Abstract
Free full text

Exercise improves body fat, lean mass and bone mass in breast cancer survivors
Abstract
Given the negative effects of a breast cancer diagnosis and its treatments on body weight and bone mass, we investigated the effects of a 6-month randomized controlled aerobic exercise intervention vs. usual care on body composition in breast cancer survivors. Secondary aims were to examine the effects stratified by important prognostic and physiologic variables. Seventy-five physically inactive postmenopausal breast cancer survivors were recruited through the Yale-New Haven Hospital Tumor Registry and randomly assigned to an exercise (n = 37) or usual care (n = 38) group. The exercise group participated in 150 min/wk of supervised gym- and home-based moderate-intensity aerobic exercise. The usual care group was instructed to maintain their current physical activity level. Body composition was assessed at baseline and 6-months via dual energy x-ray absorptiometry by one radiologist blinded to the intervention group of the participants. On average, exercisers increased moderate-intensity aerobic exercise by 129 min/wk over and above baseline levels compared with 45 min/wk among usual care participants (p < .001). Exercisers experienced decreases in percent body fat (p = .0022) and increases in lean mass (p = .047) compared with increases in body fat and decreases in lean mass in usual care participants. BMD was also maintained among exercisers compared with a loss among usual care participants (p = .043). In summary, moderate-intensity aerobic exercise, such as brisk walking, produces favorable changes in body composition that may improve breast cancer prognosis.
INTRODUCTION
Breast cancer is the most common form of cancer diagnosed among American women (1). More women are surviving breast cancer than ever before due to early diagnosis and improvements in initial treatments (1). Surviving breast cancer, however, often puts women at an increased risk for chronic diseases due in part to unfavorable changes in body composition (2).
Weight gain is common after a breast cancer diagnosis (3), and is often a result of increases in fat mass without concurrent gains in lean body mass, commonly referred to as sarcopenic obesity (4). Although not conclusive, obesity and weight gain may adversely affect the risk of breast cancer recurrence and death (5,6). Breast cancer survivors are also reported to have lower bone mineral density (BMD) and a significant increased risk for fractures compared to healthy women (7,8). Chemotherapy and endocrine therapy with aromatase inhibitors are associated with significant bone loss, osteoporosis and fractures (9,10). Thus, improving body composition is of extreme importance to breast cancer survivors.
Physical activity has been shown to be effective in the improvement of physical functioning and quality of life in breast cancer survivors (11), and may also be effective in the prevention of weight gain and bone loss. Physical activity has been shown to reduce the risk of primary breast cancer (12), and recurrence and death from breast cancer (13,14,15). The mechanisms thought to confer this reduced risk include certain metabolic and sex hormones (16).
In healthy women, aerobic exercise has been associated with favorable changes in body composition (17,18); however, few trials have examined the effect of exercise on outcomes of body composition, assessed with valid and reliable measures such as dual energy x-ray absorptiometry (DEXA), in breast cancer survivors (19–26). Fewer studies have examined the independent effect of aerobic exercise (i.e., without concomitant changes in diet or participation in resistance training) on body composition (25). Thus, there is currently no evidence to support the use of aerobic exercise, such as brisk walking, the most commonly reported physical activity among breast cancer survivors, to maintain or favorably change body fat, lean body mass (LBM) and BMD in these women.
We investigated the effects of a six-month randomized controlled aerobic exercise intervention vs. usual care on body weight and circumferences, body fat, LBM, BMD, and bone mineral content (BMC) in postmenopausal breast cancer survivors. Secondary aims were to examine the exercise effect stratified by important prognostic and physiologic variables. We hypothesized that exercise would be associated with favorable changes in body composition, and that stage at diagnosis, hormonal therapy, age, and body mass index (BMI) would modify the effect of exercise on body composition.
METHODS
Participants were recruited into the Yale Exercise and Survivorship Study, which is described in detail elsewhere (27). All study procedures were reviewed and approved by the Yale University School of Medicine Human Investigation Committee.
Study Participants
Participants were physically inactive (< 60 min/wk of recreational physical activity reported in the past six months), postmenopausal women (i.e., cessation of menses for at least 12 months) diagnosed 1–10 years ago with Stage 0 to IIIA breast cancer and who had completed chemotherapy and/or radiation at least six months prior to enrollment. Smokers, women with type 2 diabetes, and women with a previous cancer were excluded due to the potential effect of these factors on outcomes of interest.
Recruitment
Study staff used the Yale-New Haven Hospital Tumor Registry to obtain the names of Connecticut women diagnosed with breast cancer by any Yale-affiliated physician from March 2004 to January 2006 (27) (Figure 1). Staff contacted each patient’s physician to request permission to contact the participant. An invitation letter was mailed to the participant, followed by a telephone screening questionnaire, baseline and 6-month clinic visit. From 788 screening calls made, 75 (9.5%) women were eligible, interested, and randomized.
Measures
Physical Activity
At baseline and 6-months, participants completed an interview-administered physical activity questionnaire (measured at baseline only for screening purposes), assessing the past 6 months of recreational activity (27), a seven-day physical activity log (PAL) (29), and a seven-day pedometer log (30). For the PAL, women recorded the type and duration of any recreational activity performed on each day. Hours/week spent in moderate-to-vigorous intensity aerobic activity were determined using Ainsworth’s Compendium of Physical Activities (28).
Demographics & Medical History
Information was collected via an interviewer-administered questionnaire at the baseline visit. Information regarding disease stage, hormone receptor status, adjuvant therapy, and surgery was provided by participants and later confirmed by their physician and review of the medical records.
Anthropometry
Height and weight were measured at baseline and 6-months. Participants were weighed on a digital scale in light clothing, without shoes; measurements were rounded up to the next 0.1 kilogram. Height without shoes was measured using a stadiometer, rounding up to the next 0.5 centimeter. Circumference measurements were taken at the waist (minimum circumference) and hips (greatest circumference). All measurements were taken twice in succession, by the same technician, and averaged for data entry.
DEXA Scans
A total body DEXA scan was completed for each participant at baseline and 6-months (31). The DEXA measurements were made with a Hologic scanner (Hologic 4500, Hologic Inc, Waltham, Mass) that was calibrated daily. The manufacturer’s reported coefficient of variation for repeat assessment of body fat, lean mass, and BMD is less than 1%. All DEXA scans were evaluated by one radiologist blinded to the intervention group of the participant.
Food Frequency Questionnaire
All participants completed a 120-item validated food frequency questionnaire at baseline and 6-months (32). While participants were advised to maintain their current dietary habits, we measured their dietary habits in order to control for any changes in diet over the 6-month time period.
Exercise Intervention
The exercise intervention consisted of a combined supervised training program at a local health club and a home aerobic training program. Participants exercised at the health club during designated sessions three times per week and were instructed to exercise two days/week on their own, either at the health club or home. Participants were asked to perform three 15-minute sessions during Week 1, building to five 30-minute moderate-intensity sessions by Week 5, the current physical activity recommendation for adults (33). Participants wore heart rate monitors during each workout, with heart rate electronically monitored with high/low pulse rate alarms individually set for each subject. Exercise started at 50% of predicted maximal heart rate (220-age) and was gradually increased in accordance with American College of Sports Medicine guidelines to approximately 60–80% of predicted maximal heart rate. Following each exercise session, participants recorded the type, duration, perceived intensity of activity, and average heart rate during exercise in physical activity logs. Participants returned logs to the exercise physiologists at the end of each week.
The intervention consisted primarily of walking, an activity preferred by breast cancer survivors (34), although participants could choose to meet the exercise goal through other forms of aerobic activity. Activities that did not involve sustained aerobic effort, such as resistance training and yoga, could be performed but did not count toward the exercise goal for each week.
Usual Care Group
Women in the usual care group were instructed to continue with their usual activities. If a participant wanted to exercise, she was told she could, but that our exercise program and training materials would not be offered to her until the end of the study. At the end of the study, women were offered three supervised training exercise sessions, a pedometer, exercise handouts, and results of clinical tests. All study participants also received quarterly newsletters that focused on issues related to breast cancer survivorship.
Randomization
After completion of all baseline measures, each participant was randomly assigned to either the exercise or usual care group using a random number generator.
Statistical Analyses
Participants were grouped according to the intention-to-treat procedure in which all participants were grouped according to their intervention assignment at randomization. Based on a standard deviation of 4 and effect size of 5%, a sample size of 75 women provided us with at least 80% power at the 0.05 α level. We used t-tests and Chi-square analyses to assess between-group baseline differences. Intervention effects were evaluated by the differences in the mean changes at 6-months between the intervention and usual care groups using the generalized estimating equation modification to linear regression models to account for the longitudinal nature of the data. Confounders examined included disease stage, previous radiation and/or chemotherapy, hormonal therapy, age, BMI, baseline body fat, baseline BMD, current use of calcium and vitamin D supplements, and current use of bisphosphonates. However, because adjustment did not change the results and on the basis of the randomized design of the study, we have presented the unadjusted results. We also explored a priori effect modification by disease stage, previous radiation and/or chemotherapy, hormonal therapy, age, and BMI. For exercisers only, we examined changes in body composition by adherence.
Lastly, a subset of the 75 women (n – 48) were also enrolled in an ancillary study (examining the effect of exercise on mammographic density) that required them to participate in 12 months of exercise rather than just 6 months. Thus, for this subset of women, we examined the baseline to 12 month effect of exercise on body composition.
RESULTS
Baseline Characteristics
Baseline demographic and physiologic data in the exercise and usual care groups were similar (Table 1). The average age of study participants was 56 yrs. The majority (84%) of participants were non-Hispanic white. Average time since diagnosis for participants was 3.3 yrs. Women were, on average, obese with BMD within normal limits. However, at baseline, eight women (11%; three exercisers and five usual care participants) had osteopenia (t score < −1.0).
Table 1
Baseline characteristics of randomized participants in the YES Study (N=75).*
| Exercisers Mean (SD) or % | Usual Care Mean (SD) or % | |
|---|---|---|
| N | 37 | 38 |
| Age (yrs) | 56.5 (9.5) | 55.1 (7.7) |
| Ethnicity | ||
 Non-Hispanic White Non-Hispanic White | 84% | 84% |
 African-American African-American | 16% | 11% |
 Asian/Pacific Islander Asian/Pacific Islander | 0% | 3% |
| Education (%) | ||
 High school graduate High school graduate | 16% | 22% |
 Some school after high school Some school after high school | 24% | 30% |
 College graduate + College graduate + | 60% | 41% |
| Time since diagnosis (years) | 3.6 (2.2) | 3.3 (2.6) |
| Disease stage (%) | ||
 In Situ In Situ | 11% | 11% |
 Stage I Stage I | 54% | 27% |
 Stage II Stage II | 27% | 46% |
 Stage IIIA Stage IIIA | 8% | 16% |
| Treatment (%) | ||
 None None | 5% | 14% |
 Radiation only Radiation only | 41% | 24% |
 Chemotherapy only Chemotherapy only | 19% | 19% |
 Radiation and chemotherapy Radiation and chemotherapy | 35% | 43% |
| Hormone therapy | ||
 None None | 43% | 30% |
 Tamoxifen Tamoxifen | 30% | 30% |
 Aromatase Inhibitors Aromatase Inhibitors | 27% | 40% |
| Current use of calcium supplements | 58% | 58% |
| Current use of vitamin D supplements | 8% | 23% |
| Current use of bisphosphonates | 13% | 3% |
| Physical Activity Questionnaire (min/wk recreational exercise) | 13.0 (24.0) | 12.0 (20.0) |
| Daily Activity Log (min/wk recreational exercise) | 30.0 (41.1) | 11.3 (24.8) |
| Pedometer steps/day | 5,145 (2,312) | 5,342 (2,744) |
Change in Physical Activity Levels and Diet and Adherence to the Exercise Intervention
Based on information reported in the 7-Day Physical Activity Log at baseline and 6-months, exercisers, on average, increased moderate- to vigorous-intensity recreational activity by 129 min/wk at 6-months over and above baseline levels (p < .001) compared to smaller increases among usual care participants (45 min/wk at 6 months) (data not shown). Exercisers also increased their pedometer steps/day, on average, by 1621 steps (11,347 steps/wk) or approximately 0.9 miles/day (6.3 miles/wk) compared to 85 steps/day (595 steps/wk) or approximately 0.05 miles/day (0.35 miles/wk) among usual care participants (p = .032) (data not shown). Assuming a 20-min walking pace, our finding of 129 min/wk participation in exercise as measured with the physical activity log corresponds to our finding of 6.3 miles/wk as measured with the pedometer.
Exercise group participants also completed physical activity logs each week of the intervention. They performed an average of 120 min/wk of moderate- to vigorous-intensity recreational activity over 6 months. A total of 73% were completing at least 80% of the exercise goal of 150 min/wk over 6 months, respectively (data not shown). Women randomized to exercise chose weight-bearing activities most often, with 82% walking. Few women reported doing resistance training (3%).
Lastly, women did not significantly change their diet over six months; baseline to six month change in total caloric intake in women randomized to exercise was −79 kcal/day vs. −121 kcal/day in women randomized to usual care (p = .89).
Effect of Exercise vs. Usual Care on 6-Month Changes in Body Composition
Mean 6-month changes from baseline in body composition for both groups are shown in Table 2. After 6-months, exercisers experienced favorable changes in body fat and LBM compared with unfavorable changes among women randomized to usual care (p < .05). Results remained statistically significant after excluding the six women taking bisphosphonates at baseline.
Table 2
Six-Month effect of exercise on body composition in the YES Study (mean ± SD).
| Baseline | 6 months | |||
|---|---|---|---|---|
| Mean ±SD | Mean ±SD | Percent Change | Pa | |
| Weight (kg) | ||||
 Exercisers b Exercisers b | 81.28 ± 16.98 | 80.73 ± 16.88 | −0.7 | 0.39 |
 Usual Care c Usual Care c | 78.41 ± 20.01 | 78.51 ± 20.64 | 0.1 | |
| BMI (kg·m−2) | ||||
 Exercisers Exercisers | 30.57 ± 5.95 | 30.45 ± 6.00 | − 0.4 | 0.42 |
 Usual Care Usual Care | 29.74 ± 7.27 | 29.90 ± 7.59 | 0.4 | |
| Percent body fat (%) | ||||
 Exercisers Exercisers | 41.33 ± 6.46 | 40.54 ± 6.55 | −1.9 | 0.0022 |
 Usual Care Usual Care | 39.18 ± 5.90 | 39.60 ± 5.97 | 1.1 | |
| Lean Mass (kg) | ||||
 Exercisers Exercisers | 44.01 ± 6.62 | 44.35 ± 6.60 | 0.8 | 0.047 |
 Usual Care Usual Care | 43.03 ± 7.19 | 42.68 ± 7.19 | −0.8 | |
| BMD (g·cm−2) | ||||
 Exercisers Exercisers | 1.152 ± 0.109 | 1.144 ± 0.119 | −0.7 | 0.97 |
 Usual Care Usual Care | 1.120 ± 0.094 | 1.112 ± 0.101 | −0.7 | |
| BMC (g·cm) | ||||
 Exercisers Exercisers | 2258 ± 373 | 2216 ± 383 | −1.8 | 0.062 |
 Usual Care Usual Care | 2116 ± 305 | 2120 ± 308 | 0.2 | |
| Waist circumference (cm) | ||||
 Exercisers Exercisers | 91.13 ± 12.33 | 89.75 ± 12.63 | −1.5 | 0.57 |
 Usual Care Usual Care | 89.42 ± 15.13 | 88.58 ± 15.48 | −0.8 | |
| Hip circumference (cm) | ||||
 Exercisers Exercisers | 112.96 ± 13.59 | 111.92 ± 12.56 | −0.8 | 0.21 |
 Usual Care Usual Care | 110.79 ± 15.64 | 111.11 ± 14.97 | 0.6 | |
Effect of Exercise vs. Usual Care on 12-Month Changes in Body Composition
Mean 12-month changes from baseline in body composition for both groups are shown in Table 3. After 12 months, exercisers experienced favorable changes in body fat and BMD compared with unfavorable changes among women randomized to usual care (p < .05). Results remained statistically significant after excluding the six women taking bisphosphonates at baseline.
Table 3
Twelve-month effect of exercise on body composition in the YES Study (mean ± SD).
| Baseline | 12 months | |||
|---|---|---|---|---|
| Mean ±SD | Mean ±SD | Percent Change | Pa | |
| Weight (kg) | ||||
 Exercisers b Exercisers b | 81.21 ± 18.51 | 81.60 ± 18.97 | −0.2 | 0.61 |
 Usual Care c Usual Care c | 75.93 ± 17.66 | 76.58 ± 17.41 | 0.6 | |
| Percent body fat (%) | ||||
 Exercisers Exercisers | 41.21 ± 6.70 | 40.02 ± 7.06 | −2.4 | 0.043 |
 Usual Care Usual Care | 39.53 ± 6.14 | 39.50 ± 6.14 | 0.8 | |
| Lean Mass (kg) | ||||
 Exercisers Exercisers | 43.92 ± 7.06 | 44.62 ± 7.65 | 1.7 | 0.25 |
 Usual Care Usual Care | 42.30 ± 7.16 | 42.21 ± 6.74 | 0.2 | |
| BMD (g·cm−2) | ||||
 Exercisers Exercisers | 1.159 ± 0.112 | 1.167 ± 0.126 | 0.2 | 0.043 |
 Usual Care Usual Care | 1.122 ± 0.098 | 1.097 ± 0.105 | −1.7 | |
| BMC (g·cm) | ||||
 Exercisers Exercisers | 2265 ± 406 | 2267 ± 410 | −0.6 | 0.13 |
 Usual Care Usual Care | 2088 ± 300 | 2022 ± 295 | −2.2 | |
Effect of Exercise on Body Composition Stratified by Potential Effect Modifiers
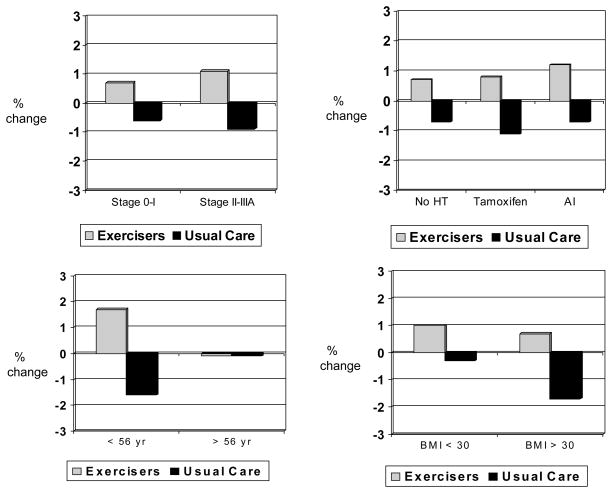
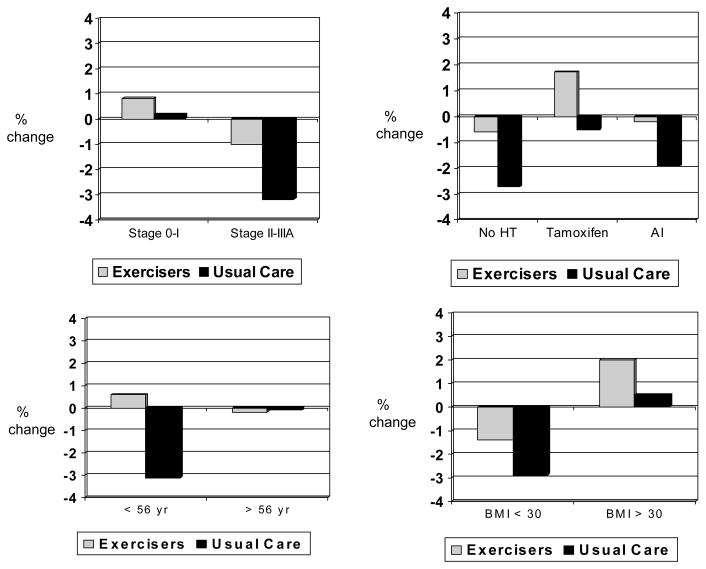
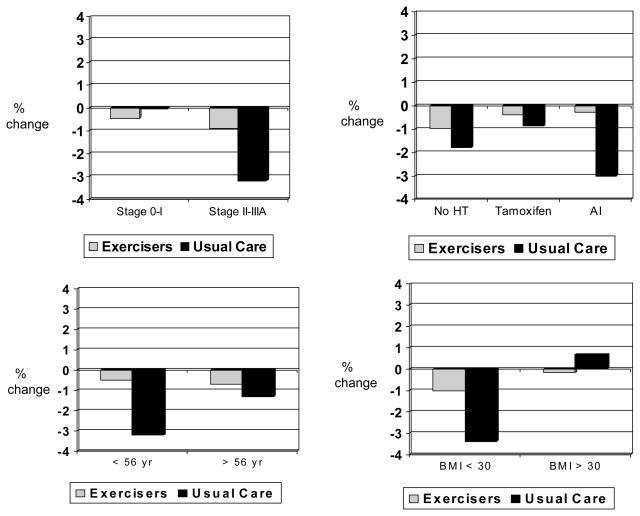
Hormone therapy modified the effect of exercise on body fat (p for interaction = .060), with greater between-group effect sizes in body fat observed among women not taking any hormone therapy vs. women taking tamoxifen or aromatase inhibitors (Figure 2). Results remained statistically significant after excluding the six women taking bisphosphonates at baseline (4 of whom were not taking any hormonal therapy). Age modified the effect of exercise on LBM (p for interaction = .044) (Figure 3) and BMD (p for interaction = .10) (Figure 4), with greater between-group effect sizes in LBM and BMD observed among younger women vs. older women. Disease stage, previous radiation or chemotherapy, and BMI did not modify the effect of exercise on body composition; however, a statistically significant between-group difference (i.e., exercisers vs. usual care) was observed for body fat, BMD, and BMC among women diagnosed with Stage II–III breast cancer (Figure 2, ,4,4, and and5).5). Statistically significant between-group differences were also observed for body fat and BMC among women with a BMI < 30.

Baseline to 6 month % change in body fat stratified by stage, hormone therapy, age, and BMI
* p < .05 difference between exercisers and controls
Sample sizes for stage 0–I (exercisers, n = 24, usual care, n = 12), stage II–IIIA (exercisers, n = 12, usual care, n = 21); no HT (exercisers, n = 15, usual care, n = 10), tamoxifen (exercisers, n = 11, usual care, n = 8), AI (exercisers, n = 10, usual care, n = 15); < 56 yr (exercisers, n = 19, usual care, n = 16), ≥56 yr (exercisers, n = 17, usual care, n = 17; BMI < 30 (exercisers, n = 16, usual care, n = 20), and BMI ≥30 (exercisers, n = 20, usual care, n = 13).
Baseline to 6 month % change in LBM stratified by stage, hormone therapy, age, and BMI
* p < .05 difference between exercisers and controls
Sample sizes for stage 0–I (exercisers, n = 24, usual care, n = 12), stage II–IIIA (exercisers, n = 12, usual care, n = 21), no HT (exercisers, n = 15, usual care, n = 10), tamoxifen (exercisers, n = 11, usual care, n = 8), AI (exercisers, n = 10, usual care, n = 15), < 56 yr (exercisers, n = 19, usual care, n = 16), ≥56 yr (exercisers, n = 17, usual care, n = 17, BMI < 30 (exercisers, n = 16, usual care, n = 20), and BMI ≥30 (exercisers, n = 20, usual care, n = 13).
Baseline to 6 month % change in BMD stratified by stage, hormone therapy, age, and BMI
* p < .05 difference between exercisers and controls
Sample sizes for stage 0–I (exercisers, n = 24, usual care, n = 12), stage II–IIIA (exercisers, n = 12, usual care, n = 21), no HT (exercisers, n = 15, usual care, n = 10), tamoxifen (exercisers, n = 11, usual care, n = 8), AI (exercisers, n = 10, usual care, n = 15), < 56 yr (exercisers, n = 19, usual care, n = 16), ≥56 yr (exercisers, n = 17, usual care, n = 17, BMI < 30 (exercisers, n = 16, usual care, n = 20), and BMI ≥30 (exercisers, n = 20, usual care, n = 13).
Baseline to 6 month % change in BMC stratified by stage, hormone therapy, age, and BMI
* p < .05 difference between exercisers and controls
Sample sizes for stage 0–I (exercisers, n = 24, usual care, n = 12), stage II–IIIA (exercisers, n = 12, usual care, n = 21), no HT (exercisers, n = 15, usual care, n = 10), tamoxifen (exercisers, n = 11, usual care, n = 8), AI (exercisers, n = 10, usual care, n = 15), < 56 yr (exercisers, n = 19, usual care, n = 16), ≥56 yr (exercisers, n = 17, usual care, n = 17, BMI < 30 (exercisers, n = 16, usual care, n = 20), and BMI ≥30 (exercisers, n = 20, usual care, n = 13).
Effect of Exercise on Body Composition Stratified by Adherence
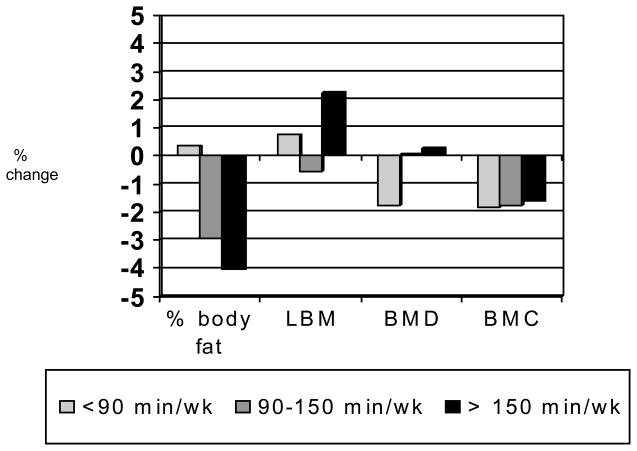
Women who exercised more experienced greater losses in body fat, yet slight increases in BMD compared with women who exercised less (p < .05) (Figure 6).
DISCUSSION
In our study, aerobic exercise, such as brisk walking, was associated with decreases in body fat, increases in LBM, and a maintenance of BMD in breast cancer survivors. The observation that aerobic exercise accompanied by fat loss was not associated with a significant loss of BMD is important. In postmenopausal women, adipose tissue is the main site of androgen conversion to estrogen by the enzyme aromatase (35). As postmenopausal women lose body fat, their serum estrogen concentrations decrease, which could cause a decrease in BMD. Thus, it is plausible that exercise accompanied by fat loss could have a negative effect on bone health in postmenopausal women. However, in our study, we observed a maintenance of BMD with exercise and subsequent fat loss.
Furthermore, women who were taking aromatase inhibitors, which significantly decrease estrogen concentrations to undetectable levels (36), and randomized to exercise, experienced decreases in body fat, increases in lean mass and maintenance of BMD and BMC, compared to no change in body fat and losses in LBM, BMD, and BMC among women randomized to usual care. These results are encouraging for the many postmenopausal women who are recommended to take aromatase inhibitors to improve their prognosis. Recently published reports show that women taking aromatase inhibitors experience greater bone loss and fractures compared to women taking tamoxifen or placebo (37–39). Aromatase inhibitor-associated bone loss may be distinct from normal postmenopausal bone loss. Estrogen deprivation that occurs during aromatase inhibitor therapy is generally abrupt compared with that occurring postmenopausally, and bone loss may occur at an accelerated rate (~2.6% bone loss/year with aromatase inhibitors compared to ~1% bone loss/year after menopause) (37). Although medications can be used to alleviate bone loss, such as bisphosphonate therapy, these drugs also cause significant side effects, such as gastrointestinal toxicity (37). Our study shows that aerobic exercise attenuates aromatase inhibitor-related bone loss. Thus, aromatase inhibitor-associated bone loss may be preventable, allowing women to benefit from endocrine therapy while also being protected against the increased risk of osteoporosis, fracture, and ultimately breast cancer recurrence or death. However, because of the small sub-group analyses, these findings should be interpreted with caution.
To our knowledge, no other study has examined whether certain prognostic or demographic factors modify the effect of exercise on body composition, and only one study has examined the main effect of aerobic exercise only on body composition assessed using valid and reliable measures such as DEXA (25). In that study, Matthews et al observed no significant changes in body fat or BMD in 10 postmenopausal women enrolled in a 12-week home-based walking intervention vs. usual care.
Three studies have examined the effect of resistance training on body composition in breast cancer survivors who have completed treatment. Schmitz et al. (20) investigated a resistance training program on body composition and observed significant increases in LBM (0.88 for exercisers vs. 0.02 for controls, p < .01) and decreases in body fat (−1.15% for exercisers vs. 0.23%, p = .023) with a twice-weekly yearlong resistance training program in 69 pre- and post-menopausal breast cancer survivors. Their observed between-group effect sizes in LBM and body fat are similar to the effect sizes observed in our study. This finding is promising because some women may prefer to control weight and LBM through aerobic exercise, such as brisk walking, rather than through resistance training. The two other studies were quasi-experimental studies of small sample sizes (< 26 women), but one of the studies observed significant improvements in BMD of the spine and hip (p < .01) with resistance training (22), and the other study reported no change in BMD (26).
Lastly, only one study has examined the effect of exercise on BMD in women with breast cancer receiving adjuvant chemotherapy. Schwartz et al. (19) examined the effects of aerobic or resistance training (therabands) vs. usual care on BMD in 66 pre- and post-menopausal women beginning adjuvant chemotherapy. The average decline in BMD was 6.23% for women randomized to usual care compared to losses of 4.92% and 0.76% among women randomized to resistance training or aerobic exercise.
In our study, stratified analyses also demonstrated greater between-group differences in body composition among women diagnosed with Stage II–III breast cancer compared to women diagnosed with Stage 0–I breast cancer. Women with higher stages of disease are often treated with radiation and chemotherapy, which may adversely affect body fat and BMD. An exercise intervention may, therefore, have stronger effects in women diagnosed with higher stages of disease. Age also modified the effect of exercise on body composition. Younger women may have experienced chemotherapy-induced menopause rather than natural menopause, resulting in greater losses of BMD and LBM during and after treatment for breast cancer. An exercise program may be more advantageous for maintenance or increases in LBM and BMD for younger breast cancer survivors compared to older breast cancer survivors. Lastly, a dose-response effect was observed with greater decreases in body fat, increases in LBM, and maintenance of BMD with higher doses of exercise per week.
The Yale Exercise and Survivorship Study was methodologically strong relative to many studies previously conducted. Our study used a population-based recruitment strategy, randomization to study groups, a physical activity prescription that met national guidelines, detailed measurement of physical activity and adherence, comprehensive assessment of body composition using validated measures, and long study duration. Our sample size is comparable, if not larger, than those of several earlier studies in breast cancer survivors; however it is still small in absolute terms, with implications for compromised statistical power with regard to some of our stratified analyses. An additional limitation of our study is that we did not measure regional bone density. Our sample was also highly-educated and mostly non-Hispanic white. However, the 75 women enrolled in the study did not differ with regard to age, race, or educational status from the non-participants that comprised the majority of women diagnosed with breast cancer at Yale-New Haven Hospital in 2004 and 2005.
Conclusions
Given that a majority of breast cancer survivors are at risk of osteoporosis and fractures (37), improving bone mass is a priority in this population. Weight gain is also common after a diagnosis, and negatively affects prognosis (5,6). In our study, aerobic exercise, such as brisk walking, was associated with favorable changes in body fat and LBM, and maintenance of BMD in postmenopausal breast cancer survivors. Future studies are needed to investigate different doses and types of exercise on body composition in breast cancer survivors and certain subgroups, such as women taking aromatase inhibitors.
Acknowledgments
We thank Rebecca Latka, Christian Stoddard, Linda Saucier, Andrew Wiley and Mary O’Neil for their assistance. We are indebted to the participants in the Yale Exercise and Survivorship Study for their dedication.
GRANT SUPPORT: American Cancer Society (MRSG-04-006-01-CPPB) and the Susan G. Komen Breast Cancer Foundation (BCTR0201916). Supported in part by a General Clinical Research Center grant from the National Center of Research Resources, National Institutes of Health (Grant # M01-RR00125) awarded to Yale University School of Medicine
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/oby.2009.18
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2841468?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/oby.2009.18
Article citations
Mitigating aging and doxorubicin induced bone loss in mature mice via mechanobiology based treatments.
Bone, 188:117235, 13 Aug 2024
Cited by: 0 articles | PMID: 39147353
Protective role of exercise on breast cancer-related osteoporosis in women undergoing aromatase inhibitors: A narrative review.
Bone Rep, 21:101756, 25 Mar 2024
Cited by: 2 articles | PMID: 38577250 | PMCID: PMC10990716
Review Free full text in Europe PMC
The efficacy and safety of exercise regimens to mitigate chemotherapy cardiotoxicity: a systematic review and meta-analysis of randomized controlled trials.
Cardiooncology, 10(1):10, 23 Feb 2024
Cited by: 0 articles | PMID: 38395955 | PMCID: PMC10885653
Review Free full text in Europe PMC
Role of Body Composition in the Prediction of Skeletal Fragility Induced by Hormone Deprivation Therapies in Cancer Patients.
Curr Oncol Rep, 25(10):1141-1152, 25 Aug 2023
Cited by: 6 articles | PMID: 37624550 | PMCID: PMC10556180
Review Free full text in Europe PMC
Post-diagnosis weight trajectories and mortality among women with breast cancer.
NPJ Breast Cancer, 9(1):98, 02 Dec 2023
Cited by: 2 articles | PMID: 38042922 | PMCID: PMC10693588
Go to all (91) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study.
Cancer Epidemiol Biomarkers Prev, 18(1):306-313, 01 Jan 2009
Cited by: 135 articles | PMID: 19124513 | PMCID: PMC2841479
The effect of exercise on body composition and bone mineral density in breast cancer survivors taking aromatase inhibitors.
Obesity (Silver Spring), 25(2):346-351, 27 Dec 2016
Cited by: 43 articles | PMID: 28026901 | PMCID: PMC5450163
Effect of exercise on bone mineral density and lean mass in postmenopausal women.
Med Sci Sports Exerc, 38(7):1236-1244, 01 Jul 2006
Cited by: 14 articles | PMID: 16826020
Exercise for health for early postmenopausal women: a systematic review of randomised controlled trials.
Sports Med, 34(11):753-778, 01 Jan 2004
Cited by: 173 articles | PMID: 15456348
Review
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: R01 CA138556
Grant ID: R01 CA132931
NCRR NIH HHS (3)
Grant ID: M01 RR000125
Grant ID: M01 RR000125-41S10028
Grant ID: M01-RR00125