Abstract
Free full text

Nicotine facilitates LTP induction in oriens-lacunosum moleculare cells via Ca2+ entry through non-α7 nicotinic acetylcholine receptors
Abstract
Hippocampal inhibitory interneurons have a central role in the control of network activity, and excitatory synapses that they receive express Hebbian and anti-Hebbian long-term potentiation (LTP). Because many interneurons in the hippocampus express nicotinic acetylcholine receptors (nAChRs), we explored whether exposure to nicotine promotes LTP induction in these interneurons. We focused on a subset of interneurons in the stratum oriens/alveus that were continuously activated in the presence of nicotine due to the expression of non-desensitizing non-α7 nAChRs. We found that, in addition to α2 subunit mRNAs, these interneurons were consistently positive for somatostatin and neuropeptide Y mRNAs, and showed morphological characteristics of oriens-lacunosum moleculare cells. Activation of non-α7 nAChRs elevated intracellular Ca2+ levels at least in part via Ca2+ entry through their channels. Presynaptic tetanic stimulation induced N-methyl-D-aspartate receptor-independent LTP in voltage-clamped interneurons at −70 mV when in the presence, but not absence, of nicotine. Intracellular application of a Ca2+ chelator blocked LTP induction, suggesting the requirement of Ca2+ signal for LTP induction. The induction of LTP was still observed in the presence of ryanodine, which inhibits Ca2+-induced Ca2+ release from ryanodine-sensitive intracellular stores, and the L-type Ca2+ channel blocker nifedipine. These results suggest that Ca2+ entry through non-α7 nAChR channels is critical for LTP induction. Thus, nicotine affects hippocampal network activity by promoting LTP induction in oriens-lacunosum moleculare cells via continuous activation of non-α7 nAChRs.
Introduction
Systemic administration of nicotine improves several hippocampal-dependent learning and memory tasks (Abdulla et al., 1996; Levin & Simon, 1998; Levin, 2002; Marti Barros et al., 2004; Gould, 2006; Dani & Bertrand, 2007; Davis et al., 2007; Kenney & Gould, 2008). However, little is known about the detailed mechanisms by which nicotine exerts its effects on learning and memory. Long-term potentiation (LTP) of excitatory synaptic transmission is thought to be a cellular substrate of learning and memory. Thus, to gain insights into the mechanisms of nicotine’s effects on learning and memory, some studies have been directed towards understanding its effects on LTP. Nicotine facilitates the induction of LTP at excitatory synapses on dentate granule cells (the perforant pathway) and CA1 pyramidal cells (the Schaffer collateral pathway) in the trisynaptic circuit of hippocampus (Sawada et al., 1994; Hamid et al., 1997; Fujii et al., 1999; Matsuyama et al., 2000; Ji et al., 2001; Mann & Greenfield, 2003; Welsby et al., 2006; Nakauchi et al., 2007a). In contrast, nicotine suppresses LTP induction at the temporoammonic pathway, a direct excitatory input from the entorhinal cortex to CA1 pyramidal cells, suggesting that nicotine influences LTP induction in a pathway-specific manner (Nakauchi et al., 2007a). It remains to be elucidated whether nicotine modulates LTP induction at excitatory synapses on interneurons in the hippocampus.
Nicotine’s behavioral and cellular effects are mediated by its interaction with nicotinic acetylcholine receptors (nAChRs). In the hippocampus, at least 4 different nAChR subtypes, non-α7 (α2*, α3β4*, α4β2*) and α7 nAChRs, appear to be expressed (Alkondon et al., 2000; Alkondon & Albuquerque, 2001; McQuiston & Madison, 1999; Sudweeks & Yakel, 2000; Ji et al., 2001; Buhler & Dunwiddie, 2001, 2002; Frazier et al., 1998a, 1998b, 2003; Jia et al., 2009). The α7 nAChR, which is the most abundant subtype in the hippocampus, is highly Ca2+-permeable (Seguela et al., 1993; Role & Berg, 1996) and, thus, has been proposed to contribute to synaptic plasticity. Indeed, activation of presynaptic or postsynaptic α7 nAChRs promotes LTP induction at the Schaffer collateral pathway via increasing glutamate release or stimulating Ca2+ signaling cascades, respectively (Ji et al., 2001). However, this subtype desensitizes in the presence of nicotine (Frazier et al., 1998a, 1998b; McQuiston & Madison, 1999; Alkondon et al., 2000; Alkondon & Albuquerque, 2005; Yamazaki et al., 2005, 2006). At the Schaffer collateral pathway, desensitization of postsynaptic α7 nAChRs on feedforward γ-aminobutyric acid (GABA)ergic interneurons can reduce feedforward inhibition via suppressing incoming excitatory nicotinic synaptic responses (Frazier et al., 1998a, 1998b; Yamazaki et al., 2005, 2006) and preventing Ca2+ signaling for inhibition of GABAA receptor-mediated responses (Wanaverbecq et al., 2007; Zhang & Berg, 2007). Suppression of feedforward inhibition could enhance N-methyl-D-aspartate receptor (NMDAR)-mediated responses in pyramidal cells (Bliss & Collingridge, 1993), providing a potential mechanism for the nicotine-induced facilitation of LTP induction via desensitization rather than activation of α7 nAChRs. It largely remains to be determined whether all of these mechanisms are working in concert during nicotine exposure to facilitate LTP induction. Activation of non-α7 nAChRs also suppresses feedforward inhibition via reducing GABA release and postsynaptic GABAA receptor-mediated responses (Yamazaki et al., 2005). The α4β2* nAChR subtype has the highest affinity for nicotine and is the primary candidate for mediating nicotine’s effects. However, it is unknown whether this subtype is involved in nicotine-induced facilitation of LTP induction. The opposing effects of nicotine on LTP induction at Schaffer collateral and temporoammonic synapses are absent or greatly reduced in α2 knockout mice, suggesting that activation of this subtype differentially modulates LTP induction at these synapses (Nakauchi et al., 2007a). The α2* nAChR is the most sparsely expressed nAChR subtype in the brain, but shows a distinct localization in a subset of GABAergic interneurons in the stratum oriens/alveus (Wada et al., 1989; Ishii et al., 2005). A subset of oriens/alveus interneurons are continuously activated in the presence of nicotine, triggering interneuronal action potentials, which in turn causes the sustained release of GABA onto specific postsynaptic membrane domains (Jia et al., 2009). Thus, the nAChR subtype expressed in these interneurons becomes a critical component in hippocampal circuitry in the presence of nicotine, and potentially serves as a molecular switch for gating LTP via altering the local balance between excitation and inhibition.
GABAergic interneurons are distributed throughout all layers of the hippocampus and selectively innervate different domains of principal cells and other interneurons, providing a powerful control of circuit activity (Freund & Buzsaki, 1996). Thus, LTP at excitatory synapses on interneurons strongly influences the operation of hippocampal circuits. In addition to the modulation of circuit activity, activation of nAChRs on oriens/alveus interneurons may cause an increase in intracellular Ca2+ concentrations, which could in turn stimulate intracellular signaling for LTP induction. In the present study, we further characterized nicotine-sensitive oriens/alveus interneurons and explored the role of nAChRs in LTP induction in these interneurons.
Materials and Methods
All animal procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with protocols approved by the Institutional Animal Care and Use Committee of the University of California at Irvine. Efforts were made to minimize numbers of animals used.
Slice preparation
Sprague-Dawley rats (18- to 54-day-old; Harlan, Indianapolis, IN, USA) were anesthetized with urethane (1.25 g/kg) and killed by decapitation. Transverse hippocampal slices (375 μm) were prepared and maintained at 30–32°C in artificial cerebrospinal fluid containing (in mM): NaCl 124, KCl 5, NaH2PO4 1.25, MgSO4 2, CaCl2 2.5, NaHCO3 22, and glucose 10 and oxygenated with 95% O2/5% CO2, for at least 1 hour before recordings.
Electrophysiological recordings
Current- and voltage-clamp recordings were made from the somatic region of pyramidal cells and interneurons as described previously (Yamazaki et al., 2005, 2006). Slices were placed in a recording chamber, submerged, and continuously perfused at 2–3 ml/min with oxygenated artificial cerebrospinal fluid at 30°C. Neurons were visualized using a 40x water-immersion objective, infrared differential interference contrast (IR-DIC) system (Axioskop, Zeiss, Germany), and a charge-coupled device camera C2400 (Hamamatsu Corporation, Hamamatsu, Japan). Patch electrodes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL, USA) using a micropipette puller (P-97, Sutter Instrument, Novato, CA, USA). The pipettes had a resistance of 3–8 MΩ after being filled with pipette solution. For voltage-clamp recordings, pipette solution contained (in mM) 117 Cs-methanesulfonate, 10 HEPES, 0.5 EGTA, 2.8 NaCl, 5 TEA-Cl, 5 QX-314, 2.5 Mg-ATP, and 0.3 Na-GTP, adjusted to pH 7.3 with CsOH. For current clamp recordings, pipette solution contained (in mM) 140 K-gluconate, 10 HEPES, 0.5 EGTA, 10 NaCl, 1 MgCl2, 2 Mg-ATP, 0.2 Na-GTP, and QX-314, adjusted to pH 7.3 with KOH. The series resistance, normally about 15–20 MΩ, was monitored throughout the experiment; if it changed more than 20%, the recording was discarded. Responses of currents and potentials were recorded using Axopatch-200B or Axoclamp-2B (Axon Instruments, Union City, CA, USA), and were filtered (1–2 kHz), digitized at 1–5 kHz, and stored on a computer. In LTP experiments, excitatory postsynaptic currents (EPSCs) were evoked in oriens/alveus interneurons using a fine tip bipolar tungsten stimulating electrode (TT201-029, Unique Medical, Tokyo, Japan), which was positioned approximately 100–150 μm from the recorded cell. Because there was a possibility that EPSCs contained not only monosynaptic EPSCs but also polysynaptic responses, we measured the amplitude of the first negative peak to evaluate changes in monosynaptic EPSCs. When the amplitude of monosynaptic EPSCs could not be measured accurately due to overlap with a second EPSC peak or an unusual long EPSC peak latency, the recording was discarded. We also applied the NMDAR antagonist 2-amino-5-phosphopentanoate (AP5; 50 μM) throughout recordings to prevent a possible passively propagated form of LTP in interneurons.
Calcium imaging
For simultaneous Ca2+ imaging and electrophysiological recordings, interneurons were first loaded with the calcium indicator calcium green 1 (100 μM; Molecular Probes, Eugene, OR, U.S.A.) via the recording pipette. It was allowed to diffuse for a minimum of 20 min before starting optical and electrophysiological recordings. Fluorescence images were obtained by using MetaMorph Imaging System (Universal Imaging Corporation, West Chester PA, USA) and a fluorescent microscope (Axioskop, Zeiss) equipped with a mercury lamp, 488-nm excitation and 520-nm emission filters, and a charge-coupled device camera M4314 (Hamamatsu Corporation). Fluorescence intensities (Fpost) over the region of interest in the soma at a given time point were normalized to baseline values [expressed as %ΔF/F = (Fpost − Frest)/(Frest −Fback) × 100, where Frest is the baseline fluorescence and Fback is the background fluorescence measured outside the cell] and plotted against time. To monitor the responsiveness of interneurons at the beginning and end of experiments, they were depolarized by 15mV by a current injection to generate calcium responses via activation of voltage-dependent Ca2+ channels.
Biocytin staining
During whole-cell recording, interneurons were passively filled with biocytin (0.5%) through the recording pipette for subsequent morphological identification (dendrite orientation, somata shape, and axon distribution) of interneurons. Following recordings, slices were incubated for 30 min and fixed overnight in 0.1 M phosphate buffer (PB, pH 7.4) containing 4% paraformaldehyde and 0.05% glutaraldehyde. Biocytin staining was processed using an avidin-peroxidase reaction (Vectastain ABC Standard Kit, Vector, Burlingame,CA, USA) with 3,3′-diaminobenzidine. The slice was then mounted on gelatin-coated glass slides for light microscopic examination.
Double-fluorescence immunohistochemistry
Hippocampal slices containing a biocytin-loaded interneuron were fixed by immersionin 4% paraformaldehyde in 0.1 M PB, pH 7.4, for several hours. After washing in 0.1 M PB, they were incubated with Alexa 546-conjugated streptavidin (Molecular Probes) diluted 1:500 with 0.1 M PB containing 0.1% Triton X-100 overnight at room temperature. Following washing in 0.1 M PB, the slices were incubated with an antibody against somatostatin-28 (IHC8004; Peninsula Laboratories, San Carlos, CA, USA; diluted 1:200) in 0.1 M PB containing 0.1% Triton X-100 for 10–12 h at room temperature. After washing, the slices were allowed to react with Alexa 488-conjugated goat anti-rabbit IgGs (Molecular Probes; diluted 1:500) in 0.1 M PB for 2 h at room temperature. The slices were then washed and coverslippedin Vectashield (Vector). Slices were observed under a confocal fluorescence microscope (LSM510 META, Zeiss).
In situ hybridization
The localization of α2 mRNA-positive cells by non-radioactive in situ hybridization was carried out as previously described (Ishii et al., 2005).
Single-cell reverse transcription (RT)-multiplex polymerase chain reaction (PCR)
To monitor the expression of molecular markers (calbindin D28k, parvalbumin, calretinin, neuropeptide Y, vasoactive intestinal polypeptide, somatostatin, and cholecystokinin) in recorded cells, single-cell RT-multiplex PCR was performed by a previously described procedure (Cauli et al., 1997). After recordings were completed, the cytoplasm of individual neurons was harvested by applying suction on a patch pipette filled with sterile intracellular pipette solution. The contents of the pipette were expelled into a PCR tube by the application of positive pressure, quickly frozen on dry ice, stored at −80°C overnight, and subjected to RT-multiplex PCR using the Titan One Tube RT-PCR System (Roche Molecular Biochemicals, Indianapolis, IN, USA) and different pairs of PCR primers for simultaneous amplification of different molecular markers. This was followed by a second round of PCR using 1 μl of the first PCR product as a template. In this second round, each target cDNA was amplified individually using its specific primer pair. Each individual PCR reaction was run on an agarose gel and visualized with ethidium bromide for identification of target cDNAs.
Statistical analysis
Data were analyzed off-line using Mini analysis program (Synaptosoft Inc., Decatur, GA, USA), Origin (OriginLab, Northampton, MA, USA), and pCLAMP 7 (Axon Instruments). Data were expressed as means ± SEM. Sample size n refers to the number of neurons analyzed in electrophysiological or optical recordings from hippocampal slices. Significant changes in action potential firing frequency, membrane depolarization, Ca2+ response, EPSC amplitude (before vs. after drug application, or between two drug groups) were assessed using a paired or an unpaired, two-tailed Student’s t-test where appropriate. The statistical tests for the data of Ca2+ response and LTP induction were carried out on the percentage data. These normalized data were compared using an unpaired t-test. The statistical tests for the data presented in Fig. 3D2 were carried out using one-way ANOVA and a post hoc Fisher test. A comparison was considered statistically significant if P < 0.05.
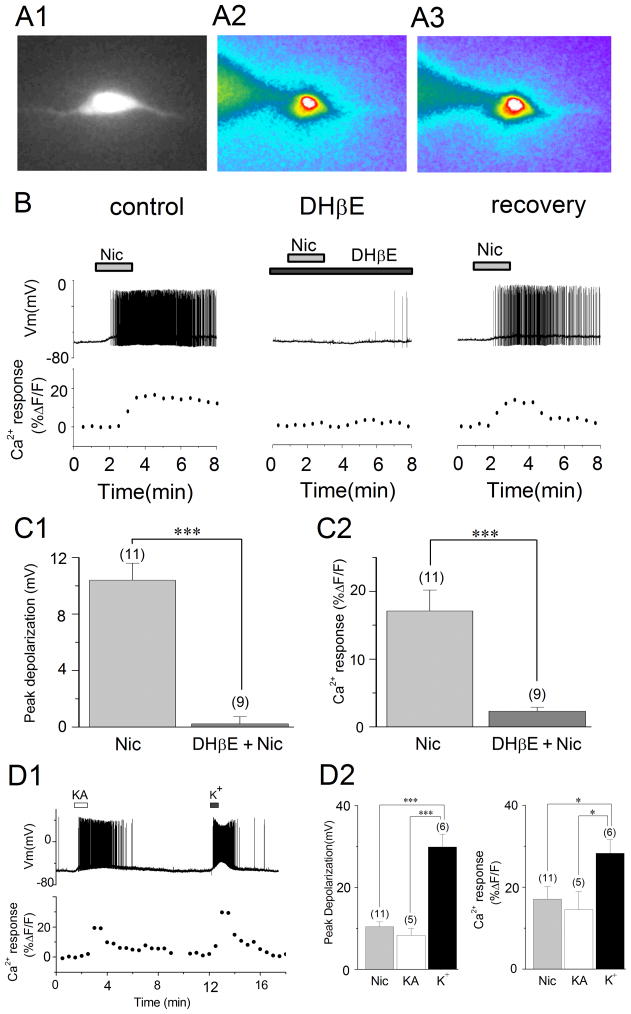
(A1–A3) Visualization of the fluorescent signal in oriens/alveus interneurons loaded with the Ca2+ indicator dye calcium green-1 through the recording pipette. (A1) A visualized oriens/alveus interneuron after dye loading. (A2, A3) Pseudo-color fluorescence imaging of a current-clamped oriens/alveus interneuron in the absence (A2) and presence (A3) of 10 μM nicotine. Note that application of nicotine produced detectable increases in fluorescent intensity at the soma. (B) Simultaneous recordings of electrical activity (Vm) and changes in Ca2+ fluorescence signal in a current-clamped oriens/alveus interneuron. Nicotine (10 μM; Nic)-induced changes in Vm and Ca2+ fluorescence signal were recorded in the absence (left) and presence of DHβE (2 μM; center) and 10 min after washout of DHβE (right). Recordings were carried out in the presence of DNQX (20 μM) and AP5 (40 μM). (C1) Summary graph showing the magnitude of depolarization observed in the presence of nicotine (10 μM) and nicotine (10 μM) + DHβE (2 μM). (C2) Summary graph showing Ca2+ fluorescence signal observed in the presence of nicotine (10 μM) and nicotine (10 μM) + DHβE (2 μM). (D1) Kainate (1 μM; KA)- and K+ (30 mM)-induced changes in Vm and Ca2+ fluorescence signal were simultaneously recorded. (D2) Summary graphs showing the magnitude of depolarization (left) and Ca2+ fluorescence signal (right) elicited by bath application of nicotine (10 μM), kainate (1 μM), and K+ (30 mM). *P < 0.05, ***P < 0.001.
Results
Distribution of nicotine-sensitive interneurons in the stratum oriens/alveus We have previously found that a subset of interneurons in the stratum oriens/alveus were continuously excited in the presence of 0.2–1 μM nicotine (Jia et al., 2009), a concentration that can be achieved in the plasma during cigarette smoking (Benowitz et al., 1989; Henningfield et al., 1993), and were consistently positive for the α2 subunit mRNA by single-cell RT-PCR (Jia et al., 2009). To further characterize these interneurons, we carried out several experiments. Non-radioactive in situ hybridization histochemistry showed that α2 mRNA-positive cells were sparsely localized at the stratum oriens/alveus border (Fig. 1A), as reported previously (Ishii et al., 2005). Many of these cells appeared to possess a horizontally oriented soma, whereas a few cells displayed a vertically oriented soma (Fig. 1A). Based on this observation, under IR-DIC visualization, we targeted horizontally oriented interneurons at the stratum oriens/alveus border for whole-cell recording. We found that bath application of nicotine (0.2–10 μM) in the presence of the glutamate receptor antagonists 6, 7-dinitro-quinoxaline-2, 3-dione (DNQX; 20 μM) and AP5 (40 μM), which eliminate excitatory synaptic activity, depolarized the cells and triggered repetitive action potential firing in these interneurons. As reported previously (Jia et al., 2009), the frequency of action potentials increased in the presence of nicotine. On average, the application of 10 μM nicotine caused a maximum increase in the frequency of action potentials (Fig. 1B1–B3; control 0.05 ± 0.03 Hz vs. nicotine 4.91 ± 0.67 Hz, n = 19, t18 = −7.27, P < 0.001). The effect was sustained during application of nicotine and was blocked by the non-α7 nAChR antagonist dihydro-β-erythroidine (DHβE; 2 μM) (Fig. 1B1,B3; nicotine 4.91 ± 0.67 Hz vs. nicotine + DHβE 0.16 ± 0.05 Hz, n = 19, t18 = 7.27, P < 0.001). As reported previously (Jia et al., 2009), this effect of nicotine was not affected in the presence of the α7 nAChR antagonist methyllycaconitine (MLA; 100 nM, data not shown). These observations suggest that the location of α2 mRNA-positive cells at least partially overlaps with the distribution of nicotine-sensitive interneurons in the stratum oriens/alveus.
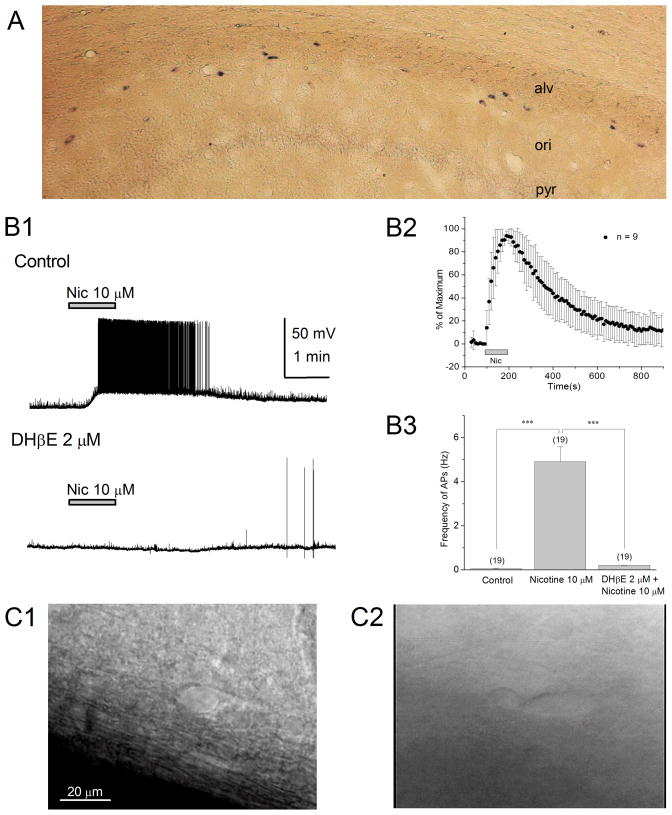
(A) Localization of α2 mRNA-containing interneurons in the hippocampal CA1 region by non-radioactive in situ hybridization. (B1) Bath application of nicotine (10 μM) in the presence of DNQX (20 μM) and AP5 (40 μM) increased the frequency of action potentials in current-clamped oriens/alveus interneurons, which was blocked by the non-α7 nAChR antagonist DHβE (2 μM). (B2) Summary plot of the effect of nicotine (10 μM) on the frequency of action potentials. The nicotine-induced change in the frequency of action potentials was normalized as a percentage change from the highest frequency of action potentials and was plotted against time. (B3) Summary plot of the effect of nicotine (10 μM) on the frequency of action potentials (mean ± SEM) in the absence and presence of DHβE (2 μM). Numbers in parentheses in this and the following figures indicate the numbers of experiments. (C1, C2) IR-DIC images of nicotine-sensitive oriens/alveus interneurons. These interneurons were covered by perineuronal glial cells (C1, the plane of focus was at the neuron under glial cells) or associated with perineuronal glial cells (C2). ***P < 0.001.
Under IR-DIC visualization, we observed that the soma of nicotine-sensitive interneurons was often, but not always, covered by perineuronal glial cells (Fig. 1C1), which were easily removed using gentle positive pressure before whole-cell recording, or associated with perineuronal glial cells (Fig. 1C2). These morphological features allowed us to identify many nicotine-sensitive interneurons before recording and, thus, approximately 80% of recorded cells were nicotine-sensitive.
Nicotine-sensitive interneurons are oriens-lacunosum moleculare cells
There are different subtypes of horizontally oriented interneurons in the stratum oriens/alveus, including oriens-lacunosum moleculare cells, basket cells, and oriens-bistratified cells (Freund & Buzsaki, 1996; Maccaferri, et al., 2000; Maccaferri, 2005). Basket cells are parvalbumin and cholecystokinin positive (Freund & Buzsaki, 1996; Maccaferri, et al., 2000). Both oriens-lacunosum moleculare cells and oriens-bistratified cells are somatostatin immunoreactive (Maccaferri, et al., 2000), and oriens-lacunosum moleculare cells also contain neuropeptide Y (Freund & Buzsaki, 1996). To gain insight into a nicotine-sensitive interneuron subtype, we combined patch-clamp recording and single-cell RT-multiplex PCR to monitor the differential expression of three calcium binding proteins (calbindin D28k, parvalbumin, and calretinin) and four neuropeptides (neuropeptide Y, vasoactive intestinal polypeptide, somatostatin, and cholecystokinin) that define interneuron types with partial overlaps (Freund & Buzsaki, 1996; Parra et al., 1998). To detect the mRNAs of these neurochemical markers simultaneously in a single cell, two steps of multiplex PCR were performed (Cauli et al., 1997; Jia et al., 2009). Initially, the efficiency and specificity of this protocol was tested on total RNA isolated from rat hippocampi. As Fig. 2A1 (top) shows, the seven specific mRNAs were detected, each generating a PCR product of the size predicted by its mRNA sequence. Subsequently, the differential expression of the seven markers was examined at the single cell level. We found that nicotine-sensitive interneurons were consistently positive for somatostatin and neuropeptide Y mRNAs (in 5 of 5 interneurons), and one of these neurons was positive for both calretinin and vasoactive intestinal polypeptide mRNAs (Fig. 2A1, bottom, and Fig. 2A2).
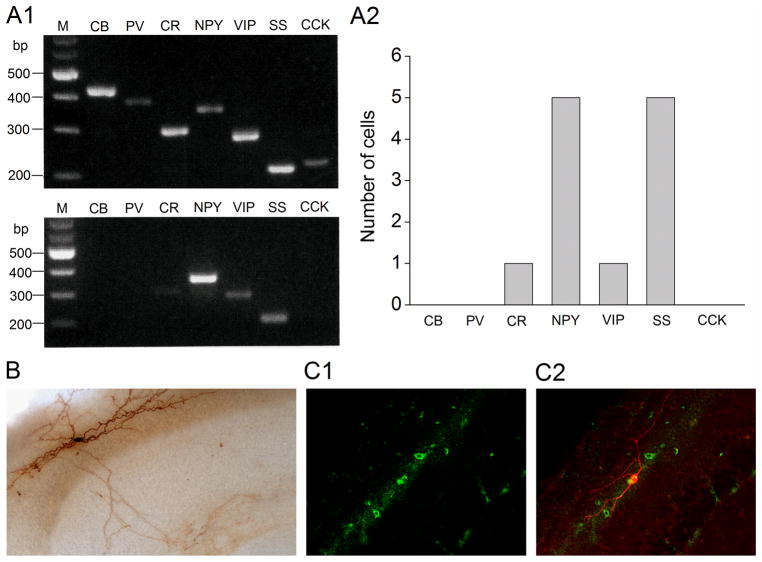
(A1, A2) Nicotine-sensitive interneurons contain somatostatin and neuropeptide Y mRNAs. (A1, top) RT-multiplex PCR amplification of different marker sequences. Hippocampal RNA (500 pg) was subjected to a RT-multiplex PCR protocol to detect the expression of calbindin D28k (CB), parvalbumin (PV), calretinin (CR), neuropeptide Y (NPY), vasoactive intestinal polypeptide (VIP), somatostatin (SS), and cholecystokinin (CCK). (A1, bottom) RT-multiplex PCR applied on a single nicotine-sensitive interneuron after electrophysiological recording to detect molecular markers. An example of single-cell RT-multiplex PCR, showing the presence of CR, NPY, VIP, and SS in a recorded cell. RT-PCR products were separated by agarose gel electrophoresis and visualized with ethidium bromide. The amplified fragments had the sizes (in bp) predicted by the mRNA sequences: 432 (CB), 388 (PV), 309 (CR), 359 (NPY), 287 (VIP), 209 (SS), 216 (CCK). A 100 bp DNA ladder was used as a molecular weight marker. (A2) Summary data of single-cell RT-multiplex PCR. (B) One biocytin-labeled nicotine-sensitive interneuron with characteristics resembling oriens-lacunosum moleculare cells. (C1, C2) Double-labeling of a nicotine-sensitive interneuron with biocytine and immunohistochemistry for somatostatin. Immunohistochemical localization of somatostatin-containing interneurons (C1) and co-localization of somatostatin (green) and biocytin (red) in a nicotine-sensitive interneuron (C2).
In a further effort to identify a nicotine-sensitive interneuron subtype, we passively filled these interneurons with biocytin through recording pipettes for subsequent morphological identification. All biocytin-filled cells had horizontally oriented dendrites. When axonal arborizations were visible (n = 10), they showed morphological characteristics of oriens-lacunosum moleculare cells (Fig. 2B), which project to the most distal dendrites of pyramidal cells (Freund & Buzsaki, 1996; Maccaferri et al., 2000; Maccaferri, 2005). To confirm the presence of somatostatin protein in nicotine-sensitive interneurons, some nicotine-responding interneurons were passively filled with biocytin for subsequent double-fluorescence immunohistochemistry with a somatostatin antibody. We found that biocytin-labeled cells were somatostatin-positive (Fig. 2C1, C2). These results demonstrate that nicotine-sensitive interneurons are oriens-lacunosum moleculare cells. However, because we were unable to follow the complete axonal arborization of some biocytin-filled interneurons, we cannot exclude the possibility that nicotine-responding interneurons include other subtypes of horizontally oriented interneurons, which express both somatostatin and neuropeptide Y.
Nicotine increases intracellular Ca2+ concentrations in oriens/alveus interneurons
Different subtypes of nAChRs are known to be differentially permeable to Ca2+ (Role & Berg, 1996; Fucile, 2004). Thus, activation of non-α7 nAChRs in oriens/alveus interneurons could elevate intracellular Ca2+ levels directly by being permeable to Ca2+ themselves, and indirectly by generating sufficient depolarizing current to activate voltage-dependent Ca2+ channels. Because increases in intracellular Ca2+ play a critical role in LTP induction (Topolnik et al., 2005, 2006), we next examined whether nicotine causes an increase in intracellular Ca2+ levels. To measure changes in intracellular Ca2+ levels in the soma, we used a fluorescent assay with calcium green-1. Current-clamped cells were loaded with calcium green-1 via the patch pipette (Fig. 3A1, A2).
Subsequent bath application of nicotine (10 μM) in the presence of DNQX (20 μM) and AP5 (40 μM) produced detectable increases in fluorescent intensity at the soma, as shown in pseudocolor images (Fig. 3A2, A3). To determine whether the increased fluorescent intensity was due to the activation of non-α7 nAChRs, we simultaneously recorded nicotine-induced changes in membrane potential and fluorescent intensity in current-clamped cells. Bath application of nicotine (10 μM) induced a depolarization in interneurons (10.4 ± 1.2 mV, n = 11) and generated action potentials, which were completely blocked in the presence of DHβE (2 μM; Fig. 3B, C1; 0.2 ± 0.5 mV, n = 9, t18 = −7.01, P < 0.001). At the same time, bath application of nicotine (10 μM) increased Ca2+ responses (17.2 ± 3.1% increase over basal levels, n = 11), which were well maintained during nicotine-induced membrane depolarization, and this effect was almost completely inhibited in the presence of DHβE (2 μM; Fig. 3B, C2; 2.3 ± 0.6% increase over basal levels, n = 9, t18 = −4.32, P < 0.001). Reapplication of nicotine after approximately 10-min drug washout reproducibly induced interneuronal depolarization and intracellular Ca2+ rises (Fig. 3B, right). These results suggest that activation of non-α7 nAChRs causes increases in intracellular Ca2+ concentrations in these interneurons.
It has been shown that bath application of kainate caused depolarization and repetitive action potential firing in oriens-lacunosum moleculare cells (Cossart et al., 1998), which resembles the effects of nicotine on oriens/alveus interneurons (Jia et al., 2009). Thus, we next examined whether activation of kainate receptors induces Ca2+ rises in nicotine-sensitive oriens/alveus interneurons. Bath application of kainate (1 μM) induced a depolarization in interneurons (8.3 ± 1.7 mV, n = 5) and generated action potentials (Fig. 3D1,D2), and simultaneously increased Ca2+ responses (Fig. 3D1,D2; 14. 5 ± 4.4% increase over basal levels, n = 5). Changes in membrane potential and Ca2+ responses caused by application of kainite were very similar to those induced by application of nicotine (Fig. 3D2). Depolarizing the cells with 30 mM K+ to activate voltage-dependent Ca2+ channels induced a membrane depolarization (Fig. 3D1,D2; 29.9 ± 3.2 mV, n = 6) that was significantly larger (Fig. 3D2; ANOVA, F(2,19) = 31.9, P < 0.001) than that caused by nicotine (P < 0.001) or kainite (P < 0.001), and caused increases in Ca2+ responses (Fig. 3D1,D2; 28.3 ± 3.3% increase over basal levels, n = 6) that were significantly higher (Fig. 3D2; ANOVA, F(2,19) = 3.54, P = 0.049) than the increases induced by nicotine (P = 0.034) or kainite (P = 0.028). Thus, nicotine-induced intracellular Ca2+ increases were much less than the Ca2+ increases that were produced by the activation of voltage-dependent Ca2+ channels. Ca2+ permeable α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptors (AMPARs) are expressed in oriens/alveus interneurons (Topolnik et al., 2005, 2006; Kullmann, & Lamsa, 2007). In our experiments, these receptors, which could be activated by kainate, were not blocked. Thus, although kainate receptors can be Ca2+ permeable depending on the degree of post-transcriptional RNA editing of their subunits (Bernard et al. 1999; Lerma et al. 2001), the observed kainate-induced Ca2+ rises might be at least in part due to the activation of Ca2+ permeable AMPARs.
Non-α7 nAChRs on oriens/alveus interneurons are Ca2+ permeable
Our results described above demonstrate that activation of non-α7 nAChRs caused intracellular Ca2+ rises in oriens/alveus interneurons. However, it was unclear whether this was due to direct entry of Ca2+ through the receptor channel, depolarization-induced activation of voltage-dependent Ca2+ channels, or both. Initially, we attempted to pharmacologically dissect out the relative contribution of voltage-dependent Ca2+ channels using Cd2+ as a voltage-gated Ca2+ channel blocker. We compared the amplitude of nicotine-induced Ca2+ elevations in current-clamped interneurons before and after addition of 100 μM Cd2+, which almost completely blocked K+ (30 mM) induced Ca2+ elevations (data not shown). However, we found that the inhibitory effect of Cd2+ on nicotine-induced Ca2+ rises was quite variable among different cells, perhaps due to differences in the relative contribution of voltage-dependent Ca2+ channels to a total Ca2+ elevation. Nevertheless, these experiments suggest the involvement of voltage-dependent Ca2+ channels in nicotine’s effects. Subsequently, we decided to simply determine whether nicotine increases intracellular Ca2+ concentrations without the contribution of voltage-dependent Ca2+ channels. Thus, we measured nicotine-induced Ca2+ responses in interneurons, which were voltage-clamped below the threshold for activation of voltage-dependent Ca2+ channels. Interneurons were loaded with calcium green-1 via the patch pipette and the cells were voltage-clamped at −70 mV in the presence of DNQX (20 μM), AP5 (40 μM). Simultaneous measurements of currents and Ca2+ responses were carried out. As reported previously (Jia et al., 2009), bath application of nicotine (0.5–10 μM) produced long-lasting inward currents that were inhibited by DHβE, but not MLA (data not shown). On average, the application of 10 μM nicotine caused a maximum response (Fig. 4A, top, 87.9 ± 2.0 pA, n = 6). Bath application of nicotine (10 μM) also produced a fluorescence response (Fig. 4A, bottom, B; 9.9 ± 1.3% increase over basal levels, n = 6), suggesting that activation of non-α7 nAChRs can produce increases in intracellular Ca2+ levels by direct passage of Ca2+ through the receptor channel. The response kinetics were different between current-clamped and voltage-clamped conditions (Fig. 3B and Fig. 4A). Furthermore, the increase of intracellular Ca2+ concentrations in voltage-clamped cells was smaller than that observed in current-clamped cells, although the difference did not reach statistical significance (Fig. 4B; t15 = 1.69, P = 0.11). These observations further support the idea that depolarization-induced activation of voltage-dependent Ca2+ channels via non-α7 nAChRs contributes to nicotine-induced intracellular Ca2+ rises in current-clamped cells.

(A) Simultaneous recordings of nicotine-induced currents (top) and changes in Ca2+ fluorescence signal (bottom) in a voltage-clamped oriens/alveus interneuron (at −70 mV). Recordings were carried out in the presence of DNQX (20 μM) and AP5 (40 μM). Nicotine (10 μM) was bath-applied as indicated by the horizontal bar. (B) Summary graph showing Ca2+ fluorescence signal (mean ± SEM) elicited in current-clamped (CC) and voltage-clamped (VC) oriens/alveus interneurons by bath application of nicotine (10 μM).
Nicotine facilitates LTP induction at excitatory synapses on oriens/alveus interneurons
Many interneurons in the stratum oriens/alveus, including oriens-lacunosum moleculare cells, show NMDAR-independent LTP (Perez et al., 2001; Lapointe et al., 2004; Topolnik et al., 2006; Lamsa et al., 2007; Kullmann & Lamsa, 2008; Oren et al., 2009), which can be induced by different LTP induction protocols. To examine whether nicotine facilitates the induction of LTP in oriens/alveus interneurons, we recorded EPSCs in voltage-clamped cells (at −70 mV). Most of the excitatory input to oriens-lacunosum moleculare cells arises from recurrent collaterals of CA1 pyramidal cells. Thus, we evoked EPSCs by stimulation in the stratum oriens, close to the patched interneurons (Fig. 5A). Tetanic stimulation (100 Hz, 100 pulses) was used to induce LTP under voltage-clamp conditions. Although NMDA receptor-dependent LTP does not occur in oriens/alveus interneurons (Perez et al. 2001; Lamsa et al. 2007), NMDAR-dependent LTP occurring at the Schaffer collateral-pyramidal cell synapses can passively propagate to these interneurons (Perez et al. 2001). Thus, to prevent a possible passively propagated form of LTP in interneurons, all recordings were performed in the presence of the NMDAR antagonist AP5 (50 μM). In addition, bicuculline (10 μM), MLA (50 nM), and atropine (1μM) were added to the bath perfusate to block GABAA receptors, α7 nAChRs, and muscarinic receptors, respectively. Under these conditions, tetanic stimulation failed to induce LTP (Fig. 5B,F; 104.3 ± 8.3% 50–55 min after tetanus, n = 4) in voltage-clamped interneurons. However, when tetanic stimulation was applied in the presence of nicotine (10 μM), LTP was induced (Fig. 5C,F; 153.3 ± 14.8% 50–55 min after tetanus, n = 4; tetanus alone vs. tetanus + 10 μM nicotine, t6 = 3.15, P = 0.020). We have previously reported that bath application of nicotine (0.5–1 μM) produced long-lasting inward currents (10–47 pA) in oriens/alveus interneurons (Jia et al., 2009). Thus, we next examined whether a lower concentration (1 μM) of nicotine facilitates LTP induction in oriens/alveus interneurons, and found that LTP was induced in nicotine-responding interneurons (Fig. 5D,F; 139.4 ± 14.8% 50–55 min after tetanus, n = 5; tetanus alone vs. tetanus + 1 μM nicotine, t7 = 2.63, P = 0.033). Bath application of nicotine for 1.5 min caused decreases in the amplitude of EPSCs and increases in holding current, indicating the presence of nicotine-induced inward currents (Fig. 5C,D). Increases in holding current reached its peak value in approximately 3 min after nicotine application, at which time the tetanus was delivered, and returned to a baseline level following washout of nicotine. The time course of changes in EPSC amplitude following nicotine application mirrored that of changes in holding current that occurred after nicotine application. This suggests that nicotine-induced inward currents suppresses EPSCs, perhaps due to reduced driving force for Na+ and decreased membrane resistance, which causes a shunting effect. To exclude the possibility that the observed effect of nicotine on LTP induction was due to a long-lasting effect of nicotine, we next examined the effect of nicotine alone (i.e., without tetanic stimulation) on EPSCs. Bath application of nicotine (10 μM) for 1.5 min caused decreases in the amplitude of EPSCs, reaching its minimum in approximately 3 min after nicotine application and returning to basal levels following washout of nicotine (Fig. 5E,F; 105.5 ± 6.1% 53–58 min after nicotine application, n = 4; tetanus + nicotine vs. nicotine alone, t6 = 3.45, P = 0.017). Thus, nicotine alone had no long-lasting effect on EPSCs. These results suggest that nicotine facilitates the induction of LTP in oriens/alveus interneurons via activation of non-α7 nAChRs.

(A) Scheme of recording setup showing the position of stimulating and recording electrodes. Oriens/alveus interneurons were voltage-clamped at −70 mV, and evoked EPSCs were recorded in the presence of AP5 (50 μM), bicuculline (10 μM), MLA (50 nM), and atropine (1 μM). (B–E) Changes in the amplitude of EPSCs were plotted as the percent change of initial baseline responses against time. Each trace above the graph in (B–E) was recorded at the time indicated. (B) A tetanus (100 pulses at 100Hz) alone failed to induce LTP. (C) A tetanus applied in the presence of nicotine (10 μM) induced LTP. (D) A tetanus applied in the presence of nicotine (1 μM) induced LTP. (E) Application of nicotine (10 μM) alone had no long-lasting effect on the amplitude of EPSCs. (C-E) Bath application of nicotine caused decreases in the amplitude of EPSCs (top) and increases in holding current (bottom). Administration of nicotine is indicated by the bar and delivery of tetanic stimulation is indicated by the arrow. (F) Histograms show the percent change (mean ± SEM) in the amplitude of EPSCs measured 50–60 min after delivery of tetanic stimulation in the absence and presence of nicotine, or after application of nicotine alone. *P < 0.05.
Ca2+ entry through non-α7 nAChR channels facilitates LTP induction in oriens/alveus interneurons
Because increases in intracellular Ca2+ concentrations have a critical role in LTP induction in oriens/alveus interneurons (Topolnik et al., 2006), we next examined whether Ca2+ entry through non-α7 nAChR channels was responsible for the nicotine-induced facilitation of LTP induction. To exclude the contribution of voltage-dependent Ca2+ channels, we recorded EPSCs in voltage-clamped oriens/alveus interneurons (at −70 mV) in the presence of AP5 (50 μM), bicuculline (10 μM), MLA (50 nM), and atropine (1μM). Tetanic stimulation was used to induce LTP under voltage-clamp conditions. We first examined the role of Ca2+ while recording from voltage-clamped interneurons with a pipette containing a high concentration of the Ca2+ chelator BAPTA (10 mM). Intracellular application of BAPTA blocked the nicotine-induced facilitation of LTP induction (Fig. 6A,E; 102.3 ± 13.0% 50–55 min after tetanus, n = 4; nicotine vs. nicotine + BAPTA, t6 = 2.80, P = 0.031). Under these conditions, nicotine still elicited inward current as evidenced by the increase in holding currents (Fig. 6A, bottom). This suggests that the nicotine-induced facilitation of LTP induction requires Ca2+ entry through non-α7 nAChRs in patched interneurons. To explore the contribution of other Ca2+ sources to the nicotine-induced facilitation of LTP induction, we carried out pharmacological experiments. Nicotine-induced enhancement of LTP in the rat dentate gyrus is dependent on entry of Ca2+ via L-type Ca2+ channels and Ca2+ release from ryanodine-sensitive intracellular stores (Welsby et al., 2006). Therefore, nicotine-induced enhancement of LTP is prevented by the blockers nifedipine and ryanodine, respectively. Because the interneurons were voltage-clamped at −70 mV, it was very unlikely that nicotine-induced responses activated voltage-dependent Ca2+ channels. However, to completely exclude the involvement of L-type Ca2+ channels, we examined the effect of nifedipine (30 μM) on nicotine-induced facilitation of LTP induction. As expected, we found that nifedipine (30 μM) did not have a significant effect on the nicotine-induced facilitation of LTP induction (Fig. 6B,E; 146.6 ± 10.6% 50–55 min after tetanus, n = 4; nicotine vs. nicotine + nifedipine, t6 = 0.01, P = 0.99), demonstrating that Ca2+ entry via L-type voltage-dependent Ca2+ channels is not involved in the effect. Another possible source of Ca2+ contributing to LTP induction is Ca2+-induced Ca2+ release (CICR). Ca2+ entry through nAChR channels can be coupled to CICR (Dajas-Bailador and Wonnacott, 2004), and CICR has been linked to LTP in oriens/alveus interneurons (Topolnik et al., 2006). Thus, we examined the possible contribution of ryanodine-sensitive internal Ca2+ stores to nicotine’s effects, and found that blockade of CICR with ryanodine had no significant effect on nicotine-induced facilitation of LTP induction (Fig. 6C–E; 10 μM ryanodine, 166.7 ± 14.1% 50–55 min after tetanus, n = 4; nicotine vs. nicotine + 10 μM ryanodine, t6 = 0.41, P = 0.69; 100 μM ryanodine, 171.2 ± 17.8% 50–55 min after tetanus, n = 4; nicotine vs. nicotine + 100 μM 2+ ryanodine, t6 = 0.88, P = 0.41). These results suggest that the major source of Ca contributing to the nicotine-induced facilitation of LTP induction is via non-α7 nAChR channels.

Oriens/alveus interneurons were voltage-clamped at −70 mV, and evoked EPSCs were recorded in the presence of AP5 (50 μM), bicuculline (10 μM), MLA (50 nM), and atropine (1 μM). (A–D) Changes in the amplitude of EPSCs were plotted as the percent change of initial baseline responses against time. Each trace above the graph in (A–D) was recorded at the time indicated. (A) Nicotine-induced facilitation of LTP induction was blocked by intracellular perfusion of the calcium chelator BAPTA through the recording pipette (10 mM). (B) Nicotine still promoted LTP induction in the presence of nifedipine (30 μM). (C, D) Nicotine still facilitated LTP induction in the presence of 10 μM (C) and 100 μM (D) of ryanodine. (A–D) Bath application of nicotine caused decreases in the amplitude of EPSCs (top) and increases in holding current (bottom). Nifedipine (30 μM) or ryanodine (10 μM) was present throughout the entire recording period in (B) or (C), respectively. In the case of 100 μM ryanodine (D), the drug was present until 5 min after tetanus. Administration of nicotine is indicated by the bar and delivery of tetanic stimulation (100 pulses at 100Hz) is indicated by the arrow. (E) Histograms show the percent change (mean ± SEM) in the amplitude of EPSCs measured 50–60 min after delivery of tetanic stimulation, to compare the effects of different drugs on nicotine-induced facilitation of LTP induction. *P < 0.05.
Discussion
The present study demonstrates that horizontally oriented interneurons at the stratum oriens/alveus border contain Ca2+-permeable non-α7 nAChRs, which are continuously activated in the presence of nicotine, and their activation facilitates the induction of LTP in these interneurons. Most, if not all, of these interneurons appeared to be oriens-lacunosum moleculare cells, which provide feedback inhibition onto the distal dendrites of pyramidal cells. Oriens-lacunosum moleculare cells are thought to be involved in the generation of theta oscillations, which typically occurs during exploratory activity, and are silenced during sharp wave ripples, which have been implicated in memory consolidation (Gillies et al., 2002; Klausberger et al., 2003; Somogyi & Klausberger, 2005; Kullmann & Lamsa, 2007). Thus, sustained activation of oriens-lacunosum moleculare cells in the presence of nicotine and LTP induction in these cells via non-α7 nAChRs may affect naturally occurring network activity, and thereby learning and memory.
A non-α7 nAChR subtype expressed in nicotine-sensitive oriens/alveus interneurons
It was previously reported that horizontally oriented interneurons in the stratum oriens/alveus, including oriens-lacunosum moleculare cells, contained both α7 and non-α7 nAChRs, which were activated by ACh puffs but were desensitized in the presence of nicotine (Frazier et al., 1998a; McQuiston & Madison, 1999; Sudweeks & Yakel, 2000). Because it was not tested whether bath application of nicotine elicited non-α7 nAChR-mediated responses in these interneurons, whether these interneurons expressed slowly or non-desensitizing nAChR subtype was unclear. Furthermore, single-cell RT-PCR analysis showed that these interneurons were consistently positive for all α subunit subtypes (α2-α7 mRNAs), making it difficult to identify a non-α7 nAChR subtype that mediated the responses (Sudweeks & Yakel, 2000). Our previous study demonstrated that bath application of nicotine produced slow inward currents in a subset of horizontally oriented interneurons in the stratum oriens/alveus, which were well maintained and inhibited by the non-α7 antagonist DHβE, but not the α7 antagonist MLA (Jia et al., 2009). In situ hybridization demonstrate that the α2 mRNA is uniquely expressed in a subset of interneurons in the stratum oriens/alveus (Ishii et al., 2005; Jia et al., 2009). Furthermore, single-cell RT-PCR analysis demonstrated that these nicotine-responding interneurons were consistently positive for the α2 subunit mRNA (in 8 of 8 interneurons) and two of these cells were positive for the α3 subunit mRNA, but not other α subunit mRNAs (Jia et al., 2009). Thus, the nicotine-induced facilitation of LTP induction is most likely mediated via activation of α2* nAChRs. However, the absence of α3 and α4 subunit mRNAs in many or all nicotine-sensitive interneurons, respectively, can easily be due to a lack of the sensitivity needed to detect less-abundant nAChR subunit mRNAs than α2 subunit mRNAs in these interneurons. It remains to be determined whether these cells contain functional α2* nAChRs that mediate the effects of nicotine.
An interneuron subtype containing non-desensitizing non-α7 nAChRs
The present study demonstrated that biocytin-filled nicotine-sensitive cells showed morphological characteristics of oriens-lacunosum moleculare cells, when axonal arborizations were visible. Furthermore, all non-α7 nAChR-containing interneurons tested in the present study were positive for somatostatin and neuropeptide Y, both of which are known to be expressed in oriens-lacunosum moleculare cells (Freund & Buzsaki, 1996; Maccaferri et al., 2000).
Nicotine-sensitive horizontally oriented interneurons in the stratum oriens/alveus are connected to pyramidal cells (Jia et al., 2009). In the literature (Freund & Buzsaki, 1996; Maccaferri et al., 2000; Maccaferri, 2005), at least three different subtypes of horizontally oriented interneurons (oriens-lacunosum moleculare cells, basket cells, and oriens-bistratified cells) that innervate pyramidal cells have been identified and characterized. Both oriens-lacunosum moleculare cells and oriens-bistratified cells are somatostatin immunoreactive, but basket cells, which innervate the soma, are not (Freund & Buzsaki, 1996; Maccaferri et al., 2000). Thus, although rigid classification of the subtypes of interneurons may be difficult, it is most likely that interneurons activated by nicotine are not basket cells. Because a previous study demonstrated that non-α7 nAChR-mediated responses in oriens-lacunosum moleculare cells desensitized in the presence of nicotine (McQuiston & Madison, 1999), our results suggest the presence of a separate population of oriens-lacunosum moleculare cells, expressing non-desensitizing non-α7 nAChRs.
Oriens-lacunosum moleculare cells project to the most distal dendrites of pyramidal cells where the temporoammonic path terminates (Freund & Buzsaki, 1996; Maccaferri et al., 2000; Maccaferri, 2005). Bath application of nicotine inhibits field excitatory postsynaptic potentials at the temporoammonic pathway, which requires GABAergic inhibition (Nakauchi et al., 2007a,b). This effect of nicotine is prevented, if a knife cut is made along the pyramidal layer in the stratum oriens to sever the axons of interneurons projecting to the apical dendrites of pyramidal cells (Nakauchi et al., 2007b). These observations suggest that the effect of nicotine is attributed to GABA release from the terminals of oriens-lacunosum moleculare cells. The axons of oriens-bistratified cells primarily terminate in the stratum radiatum and overlap the dendritic trees of pyramidal cells (Maccaferri et al., 2000). Bath application of nicotine has no significant effect on field excitatory postsynaptic potentials at the Schaffer collateral pathway (Fujii et al., 1999; Nakauchi et al., 2007a), suggesting that there is no significant GABA release from the terminals of oriens-bistratified cells during nicotine application. These observations also support the idea that oriens-lacunosum moleculare cells are a major subtype of nicotine-sensitive interneurons in the stratum oriens/alveus, which express α2 mRNAs. Identification of a nicotine-sensitive interneuron subtype as an oriens-lacunosum moleculare cell is significant, because nicotine-induced tonic inhibition at the most distal dendrites would depress direct information flow into CA1 pyramidal cells from entorhinal input (the temporoammonic path) and might preferentially direct information flow to CA1 pyramidal cells through the trisynaptic circuitry in the presence of nicotine.
Roles of Ca2+ entering through nAChR channels in circuit activity and plasticity
The α7 nAChR is expressed at high levels by interneurons in the hippocampal CA1 region (Alkondon & Albuquerque, 2001; Alkondon et al., 2000; McQuiston & Madison, 1999; Sudweeks & Yakel, 2000; Ji et al., 2001; Buhler & Dunwiddie, 2001, 2002; Frazier et al., 1998a, 1998b, 2003), in which activation of α7 nAChRs causes an increase in intracellular Ca2+ concentrations without involving CICR or activation of voltage-dependent Ca2+ channels (Khiroug et al., 2003; Fayuk & Yakel., 2005, 2007). This source of Ca2+ triggers signaling, leading to the suppression of GABAA receptor-mediated responses in the interneurons (Wanaverbecq et al., 2007; Zhang & Berg, 2007).
Oriens/alveus interneurons in the hippocampal CA1 region express non-α7 nAChRs, which desensitize in the presence of nicotine (McQuiston & Madison, 1999). The activation of these non-α7 nAChRs via 5 sec pressure pulses of ACh (2 mM) in the presence of MLA does not significantly change intracellular Ca2+ concentrations (Fayuk & Yakel., 2005). However, in the present study, we found that bath application of nicotine increases Ca2+ levels in nicotine-sensitive interneurons at the stratum oriens/alveus border. This increase is at least in part attributed to Ca2+ entry through non-α7 nAChRs, which are continuously activated in the presence of nicotine. The change in intracellular Ca2+ concentrations caused by a brief activation of non-α7 nAChRs in oriens/alveus interneurons may be too small to be detected, and only becomes detectable when they are continuously activated in the presence of nicotine. In this case, Ca2+ signals leading to LTP induction can be only elicited in interneurons expressing non-desensitizing non-α7 nAChRs, because they are continuously activated in the presence of nicotine.
Nicotine-induced facilitation of LTP induction in oriens/alveus interneurons
Oriens/alveus interneurons show both Hebbian and anti-Hebbian forms of LTP, both of which are independent of the activation of NMDARs and appear to be expressed presynaptically (Perez et al., 2001; Lapointe et al., 2004; Lamsa et al., 2007; Kullmann & Lamsa, 2007). In the present study, high frequency presynaptic stimulation with the activation of non-α7 nAChRs induced LTP in oriens/alveus interneurons that were voltage-clamped at −70 mV. Thus, this LTP, which is also independent of the activation of NMDARs, is arguably anti-Hebbian.
Nicotine-induced LTP in oriens/alveus interneurons requires postsynaptic Ca2+ rises, as in the cases of Hebbian and anti-Hebbian forms of LTP, both of which occur at synapses containing Ca2+ permeable AMPARs (Perez et al., 2001; Lapointe et al., 2004; Lamsa et al., 2007; Kullmann & Lamsa, 2007). The mGluR1a subtype is highly expressed in oriens/alveus interneurons (Masu et al., 1991; Lujan et al., 1996) and can be activated synaptically (Huang et al., 2004). Activation of this subtype induces Ca2+ influx via transient receptor potential channels and Ca2+ release from intracellular stores (Topolnik et al., 2006). These sources of Ca2+ contribute to a Hebbian form of LTP (Topolnik et al., 2006). A well-established pathway of mGluR1 Ca2+ signaling is 1,4,5-trisphosphate-dependent intracellular Ca2+ release (Pin & Duvoisin, 1995). However, because 1,4,5-trisphosphate receptors appear to be absent in oriens/alveus interneurons (Fotuhi et al., 1993), mGluR1/5-mediated Ca2+ signaling in these interneurons involves Ca2+ release from ryanodine-sensitive Ca2+ stores (Woodhall et al., 1999). This source of Ca2+ appears to be also necessary for anti-Hebbian forms of LTP induction (Lamsa et al., 2007). In contrast, this source of Ca2+ appears to be unnecessary for nicotine-induced LTP induction, because it still occurs after ryanodine receptors are blocked. One possible explanation is that Ca2+ from ryanodine-sensitive Ca2+ stores lowers the threshold for LTP induction, but is not required for LTP induction when in the presence of Ca2+ entering through non-α7 nAChRs. The relative contribution of different Ca2+ signals, arising from Ca2+ permeable AMPARs and non-α7 nAChRs, to LTP induction remains to be determined.
Oriens-lacunosum moleculare cells receive excitatory synaptic input from the recurrent collaterals of CA1 pyramidal cells, which fire at high frequency during sharp wave ripple activity (Klausberger et al., 2003; Somogyi & Klausberger, 2005; Kullmann & Lamsa, 2007). As suggested by others, this network activity may trigger LTP induction in oriens-lacunosum moleculare cells (Kullmann & Lamsa, 2007). Because the nicotine levels achieved after smoking can continuously activate oriens-lacunosum moleculare cells, smoking may affect hippocampal network activity by promoting a long-term alteration of pyramidal cell excitation of these cells.
Acknowledgments
This research was supported by the National Institute on Drug Abuse (DA014542, DA025269, DA026458).
Abbreviations
- ACh
- acetylcholine
- AMPAR
- α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor
- AP5
- 2-amino-5-phosphopentanoate
- CICR
- Ca2+-induced Ca2+ release
- DHβE
- dihydro-β-erythroidine
- DNQX
- 6, 7-dinitroquinoxaline-2, 3-dione
- EPSCs
- excitatory postsynaptic currents
- GABA
- γ-aminobutyric acid
- IR-DIC
- infrared-differential interference contrast
- LTP
- long-term potentiation
- mGluRs
- metabotropic glutamate receptors
- MLA
- methyllycaconitine
- nAChRs
- nicotinic acetylcholine receptors
- NMDARs
- N-methyl-D-aspartate receptors
- PB
- phosphate buffer
- PCR
- polymerase chain reaction
- RT
- reverse transcription
References
- Abdulla FA, Bradbury E, Calaminici MR, Lippiello PM, Wonnacott S, Gray JA, Sinden JD. Relationship between up-regulation of nicotine binding sites in rat brain and delayed cognitive enhancement observed after chronic or acute nicotinic receptor stimulation. Psychopharmacology. 1996;124:323–331. [Abstract] [Google Scholar]
- Alkondon M, Pereira EF, Almeida LE, Randall WR, Albuquerque EX. Nicotine at concentrations found in cigarette smokers activates and desensitizes nicotinic acetylcholine receptors in CA1 interneurons of rat hippocampus. Neuropharmacology. 2000;39:2726–2739. [Abstract] [Google Scholar]
- Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–55. [Abstract] [Google Scholar]
- Alkondon M, Albuquerque EX. Nicotinic receptor subtypes in rat hippocampal slices are differentially sensitive to desensitization and early in vivo functional up-regulation by nicotine and to block by bupropion. J Pharmacol Exp Ther. 2005;313:740–750. [Abstract] [Google Scholar]
- Benowitz NL, Porchet H, Jacob P. Nicotine dependence and tolerance in man: pharmacokinetic and pharmacodynamic investigations. Prog Brain Res. 1989;79:279–287. [Abstract] [Google Scholar]
- Bernard A, Ferhat L, Dessi F, Charton G, Represa A, Ben-Ari Y, Khrestchatisky M. Q/R editing of the rat GluR5 and GluR6 kainate receptors in vivo and in vitro: evidence for independent developmental, pathological and cellular regulation. Eur J Neurosci. 1999;11:604–616. [Abstract] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. [Abstract] [Google Scholar]
- Buhler AV, Dunwiddie TV. alpha7 nicotinic acetylcholine receptors on GABAergic interneurons evoke dendritic and somatic inhibition of hippocampal neurons. J Neurophysiol. 2002;87:548–557. [Abstract] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci. 1997;17:3894–3906. [Europe PMC free article] [Abstract] [Google Scholar]
- Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nat Neurosci. 1998;1:470–478. [Abstract] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. [Abstract] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci. 2007;27:10870–10877. [Europe PMC free article] [Abstract] [Google Scholar]
- Fayuk D, Yakel JL. Ca2+ permeability of nicotinic acetylcholine receptors in rat hippocampal CA1 interneurones. J Physiol (Lond) 2005;566:759–768. [Abstract] [Google Scholar]
- Fayuk D, Yakel JL. Dendritic Ca2+ signalling due to activation of alpha 7-containing nicotinic acetylcholine receptors in rat hippocampal neurons. J Physiol (Lond) 2007;582:597–611. [Abstract] [Google Scholar]
- Fotuhi M, Sharp AH, Glatt CE, Hwang PM, von Krosigk M, Snyder SH, Dawson TM. Differential localization of phosphoinositide-linked metabotropic glutamate receptor (mGluR1) and the inositol 1,4,5-trisphosphate receptor in rat brain. J Neurosci. 1993;13:2001–2012. [Europe PMC free article] [Abstract] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an α-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998a;18:1187–1195. [Europe PMC free article] [Abstract] [Google Scholar]
- Frazier CJ, Buhler AV, Weiner JL, Dunwiddie TV. Synaptic potentials mediated via α-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. J Neurosci. 1998b;18:8228–8235. [Europe PMC free article] [Abstract] [Google Scholar]
- Frazier CJ, Strowbridge BW, Papke RL. Nicotinic receptors on local circuit neurons in dentate gyrus: a potential role in regulation of granule cell excitability. J Neurophysiol. 2003;89:3018–3028. [Abstract] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. [Abstract] [Google Scholar]
- Fucile S. Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium. 2004;35:1–8. [Abstract] [Google Scholar]
- Fujii S, Ji Z, Morita N, Sumikawa K. Acute and chronic nicotine exposure differentially facilitate the induction of LTP. Brain Res. 1999;846:137–143. [Abstract] [Google Scholar]
- Gillies MJ, Traub RD, LeBeau FE, Davies CH, Gloveli T, Buhl EH, Whittington MA. A model of atropine-resistant theta oscillations in rat hippocampal area CA1. J Physiol (Lond) 2002;543:779–793. [Abstract] [Google Scholar]
- Gould TJ. Nicotine and hippocampus-dependent learning: implications for addiction. Mol Neurobiol. 2006;34:93–107. [Europe PMC free article] [Abstract] [Google Scholar]
- Hamid S, Dawe GS, Gray JA, Stephenson JD. Nicotine induces long-lasting potentiation in the dentate gyrus of nicotine-primed rats. Neurosci Res. 1997;29:81–85. [Abstract] [Google Scholar]
- Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, London ED. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug Alcohol Depend. 1993;33:23–29. [Abstract] [Google Scholar]
- Huang YH, Sinha SR, Tanaka K, Rothstein JD, Bergles DE. Astrocyte glutamate transporters regulate metabotropic glutamate receptor-mediated excitation of hippocampal interneurons. J Neurosci. 2004;24:4551–4559. [Europe PMC free article] [Abstract] [Google Scholar]
- Ishii K, Wong JK, Sumikawa K. A comparison of α2 nicotinic acetylcholine receptor subunit mRNA expression in the central nervous system of rats and mice. J Comp Neurol. 2005;493:241–260. [Europe PMC free article] [Abstract] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. [Abstract] [Google Scholar]
- Jia Y, Yamazaki Y, Nakauchi S, Sumikawa K. Alpha2 nicotine receptors function as a molecular switch to continuously excite a subset of interneurons in rat hippocampal circuits. Eur J Neurosci. 2009;29:1588–1603. [Europe PMC free article] [Abstract] [Google Scholar]
- Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol. 2008;38:101–121. [Europe PMC free article] [Abstract] [Google Scholar]
- Khiroug L, Giniatullin R, Klein RC, Fayuk D, Yakel JL. Functional mapping and Ca2+ regulation of nicotinic acetylcholine receptor channels in rat hippocampal CA1 neurons. J Neurosci. 2003;23:9024–9031. [Europe PMC free article] [Abstract] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. [Abstract] [Google Scholar]
- Kullmann DM, Lamsa KP. Long-term synaptic plasticity in hippocampal interneurons. Nat Rev Neurosci. 2007;8:687–699. [Abstract] [Google Scholar]
- Kullmann DM, Lamsa K. Roles of distinct glutamate receptors in induction of anti-Hebbian long-term potentiation. J Physiol (Lond) 2008;586:1481–1486. [Abstract] [Google Scholar]
- Lamsa KP, Heeroma JH, Somogyi P, Rusakov DA, Kullmann DM. Anti-Hebbian long-term potentiation in the hippocampal feedback inhibitory circuit. Science. 2007;315:1262–1266. [Europe PMC free article] [Abstract] [Google Scholar]
- Lapointe V, Morin F, Ratté S, Croce A, Conquet F, Lacaille JC. Synapse-specific mGluR1-dependent long-term potentiation in interneurones regulates mouse hippocampal inhibition. J Physiol (Lond) 2004;555:125–135. [Abstract] [Google Scholar]
- Lerma J, Paternain AV, Rodríguez-Moreno A, López-García JC. Molecular physiology of kainate receptors. Physiol Rev. 2001;81:971–998. [Abstract] [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–640. [Abstract] [Google Scholar]
- Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology. 1998;138:217–230. [Abstract] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. [Abstract] [Google Scholar]
- Maccaferri G, Roberts JDB, Szucs P, Cottingham CA, Somogyi P. Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurons in rat hippocampus in vitro. J Physiol (Lond) 2000;524:91–116. [Abstract] [Google Scholar]
- Maccaferri G. Stratum oriens horizontal interneurone diversity and hippocampal network dynamics. J Physiol (Lond) 2005;562:73–80. [Abstract] [Google Scholar]
- Mann EO, Greenfield SA. Novel modulatory mechanisms revealed by the sustained application of nicotine in the guinea-pig hippocampus in vitro. J Physiol (Lond) 2003;551:539–550. [Abstract] [Google Scholar]
- Marti Barros D, Ramirez MR, Dos Reis EA, Izquierdo I. Participation of hippocampal nicotinic receptors in acquisition, consolidation and retrieval of memory for one trial inhibitory avoidance in rats. Neuroscience. 2004;126:651–656. [Abstract] [Google Scholar]
- Masu M, Tanabe Y, Tsuchida K, Shigemoto R, Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991;349:760–765. [Abstract] [Google Scholar]
- Matsuyama S, Matsumoto A, Enomoto T, Nishizaki T. Activation of nicotinic acetylcholine receptors induces long-term potentiation in vivo in the intact mouse dentate gyrus. Eur J Neurosci. 2000;12:3741–3747. [Abstract] [Google Scholar]
- McQuiston AR, Madison DV. Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. J Neurosci. 1999;19:2887–2896. [Europe PMC free article] [Abstract] [Google Scholar]
- Nakauchi S, Brennan RJ, Boulter J, Sumikawa K. Nicotine gates long-term potentiation in the hippocampal CA1 region via the activation of alpha2* nicotinic ACh receptors. Eur J Neurosci. 2007a;25:2666–2681. [Abstract] [Google Scholar]
- Nakauchi S, Yamazaki Y, Sumikawa K. Chronic nicotine exposure affects the normal operation of hippocampal circuits. NeuroReport. 2007b;18:87–91. [Abstract] [Google Scholar]
- Oren I, Nissen W, Kullmann DM, Somogyi P, Lamsa KP. Role of ionotropic glutamate receptors in long-term potentiation in rat hippocampal CA1 oriens-lacunosum moleculare interneurons. J Neurosci. 2009;29:939–950. [Europe PMC free article] [Abstract] [Google Scholar]
- Parra P, Gulyas AI, Miles R. How many subtypes of inhibitory cells in the hippocampus? Neuron. 1998;20:983–993. [Abstract] [Google Scholar]
- Perez Y, Morin F, Lacaille JC. A hebbian form of long-term potentiation dependent on mGluR1a in hippocampal inhibitory interneurons. Proc Natl Acad Sci USA. 2001;98:9401–9406. [Europe PMC free article] [Abstract] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. [Abstract] [Google Scholar]
- Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. [Abstract] [Google Scholar]
- Sawada S, Yamamoto C, Ohno-Shosaku T. Long-term potentiation and depression in the dentate gyrus, and effects of nicotine. Neurosci Res. 1994;20:323–329. [Abstract] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. [Europe PMC free article] [Abstract] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol (Lond) 2005;562:9–26. [Abstract] [Google Scholar]
- Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J Physiol (Lond) 2000;527:515–528. [Abstract] [Google Scholar]
- Topolnik L, Congar P, Lacaille JC. Differential regulation of metabotropic glutamate receptor- and AMPA receptor-mediated dendritic Ca2+ signals by presynaptic and postsynaptic activity in hippocampal interneurons. J Neurosci. 2005;25:990–1001. [Europe PMC free article] [Abstract] [Google Scholar]
- Topolnik L, Azzi M, Morin F, Kougioumoutzakis A, Lacaille JC. mGluR1/5 subtype-specific calcium signalling and induction of long-term potentiation in rat hippocampal oriens/alveus interneurones. J Physiol (Lond) 2006;575:115–131. [Abstract] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. [Abstract] [Google Scholar]
- Wanaverbecq N, Semyanov A, Pavlov I, Walker MC, Kullmann DM. Cholinergic axons modulate GABAergic signaling among hippocampal interneurons via postsynaptic alpha 7 nicotinic receptors. J Neurosci. 2007;27:5683–5693. [Europe PMC free article] [Abstract] [Google Scholar]
- Welsby P, Rowan M, Anwyl R. Nicotinic receptor-mediated enhancement of long-term potentiation involves activation of metabotropic glutamate receptors and ryanodine-sensitive calcium stores in the dentate gyrus. Eur J Neurosci. 2006;24:3109–3118. [Abstract] [Google Scholar]
- Woodhall G, Gee CE, Robitaille R, Lacaille JC. Membrane potential and intracellular Ca2+ oscillations activated by mGluRs in hippocampal stratum oriens/alveus interneurons. J Neurophysiol. 1999;81:371–382. [Abstract] [Google Scholar]
- Yamazaki Y, Jia Y, Hamaue N, Sumikawa K. Nicotine-induced switch in the nicotinic cholinergic mechanisms of facilitation of long-term potentiation. Eur J Neurosci. 2005;22:845–860. [Abstract] [Google Scholar]
- Yamazaki Y, Fujii S, Jia Y, Sumikawa K. Nicotine withdrawal suppresses nicotinic modulation of long-term potentiation induction in the hippocampal CA1 region. Eur J Neurosci. 2006b;24:2903–2916. [Abstract] [Google Scholar]
- Zhang J, Berg DK. Reversible inhibition of GABAA receptors by alpha7-containing nicotinic receptors on the vertebrate postsynaptic neurons. J Physiol (Lond) 2007;579:753–763. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1111/j.1460-9568.2009.07058.x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2852628?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1111/j.1460-9568.2009.07058.x
Article citations
Nicotine-mediated activation of α2 nAChR-expressing OLM cells in developing mouse brains disrupts OLM cell-mediated control of LTP in adolescence.
Neurobiol Learn Mem, 194:107674, 24 Aug 2022
Cited by: 0 articles | PMID: 36029955 | PMCID: PMC9835838
The Engram's Dark Horse: How Interneurons Regulate State-Dependent Memory Processing and Plasticity.
Front Neural Circuits, 15:750541, 13 Sep 2021
Cited by: 6 articles | PMID: 34588960 | PMCID: PMC8473837
Review Free full text in Europe PMC
Sleep loss drives acetylcholine- and somatostatin interneuron-mediated gating of hippocampal activity to inhibit memory consolidation.
Proc Natl Acad Sci U S A, 118(32):e2019318118, 01 Aug 2021
Cited by: 27 articles | PMID: 34344824 | PMCID: PMC8364159
Effects of 3-Month Exposure to E-Cigarette Aerosols on Glutamatergic Receptors and Transporters in Mesolimbic Brain Regions of Female C57BL/6 Mice.
Toxics, 8(4):E95, 29 Oct 2020
Cited by: 7 articles | PMID: 33137879 | PMCID: PMC7712012
Nicotinic receptor activation induces NMDA receptor independent long-term potentiation of glutamatergic signalling in hippocampal oriens interneurons.
J Physiol, 599(2):667-676, 13 Dec 2020
Cited by: 6 articles | PMID: 33251594 | PMCID: PMC7839446
Go to all (36) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Early postnatal nicotine exposure disrupts the α2* nicotinic acetylcholine receptor-mediated control of oriens-lacunosum moleculare cells during adolescence in rats.
Neuropharmacology, 101:57-67, 16 Sep 2015
Cited by: 8 articles | PMID: 26386153 | PMCID: PMC4681641
In hippocampal oriens interneurons anti-Hebbian long-term potentiation requires cholinergic signaling via α7 nicotinic acetylcholine receptors.
J Neurosci, 33(3):1044-1049, 01 Jan 2013
Cited by: 19 articles | PMID: 23325242 | PMCID: PMC6704869
Nicotinic receptor-mediated enhancement of long-term potentiation involves activation of metabotropic glutamate receptors and ryanodine-sensitive calcium stores in the dentate gyrus.
Eur J Neurosci, 24(11):3109-3118, 01 Dec 2006
Cited by: 86 articles | PMID: 17156372
The alpha2 nicotinic acetylcholine receptor, a subunit with unique and selective expression in inhibitory interneurons associated with principal cells.
Pharmacol Res, 196:106895, 29 Aug 2023
Cited by: 1 article | PMID: 37652281
Review
Funding
Funders who supported this work.
NIDA NIH HHS (9)
Grant ID: DA014542
Grant ID: R01 DA014542-06A2
Grant ID: R01 DA026458-01A1
Grant ID: R01 DA014542
Grant ID: R01 DA026458
Grant ID: DA025269
Grant ID: R21 DA025269
Grant ID: DA026458
Grant ID: R21 DA025269-01




