article] [Abstract] [Google Scholar]
- Powell DJ Jr, Dudley ME, Hogan KA, Wunderlich JR., and Rosenberg SA. Adoptive transfer of vaccine-induced peripheral blood mononuclear cells to patients with metastatic melanoma following lymphodepletion. J Immunol. 2006;177:6527–6539. [Europe PMC free article] [Abstract] [Google Scholar]
- Singh S, Ross SR, Acena M, Rowley DA., and Schreiber H. Stroma is critical for preventing or permitting immunological destruction of antigenic cancer cells. J Exp Med. 1992;175:139–146. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. [Europe PMC free article] [Abstract] [Google Scholar]
- Spiotto MT., and Schreiber H. Rapid destruction of the tumor microenvironment by CTLs recognizing cancer-specific antigens cross-presented by stromal cells. Cancer Immun. 2005;5:8. [Abstract] [Google Scholar]
- Wan YY., and Flavell RA. Regulatory T cells, transforming growth factor-β, and immune suppression. Proc Am Thorac Soc. 2007;4:271–276. [Europe PMC free article] [Abstract] [Google Scholar]
- Chaput N, Darrasse-Jeze G, Bergot A-S, Cordier C, Ngo-Abdalla S, Klatzmann D, et al. Regulatory T cells prevent CD8 T cell maturation by inhibiting CD4 Th cells at tumor sites. J Immunol. 2007;179:4969–4978. [Abstract] [Google Scholar]
- Frederiksen KS, Lundsgaard D, Freeman JA, Hughes SD, Holm TL, Skrumsager BK. IL-21 induces in vivo immune activation of NK cells and CD8(+) T cells in patients with metastatic melanoma and renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1439–1449. [Europe PMC free article] [Abstract] [Google Scholar]
- Li Y., and Yee C. IL-21 mediated Foxp3 suppression leads to enhanced generation of antigen-specific CD8+ cytotoxic T lymphocytes. Blood. 2008;111:229–235. [Europe PMC free article] [Abstract] [Google Scholar]
- Peluso I, Fantini MC, Fina D, Caruso R, Boirivant M, MacDonald TT, et al. IL-21 Counteracts the Regulatory T cell-Mediated Suppression of Human CD4+ T Lymphocytes. J Immunol. 2007;178:732–739. [Abstract] [Google Scholar]
- Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–286. [Europe PMC free article] [Abstract] [Google Scholar]
- Dudley ME, Wunderlich JR, Shelton TE, Even J., and Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–342. [Europe PMC free article] [Abstract] [Google Scholar]
- Schultze JL, Seamon MJ, Michalak S, Gribben JG., and Nadler LM. Autologous tumor infiltrating T cells cytotoxic for follicular lymphoma cells can be expanded in vitro. Blood. 1997;89:3806–3816. [Abstract] [Google Scholar]
- Rosenberg SA, Spiess P., and Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. [Abstract] [Google Scholar]
- Nitta T, Oksenberg JR, Rao NA., and Steinman L. Predominant expression of T cell receptor V alpha 7 in tumor-infiltrating lymphocytes of uveal melanoma. Science. 1990;249:672–674. [Abstract] [Google Scholar]
- Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nature Med. 2008;14:518–527. [Abstract] [Google Scholar]
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. [Abstract] [Google Scholar]
- Petrulio CA, Kim-Schulze S., and Kaufman HL. The tumour microenvironment and implications for cancer immunotherapy. Expert Opin Biol Ther. 2006;6:671–684. [Abstract] [Google Scholar]
- Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. [Abstract] [Google Scholar]
- Kaufman HL, DeRaffele G, Mitcham J, Moroziewicz D, Cohen SM, et al. Targeting the local tumor microenvironment with vaccinia virus expressing B7.1 for the treatment of melanoma. J Clin Invest. 2005;115:1903–1912. [Europe PMC free article] [Abstract] [Google Scholar]
- Wei S, Shreiner AB, Takeshita N, Chen L, Zou W., and Chang AE. Tumor-induced immune suppression of in vivo effector T-cell priming is mediated by the B7-H1/PD-1 axis and transforming growth factor β Cancer Res. 2008;68:5432–5438. [Europe PMC free article] [Abstract] [Google Scholar]
- Bach JF. Regulatory T cells under scrutiny. Nat Rev Immunol. 2003;3:189–198. [Abstract] [Google Scholar]
- Marie JC, Letterio JJ, Gavin M., and Rudensky AY. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. [Europe PMC free article] [Abstract] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. [Europe PMC free article] [Abstract] [Google Scholar]
- Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-β signals in vivo. Proc Natl Acad Sci USA. 2005;102:419–424. [Europe PMC free article] [Abstract] [Google Scholar]
- Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878–4886. [Abstract] [Google Scholar]
- Koneru M, Schaer D, Monu N, Ayala A., and Frey AB. Defective proximal TCR signaling inhibits CD8+ tumor-infiltrating lymphocyte lytic function. J Immunol. 2005;174:1830–1840. [Abstract] [Google Scholar]
- Nakagomi H, Petersson M, Magnusson I, Juhlin C, Matsuda M, Mellstedt H, et al. Decreased expression of the signal-transducing zeta chains in tumor-infiltrating T-cells and NK cells of patients with colorectal carcinoma. Cancer Res. 1993;53:5610–5612. [Abstract] [Google Scholar]
- Takaki R, Hayakawa Y, Nelson A, Sivakumar PV, Hughes S, Smyth MJ, et al. IL-21 enhances tumor rejection through a NKG2D-dependent mechanism. J Immunol. 2005;175:2167–2173. [Abstract] [Google Scholar]
- Smyth MJ, Wallace ME, Nutt SL, Yagita H, Godfrey DI., and Hayakawa Y. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. J Exp Med. 2005;201:1973–1985. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang G, Tschoi M, Spolski R, Lou Y, Ozaki K, Feng C, et al. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res. 2003;63:9016–9022. [Abstract] [Google Scholar]
- Strengell M, Lehtonen A, Matikainen S., and Julkunen I. IL-21 enhances SOCS gene expression and inhibits LPS-induced cytokine production in human monocyte-derived dendritic cells. J Leukoc Biol. 2006;79:1279–1285. [Abstract] [Google Scholar]
- Desai S, Liu H., and Pahwa S. IL-21 augments monocyte derived dendritic cell (mDC) maturation and upregulates PD-L1 gene expression. J Immunol. 2007;178:73–74. [Google Scholar]
- Burgess SJ, Marusina AI, Pathmanathan I, Borrego F., and Coligan JE. IL-21 down-regulates NKG2D/DAP10 expression on human NK and CD8+ T cells. J Immunol. 2006;176:1490–1497. [Abstract] [Google Scholar]
- Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105:3005–3010. [Europe PMC free article] [Abstract] [Google Scholar]
- Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–791. [Europe PMC free article] [Abstract] [Google Scholar]
- Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. [Europe PMC free article] [Abstract] [Google Scholar]
- He H, Wisner P, Yang G, Hu HM, Haley D, Miller W, et al. Combined IL-21 and low-dose IL-2 therapy induces anti-tumor immunity and long-term curative effects in a murine melanoma tumor model. J Transl Med. 2006;4:24. [Europe PMC free article] [Abstract] [Google Scholar]
Articles from Molecular Therapy are provided here courtesy of The American Society of Gene & Cell Therapy
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/141643980
Article citations
TCR-engaging scaffolds selectively expand antigen-specific T-cells with a favorable phenotype for adoptive cell therapy.
J Immunother Cancer, 11(8):e006847, 01 Aug 2023
Cited by: 2 articles | PMID: 37586765 | PMCID: PMC10432666
Implications of IL-21 in solid tumor therapy.
Med Oncol, 40(7):191, 30 May 2023
Cited by: 3 articles | PMID: 37249661
Review
Engineered murine IL-21-secreting leukemia cells induce granzyme B+ T cells and CD4+CD44+CD62L- effector memory cells while suppressing regulatory T cells, leading to long-term survival.
Cancer Immunol Immunother, 72(8):2597-2612, 15 Apr 2023
Cited by: 0 articles | PMID: 37061631 | PMCID: PMC10991896
Interleukin gene delivery for cancer gene therapy: In vitro and in vivo studies.
Iran J Basic Med Sci, 26(2):128-136, 01 Feb 2023
Cited by: 0 articles | PMID: 36742134 | PMCID: PMC9869882
Review Free full text in Europe PMC
Analysis of causes for poor persistence of CAR-T cell therapy in vivo.
Front Immunol, 14:1063454, 25 Jan 2023
Cited by: 4 articles | PMID: 36761742 | PMCID: PMC9905114
Review Free full text in Europe PMC
Go to all (42) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Tc1 and Tc2 effector cell therapy elicit long-term tumor immunity by contrasting mechanisms that result in complementary endogenous type 1 antitumor responses.
J Immunol, 172(3):1380-1390, 01 Feb 2004
Cited by: 35 articles | PMID: 14734713
Role of effector cell-derived IL-4, IL-5, and perforin in early and late stages of type 2 CD8 effector cell-mediated tumor rejection.
J Immunol, 167(1):424-434, 01 Jul 2001
Cited by: 30 articles | PMID: 11418679
CD8-mediated type 1 antitumor responses selectively modulate endogenous differentiated and nondifferentiated T cell localization, activation, and function in progressive breast cancer.
J Immunol, 177(11):8191-8201, 01 Dec 2006
Cited by: 23 articles | PMID: 17114496



