Abstract
Free full text

Bcl6 Mediates the Development of T Follicular Helper Cells
Abstract
A fundamental function of CD4+ helper T (TH) cells is the regulation of B cell–mediated humoral immunity. Development of T follicular helper (TFH) cells that provide help to B cells is mediated by the cytokines interleukin-6 and interleukin-21 but is independent of TH1, TH2, and TH17 effector cell lineages. Here, we characterize the function of Bcl6, a transcription factor selectively expressed in TFH cells. Bcl6 expression is regulated by interleukin-6 and interleukin-21. Bcl6 overexpression induced TFH-related gene expression and inhibited other TH lineage cell differentiation in a DNA binding–dependent manner. Moreover, Bcl6 deficiency in T cells resulted in impaired TFH cell development and germinal center reactions, and altered production of other effector T cell subsets. Our data thus illustrate that Bcl6 is required for programming of TFH cell generation.
A critical function of CD4+ helper T (TH) cells is to provide “help” to B cells, especially in the germinal center structures where activated B cells proliferate and undergo antibody affinity maturation. Recently, T follicular helper (TFH) cells have been characterized by their expression of chemokine (C-X-C motif) receptor 5 (CXCR5) (1–3).We, as well as others, recently reported that TFH cell development is mediated by interleukin (IL)–6 or IL-21 but is independent of TH1, TH2, and TH17 cells (4, 5).
The B cell lymphoma 6 (Bcl6) transcription factor is selectively expressed by TFH cells (2, 3). Bcl6 was previously shown to be inhibitory to TH2 responses by blocking signal transducer and activator of transcription 6 (STAT6) binding to DNA (6, 7), whereas Bcl6-deficient mice developed multiorgan inflammatory diseases, enhanced immunoglobulin E (IgE) production, and defective germinal center reaction (6, 8). It is not clear whether the germinal center defect in these mice is caused by lack of proper T and/or B cell function because Bcl6 is also expressed by germinal center B cells (9). To analyze the function of Bcl6 in TFH cells, we activated naïve CD4+ T cells (CD44lowCD62LhighCD25−) from C57BL/6 mice with antibodies to CD3 and CD28 in the presence or absence of various cytokines for 1 or 2 days, and Bcl6 mRNA expression was assessed by real-time reverse transcription polymerase chain reaction (RT-PCR) analysis (10). Treatment with IL-6 or IL-21 significantly up-regulated Bcl6 expression, which was strongly inhibited by the addition of exogenous transforming growth factor beta (TGFβ) (Fig. 1A). These results correlate with our previous observations that IL-6 or IL-21 alone induces TFH cell development and Bcl6 expression, whereas treatment, together with TGFβ, promotes TH17 differentiation instead (4). To determine whether IL-21 is necessary for IL-6–induced Bcl6 expression, we activated naïve wild-type and IL-21– or IL-21 receptor (IL-21R)–deficient CD4+ T cells in the presence of IL-6. IL-21– and IL-21R–deficient T cells showed significantly reduced expression of Bcl6 (fig. S1).
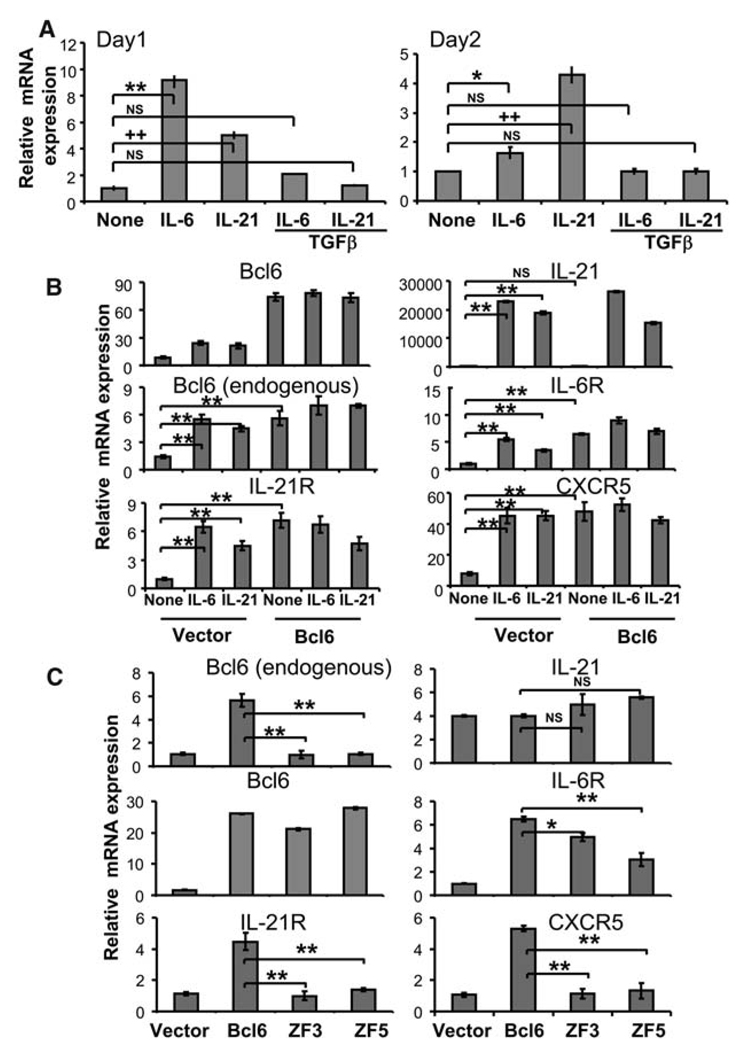
Bcl6 regulates TFH-specific gene expression. (A) Naïve T cells were activated with antibodies to CD3 and CD28, and with or without indicated cytokines for 1 or 2 days. Bcl6 mRNA expression was analyzed by real-time RT-PCR. The graph shows means ± SD. *P < 0.005; **P < 0.001 when comparing IL-6 and IL-6 plus TGFβ to nontreated sample, analysis of variance (ANOVA) test. ++P < 0.001 when comparing IL-21 and IL-21 plus TGFβ to nontreated sample, ANOVA test. (B) Naïve OT-II T cells were activated with antibodies to CD3 and CD28 alone or together with IL-6 or IL-21 and with antibodies to IL-4, IFNγ, and TGFβ, and infected with a bicistronic retrovirus containing internal ribosomal entry site green fluorescent protein (GFP) expressing Bcl6 or a vector control virus. mRNA expression of indicated genes was assessed by real-time RT-PCR. (C) Naïve OT-II T cells were activated with antibodies to CD3 and CD28 alone and infected with retroviruses expressing Bcl6, Bcl6 mutants (ZF3 and ZF5), or vector alone. GFP+ cells were sorted from (B) and (C) and restimulated for 4 hours with antibody to CD3. mRNA expression of indicated genes was analyzed by real-time RT-PCR. The data shown were normalized to the expression of a reference gene, Actb. The graph shows means ± SD. *P < 0.005; **P < 0.001, t test. The data represent at least three independent experiments with consistent results.
We next assessed whether overexpression of Bcl6 promoted TFH cell development in the absence of exogenous cytokines. Bcl6 overexpression led to increased expression of endogenous Bcl6 mRNA as well as IL-21R, IL-6R, and CXCR5 mRNA, similar to cells treated with IL-6 or IL-21 (Fig. 1B and fig. S2A). Interestingly, IL-21 expression was not up-regulated by Bcl6 overexpression.
Bcl6 has multiple zinc finger (ZF) domains, and the mutation of two of these (ZF3 and ZF5) was previously shown to abolish DNA binding but not nuclear localization (11). We thus assessed the function of Bcl6 with a mutation in either domain in the induction of TFH-specific genes. ZF3 and ZF5 mutations completely abrogated the ability of Bcl6 to up-regulate endogenous Bcl6, IL-21R, and CXCR5 expression, whereas the ZF3 exhibited less efficient inhibition of IL-6R expression than ZF5 (Fig. 1C). Thus, the regulation of TFH gene expression by Bcl6 appears to depend largely on its ability to bind DNA.
We then assessed whether Bcl6 overexpression antagonizes the differentiation of other TH lineage cells. We first overexpressed Bcl6 in cells undergoing TH17 differentiation in the presence of TGFβ and IL-6. We found that Bcl6 overexpression induced endogenous Bcl6 expression and also moderately increased the expression of CXCR5, IL-21R, and IL-6R (Fig. 2A); however, the expression of these genes was significantly lower than that induced by Bcl6 under neutral conditions or in TFH cells (Fig. 1B). Bcl6 also inhibited IL-17 protein expression when measured by intracellular staining, as well as IL-17 and IL-17F mRNA expression, in a DNA binding–dependent manner (Fig. 2, A and B). In contrast, RORγt or IL-21 expression was not affected by Bcl6. We further analyzed whether Bcl6 might influence RORγt-dependent transcription using a luciferase reporter driven by the IL-17 gene promoter and the conserved noncoding sequence 2 (CNS2) element (12). Wild-type Bcl6, but not the DNA-binding mutants ZF3 and ZF5, strongly inhibited RORγt-induced luciferase activity (Fig. 2C). Thus, similar to Foxp3, Bcl6 inhibits RORγt function but not its expression. Unlike Foxp3 (13), however, Bcl6 function appears to be dependent on its binding to DNA.
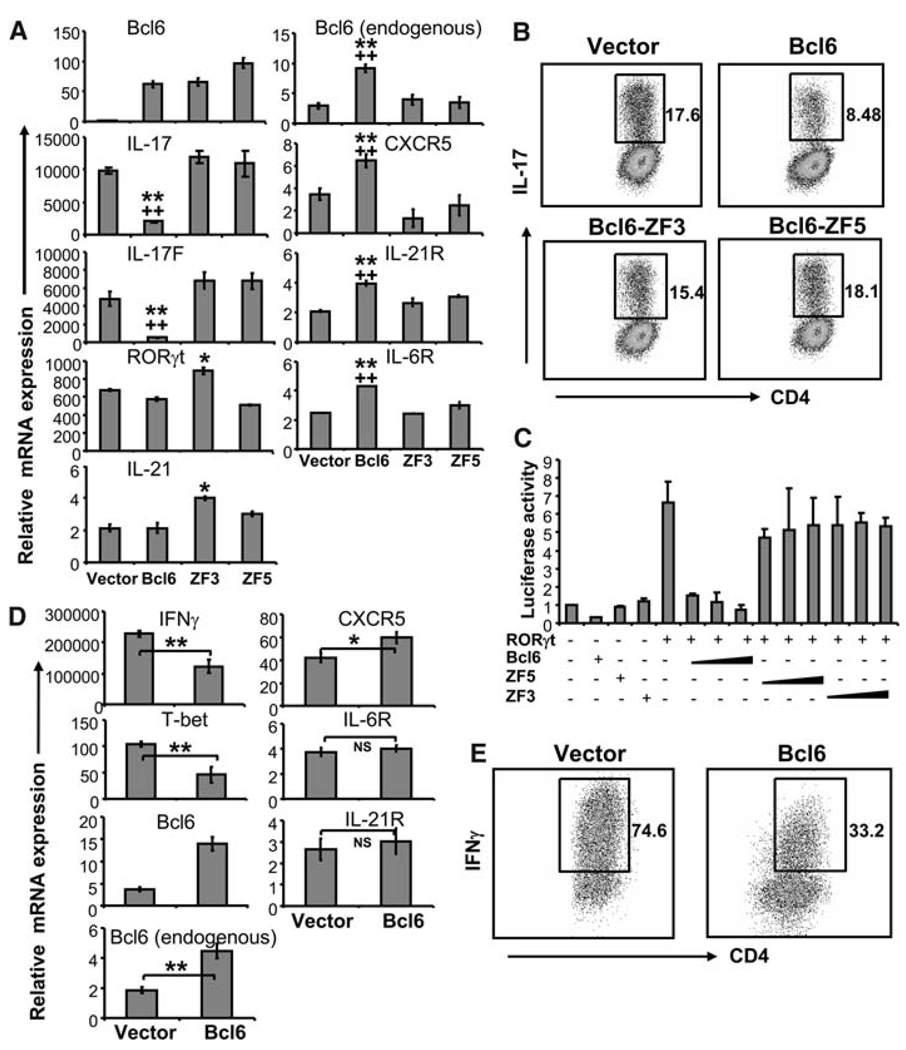
Bcl6 suppresses TH17 and TH1 differentiation. (A and B) Naïve OT-II T cells were activated under TH17 conditions and infected with retroviruses expressing Bcl6, Bcl6 mutants, or vector alone. GFP+ cells sorted by fluorescence-activated cell sorting (FACS) were restimulated with antibody to CD3, and mRNA expression of indicated genes was analyzed by real-time RT-PCR (A). The data shown were normalized to the expression of reference gene Actb. The graph shows means ± SD. *P < 0.005; **P < 0.001 when comparing Bcl6 and ZF3 to vector alone, ANOVA test. ++P < 0.001 when comparing Bcl6 and ZF5 to vector alone, ANOVA test. IL-17–expressing cells were measured by intracellular staining of the sorted GFP+ population (B). Numbers in dot-plot quadrants represent the percentages. (C) EL-4 cells were transfected with a vector containing the firefly luciferase gene under the control of the IL-17 promoter and the CNS2 region, a vector expressing Renilla luciferase, and vectors expressing RORγt, Bcl6 wild-type, various Bcl6 mutants, or vector alone. The firefly luciferase activity was determined and normalized to Renilla luciferase. Values were also normalized to vector alone. The graph shows means ± SD. (D and E) Naïve OT-II T cells were activated under TH1 conditions and infected with the indicated viruses. GFP+ cells were restimulated with antibody to CD3, and mRNA expression of the indicated genes was analyzed by real-time RT-PCR (D). The data shown were normalized to the expression of reference gene Actb. The graph shows means ± SD. *P < 0.005; **P < 0.001, t test. IFNγ expression was analyzed by intracellular staining on FACS-sorted GFP+ cells (E). Numbers in dot-plot quadrants represent the percentages. The data represent at least three independent experiments with consistent results.
In addition to TH17 cells, we also analyzed the effect of Bcl6 in developing TH1 and TH2 cells and found that overexpression of Bcl6 increased endogenous Bcl6 expression and CXCR5 (Fig. 2D and fig. S2, B and C). Bcl6, but not ZF3 and ZF5 mutants, also significantly inhibited the expression of TH1 (IFNγ and T-bet) and TH2 (IL-4 and GATA3) genes (Fig. 2, D and E, and fig. S2, B and C). Together, these data suggest that Bcl6 autoregulates its own expression. Moreover, Bcl6 not only induces TFH gene expression in neutral conditions, similar to IL-6 or IL-21, but also inhibits the differentiation of other TH lineage cells.
To understand whether Bcl6 is necessary for TFH cell development, we used a previously reported Bcl6 knockout mouse (Bcl6−/−) (14) and crossed it with OT-II T cell receptor (TCR) transgenic mice. The OT-II TCR is specific for a peptide derived from chicken ovalbumin presented by major histocompatability complex class II; thus, the majority of T cells in these mice are CD4+. Naïve CD4+ T cells were first differentiated to promote TFH cell differentiation in vitro (4). Bcl6 deficiency resulted in substantial reduction of CXCR5 and IL-6R expression, although the expression of IL-21R and IL-21 was partially reduced (Fig. 3A).
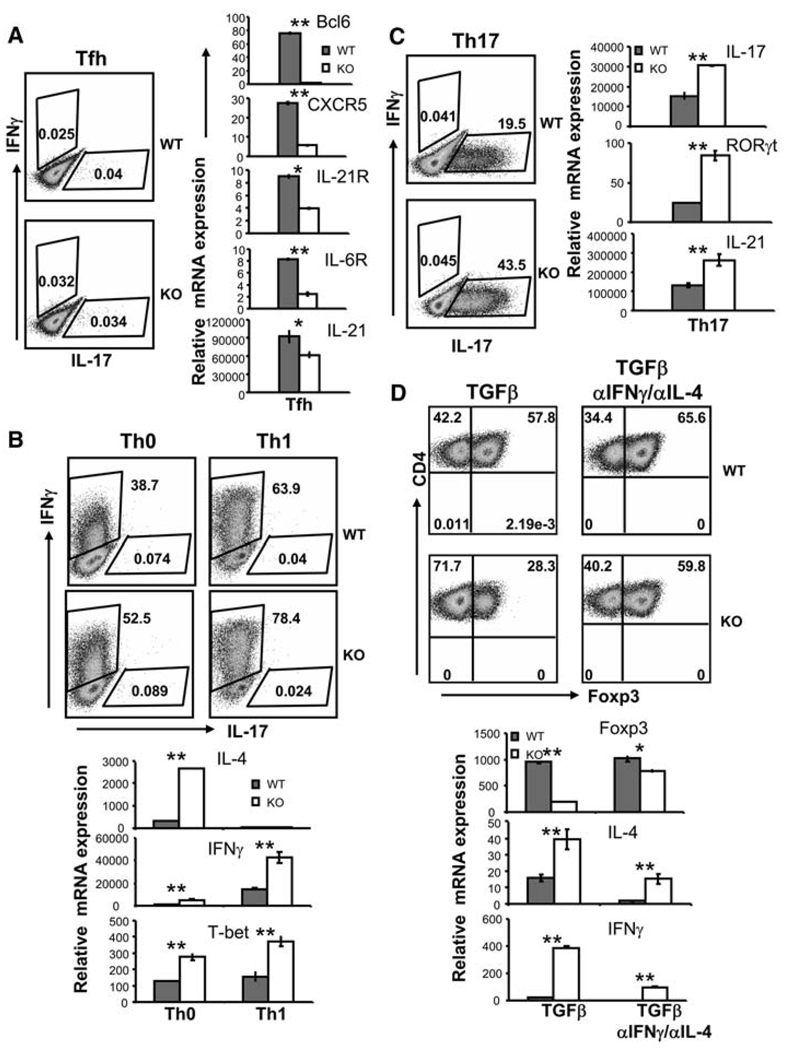
Bcl6 deficiency causes defective T cell differentiation in vitro. Naïve CD4+ T cells from Bcl6−/− mice and their littermate control mice carrying the OT-II TcR transgene were activated under TFH (A), TH0 and TH1 (B), TH17 (C), and Treg (TGFβ or TGFβ with antibodies to IL-4 and IFNγ) (D) conditions. Five days later, cells were assessed for IFNγ and IL-17 production [(A) to (C)] or Foxp3 expression (D) using intracellular staining. Numbers in FACS plot quadrants represent the percentages. mRNA expression of various genes was analyzed by real-time RT-PCR, and the data shown were normalized to the expression of reference gene β-actin. The graph shows means ± SD. P values were calculated using a t test comparing wild-type and Bcl6−/− CD4+ T cells and are indicated as follows: *P < 0.005; **P < 0.001. The experiments were repeated three times with consistent results.
We also subjected control and Bcl6−/− naïve T cells to TH1 and TH17 differentiation. As expected, absence of Bcl6 resulted in enhanced TH1 and TH17 differentiation and increased expression of T-bet and RORγt, respectively (Fig. 3, B and C, and fig. S3). Interestingly, IL-21 mRNA expression in Bcl6−/− cells differentiated under TH17 condition was enhanced, correlating with increased expression of IL-17 and RORγt (Fig. 3C). When activated in the presence of TGFβ to induce Foxp3 expression, Bcl6−/− T cells exhibited reduced levels of Foxp3 expression but enhanced IL-4 and IFNγ expression. Moreover, Foxp3 expression was partially restored by treatment with antibodies to IFNγ and IL-4 (Fig. 3D).
To analyze the function of Bcl6 in TFH cell generation in vivo, we first analyzed germline wild-type and Bcl6−/− mice. We found that memory T cells from aged Bcl6−/− mice produced significantly elevated amounts of IL-17, but their production of IFNγ and IL-4 was comparable to that of wild-type cells (fig. S4, A and B). IL-21 expression in IL-17+ T cells was also increased in these mice (fig. S4C). We then immunized wild-type and Bcl6−/− mice with keyhole limpet hemocyanin (KLH) protein (4), which induces an immune response where B cell activation requires T cell help. Bcl6−/− mice exhibited severely reduced CXCR5 expression on T cells and numbers of germinal center B cells (fig. S5) 7 days after immunization. Immunohistochemistry analysis also revealed that the germinal center B cells were greatly reduced in the Bcl6−/− mice when compared with immunized wild-type mice (fig. S6A). The production of KLH-specific IgG, IgM, and IgG2a was also reduced in Bcl6−/− mice, whereas IgE was enhanced (fig. S7A). In contrast, we observed increased expression of TH1, TH2, and TH17 cytokines in Bcl6−/− mice, whereas IL-21 production was not affected (fig. S5). To substantiate the above results, we also purified CD4+CD44high cells from immunized mice and measured their cytokine secretion after activation ex vivo with KLH and irradiated wild-type splenic antigen-presenting cells. We observed greatly increased expression of IL-4, IFNγ, and IL-17 in Bcl6−/− T cells when compared with wild-type T cells (fig. S8A). Furthermore, Bcl6−/− T cells exhibited defective expression of TFH-related genes, but their expression of TH1, TH2, and TH17 genes was significantly enhanced (fig. S8B). Interestingly, Bcl6 deficiency did not result in defective IL-21 production, and TH17 cells are likely the source of IL-21 in knockout mice.
To specifically analyze the function of Bcl6 in T cells, we mixed wild-type or Bcl6−/− naïve CD4+ T cells with wild-type B cells and transferred them into Rag1−/− mice, which lack T and B cells, and immunized the mice with KLH. Absence of Bcl6 in T cells again resulted in greatly reduced numbers of CXCR5+ T cells (Fig. 4A). Moreover, germinal center B cells (GL7+ FAS+) were greatly reduced in these animals (Fig. 4A), which was consistent with our immunohistochemistry analysis (fig. S6B). KLH-specific IgG, IgM, and IgG2a production was also reduced (fig. S7B). We also observed that Bcl6 deficiency resulted in enhanced production of IL-4 by T cells and KLH-specific IgE by B cells, but only moderately decreased IL-17 and IL-21 expression by knockout T cells (Fig. 4A and fig. S7B). In another experiment, we transferred wild-type and Bcl6−/− total CD4+ T cells with wild-type B cells into Rag1−/− mice and immunized them with KLH. The analysis of sorted effector-phenotype CD4+CD44high cells from the immunized mice revealed that Bcl6 deficiency in T cells resulted in the reduction of mRNA expression of most of the TFH-related genes except for IL-21 (fig. S9), which was increased together with IL-17 and RORγt, suggesting that the source of IL-21 may be TH17 cells.
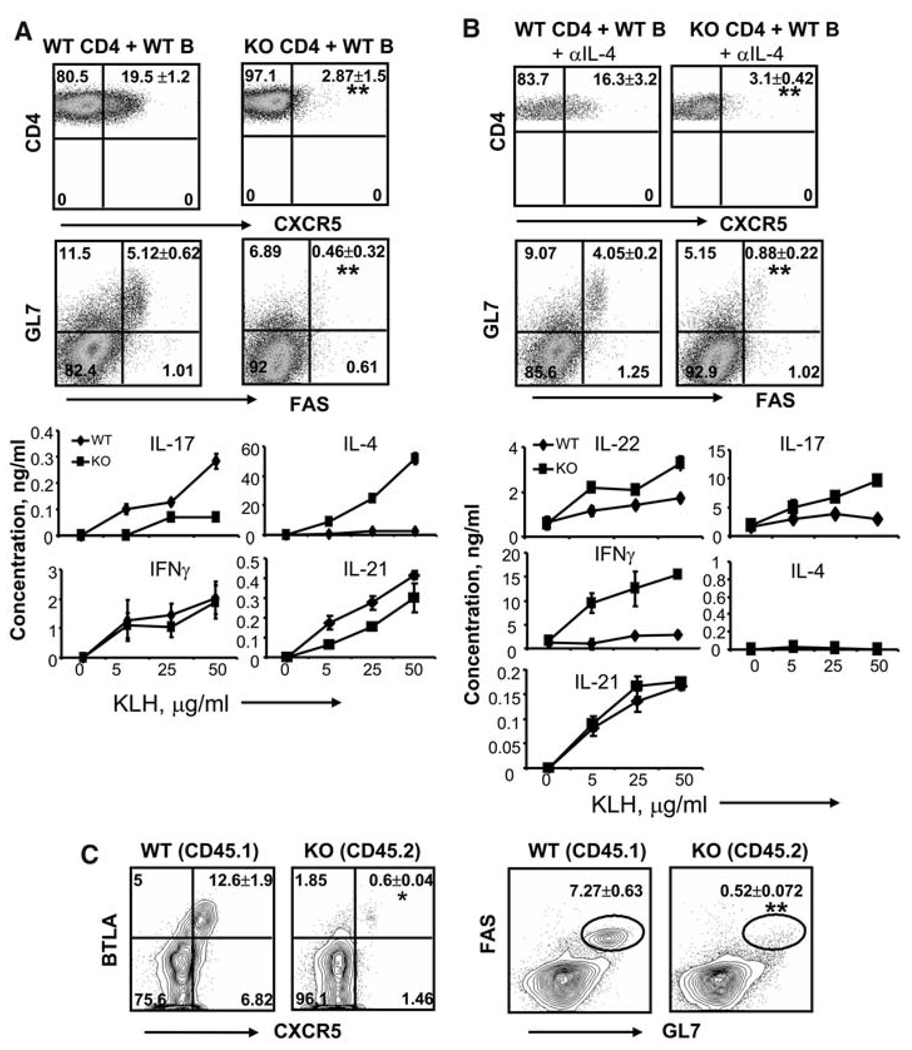
Bcl6 is necessary for the generation of TFH cells in vivo. (A and B) Naïve CD4+ cells from wild-type (WT) or Bcl6−/− mice were mixed with WT B cells and transferred into Rag1−/− mice (3 mice per group). The recipient mice were immunized subcutaneously with KLH emulsified in Freund’s complete adjuvant (FCA). In (B), recipient mice were also treated with antibody to IL-4. Seven days after the immunization, germinal center B cells were determined by staining with GL-7, FAS and B220 antibodies, and TFH cells by CD4 and CXCR5 antibodies. Numbers in dot-plot quadrants represent the percentages in gated T and B cells, respectively. Spleen cells from immunized mice were stimulated with the indicated concentration of KLH. Effector cytokines were measured after 4 days of treatment. The graph shows means ± SD. P values were calculated using a t test comparing CXCR5 expression on T cells or GL7/Fas expression on B cells between mice transferred with WT or Bcl6-deficient T and B cells, respectively, and are indicated as follows: *P < 0.005; **P < 0.001. The results are representative of multiple mice (n = 6 per group) from two independent experiments with similar results. (C) Mixed–bone marrow chimeric mice (n = 15) were generated and immunized with KLH in FCA. Spleen cells were stained with antibody to CD45.2 to distinguish WT and Bcl6 knockout compartments and then analyzed for the germinal center B cells and TFH cells. P values were calculated using a t test comparing CXCR5 and GL7/Fas expression between WT and Bcl6-deficient T and B cells, respectively, and are indicated as follows: *P < 0.005; **P < 0.001. The results are a representative of multiple mice (n = 15) from three independent experiments with similar results.
Because Bcl6 deficiency also caused hyper-IL-4 production in the above experiment, we examined the effect of IL-4 neutralization. Treatment with antibody to IL-4 almost completely blocked IL-4 production by T cells, whereas defective CXCR5 expression was still observed in Bcl6−/− T cells (Fig. 4B). We also observed reduced generation of germinal center B cells and KLH-specific IgG, IgM, and IgG2a production (Fig. 4B, fig. S6C, and fig. S7C). In addition, Bcl6−/− T cells also exhibited increased IL-22, IL-17, and IFNγ production (Fig. 4B). Despite the absence of TFH cells in Bcl6−/− mice, we detected IL-21 production that was comparable to wild-type mice, likely derived from TH17 cells.
To further demonstrate the role of Bcl6 in T and B cells, we generated mixed bone marrow chimeras by transferring a mixture of congenic CD45.1+ wild-type and CD45.2+ Bcl6-deficient bone marrow cells into sublethally irradiated Rag1−/− mice. Eight weeks after reconstitution, we immunized the mice with KLH. Bcl6−/− CD4+ T cells did not mature into CXCR5+ TFH cells that also express B and T lymphocyte attenuator (BTLA) (Fig. 4C), and did not exhibit germinal center B cells. CD4+CD44high Bcl6−/− cells exhibited greatly reduced TFH gene expression; however, the expression of TH1, TH2, and TH17 genes were all increased when compared with wild-type cells (fig. S10). Moreover, Bcl6-deficient B cells did not produce germinal center B cells. On the basis of the above data, we conclude that Bcl6 expression in both T and B cells is required for the germinal center reactions.
In summary, we have demonstrated that Bcl6 is regulated by IL-6 and IL-21 but that, similar to RORγt in TH17 cells, it does not regulate the expression of IL-21. Bcl6 deficiency in vivo did not inhibit IL-21 expression, likely by TH17 cells. Thus, IL-21 expression by non-TFH cells is not sufficient to induce germinal center reactions. Our data overall indicate that Bcl6, selectively induced by IL-21 or IL-6 in the absence of TGFβ signaling, serves as a master transcriptional factor in TFH cell differentiation, analogous to RORγt and RORα, both of which are induced by IL-6 or IL-21 in the presence of TGFβ and function to promote TH17 differentiation (fig. S11). IL-6 alone induces the expression of RORγt (15), whose function is inhibited by Bcl6, similar to regulatory T cells (Treg), where the function of TGFβ-induced RORγt is suppressed by Foxp3 (13, 16). The combination of TGFβ and IL-6 not only induces RORγt expression in T cells, but in the meantime, Foxp3 and Bcl6 expression is suppressed by IL-6 and TGFβ signaling, respectively, allowing RORγt to function properly in promoting TH17 cell differentiation.
Although we proposed in the past year that TFH cell differentiation mediated by IL-6 and IL-21 is a novel lineage of TH cell differentiation, subsequently several groups reported that CXCR5+ T cells also express TH2 or TH17 cytokines in vivo (17, 18). Our current study indicates that Bcl6 promotes the expression of TFH-related genes but inhibits the differentiation of TH1, TH2, and TH17 cells. Our data thus support Bcl6-dependent TFH cell generation as a pathway that is independent of other TH cell lineages.
Footnotes
Supporting Online Material
www.sciencemag.org/cgi/content/full/1176676/DC1
Materials and Methods
Figs. S1 to S11
References
Reference and Notes
Full text links
Read article at publisher's site: https://doi.org/10.1126/science.1176676
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2857334?pdf=render
Subscription required at www.sciencemag.org
http://www.sciencemag.org/cgi/content/full/325/5943/1001
Subscription required at www.sciencemag.org
http://www.sciencemag.org/cgi/reprint/325/5943/1001.pdf
Free to read at www.sciencemag.org
http://www.sciencemag.org/cgi/content/abstract/325/5943/1001
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
B Cell Lymphoma 6 (BCL6): A Conserved Regulator of Immunity and Beyond.
Int J Mol Sci, 25(20):10968, 11 Oct 2024
Cited by: 0 articles | PMID: 39456751 | PMCID: PMC11507070
Review Free full text in Europe PMC
Clinical significance of T helper cell subsets in the peripheral blood and bone marrow of patients with multiple myeloma.
Front Immunol, 15:1445530, 11 Sep 2024
Cited by: 0 articles | PMID: 39324138 | PMCID: PMC11422089
Generation of antigen-specific memory CD4 T cells by heterologous immunization enhances the magnitude of the germinal center response upon influenza infection.
PLoS Pathog, 20(9):e1011639, 16 Sep 2024
Cited by: 1 article | PMID: 39283916 | PMCID: PMC11404825
Clinical and prognostic significance of follicular helper and regulatory T cells in bladder cancer draining lymph nodes.
Sci Rep, 14(1):20358, 02 Sep 2024
Cited by: 0 articles | PMID: 39223192 | PMCID: PMC11369110
Tertiary lymphoid structures in diseases: immune mechanisms and therapeutic advances.
Signal Transduct Target Ther, 9(1):225, 28 Aug 2024
Cited by: 0 articles | PMID: 39198425 | PMCID: PMC11358547
Review Free full text in Europe PMC
Go to all (966) article citations
Other citations
Wikipedia
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation.
Science, 325(5943):1006-1010, 16 Jul 2009
Cited by: 1049 articles | PMID: 19608860 | PMCID: PMC2766560
Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages.
Immunity, 29(1):138-149, 03 Jul 2008
Cited by: 853 articles | PMID: 18599325 | PMCID: PMC2556461
Insights into the role of Bcl6 in follicular Th cells using a new conditional mutant mouse model.
J Immunol, 191(7):3705-3711, 26 Aug 2013
Cited by: 95 articles | PMID: 23980208 | PMCID: PMC3783642
IL-21 and T follicular helper cells.
Int Immunol, 22(1):7-12, 23 Nov 2009
Cited by: 114 articles | PMID: 19933709 | PMCID: PMC2795365
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIAID NIH HHS (7)
Grant ID: R01 AI050761
Grant ID: R01 AI050761-05
Grant ID: R01 AI050761-07A1
Grant ID: R01 AI050761-06
Grant ID: R01 AI050746
Grant ID: R01 AI050746-05
Grant ID: R01 AI083761
NIAMS NIH HHS (3)
Grant ID: R01 AR050772
Grant ID: R01 AR050772-07
Grant ID: R01 AR050772-08





