Abstract
Free full text

Role of tissue factor and protease-activated receptors in a mouse model of endotoxemia
Abstract
Sepsis is associated with a systemic activation of coagulation and an excessive inflammatory response. Anticoagulants have been shown to inhibit both coagulation and inflammation in sepsis. In this study, we used both genetic and pharmacologic approaches to analyze the role of tissue factor and protease-activated receptors in coagulation and inflammation in a mouse endotoxemia model. We used mice expressing low levels of the procoagulant molecule, tissue factor (TF), to analyze the effects of TF deficiency either in all tissues or selectively in hematopoietic cells. Low TF mice had reduced coagulation, inflammation, and mortality compared with control mice. Similarly, a deficiency of TF expression by hematopoietic cells reduced lipopolysaccharide (LPS)–induced coagulation, inflammation, and mortality. Inhibition of the downstream coagulation protease, thrombin, reduced fibrin deposition and prolonged survival without affecting inflammation. Deficiency of either protease activated receptor-1 (PAR-1) or protease activated receptor-2 (PAR-2) alone did not affect inflammation or survival. However, a combination of thrombin inhibition and PAR-2 deficiency reduced inflammation and mortality. These data demonstrate that hematopoietic cells are the major pathologic site of TF expression during endotoxemia and suggest that multiple protease-activated receptors mediate crosstalk between coagulation and inflammation.
Introduction
Sepsis is induced by a systemic infection, mostly by Gram-negative bacteria, and is associated with activation of coagulation and inflammation.1 In sepsis, an excessive stimulation of the host immune system by lipopolysaccharide (LPS) results in high levels of inflammatory cytokines in the circulation. However, clinical trials that targeted specific inflammatory mediators have not been successful.2 Despite extensive research, sepsis remains a major cause of death in intensive care units, with mortality rates between 30% and 70%.2 Three natural anticoagulants, tissue factor pathway inhibitor-1 (TFPI-1), antithrombin-III (AT-III), and activated protein C (APC), have been shown to have both anticoagulant and antiinflammatory properties in different animal models of sepsis.3–5 All 3 anticoagulants showed positive results in phase 2 clinical trials for sepsis, although only human recombinant APC was protective in a phase 3 clinical trial.2,6–9
LPS induction of the procoagulant molecule, tissue factor (TF), within the vasculature causes systemic activation of coagulation and disseminated intravascular coagulation.10 In animal models of sepsis, TF is expressed by circulating monocytes and by splenic microvascular endothelial cells but not by endothelial cells in other vascular beds.11–13 Intravascular fibrin deposition contributes to multiple organ failure and mortality in different animal models of endotoxemia and human sepsis.10 In endotoxemic rabbits, selective inhibition of thrombin with hirudin decreased fibrin deposition and decreased mortality.14 Moreover, endotoxemicAT-III+/− and PC+/− heterozygous mice exhibited increased fibrin deposition and mortality compared with wild-type (WT) controls, presumably due to reduced levels of the anticoagulants.15,16
In a lethal baboon model of Escherichia coli–induced sepsis, inhibition of the TF–factor VIIa (FVIIa) complex with either an inhibitory anti-TF monoclonal antibody, active site-inhibited FVIIa (FVIIai), or TFPI-1 decreased coagulation and prevented death.3,17,18 Importantly, FVIIai and TFPI-1 reduced the expression of the inflammatory mediators interleukin-6 (IL-6) and IL-8 at later times (more than 4 hours after administration of E coli) but did not affect tumor necrosis factor-α (TNF-α) expression,3,18 indicating that IL-6 expression can be used to monitor crosstalk between coagulation and inflammation. In contrast, FXai blocked coagulation in septic baboons without reducing mortality.19 These studies suggest that the TF-FVIIa complex mediates crosstalk between coagulation and inflammation in sepsis.
Studies on the crosstalk between coagulation and inflammation have focused on the protease activated receptor (PAR) family, which comprises 4 members, PAR-1 to PAR-4.20,21 Several studies using cultured cells have shown that the TF-FVIIa and TF-FVIIa-FXa complexes, as well as FXa, can activate PAR-2.22–24 In mice, thrombin activates both PAR-1 and PAR-4 on vascular cells and a PAR-3–PAR-4 complex on platelets.20,21 PAR-1 can also be activated by FXa in vitro.24,25 LPS and inflammatory cytokines induce PAR-2 expression on endothelial cells, and IL-1 induces both PAR-2 and PAR-4 in human coronary artery tissue.26,27 In vivo studies have shown that PAR-2 plays a role in leukocyte rolling and in mediating chronic inflammation associated with arthritis.28,29 Thrombin activation of PAR-1 has been shown to induce the expression of proinflammatory cytokines and chemokines in vitro and to contribute to renal inflammation in vivo.20,30 In addition, LPS and TNF-α induction of IL-6 expression by cultured endothelial cells is enhanced by activation of PAR-1 and PAR-2.31 Taken together, these studies suggest that activation of PARs by coagulation proteases may contribute to inflammation in endotoxemia and sepsis.
In this study, we used genetic and pharmacologic approaches to analyze the role of TF and PARs in coagulation and inflammation in a mouse model of endotoxemia. In addition, we analyzed the role of hematopoietic cell TF expression in endotoxemia by reconstituting wild-type mice with bone marrow from low TF mice. Our results indicate that hematopoietic cells are the major site of pathologic TF expression during endotoxemia and that PARs mediate the crosstalk between coagulation and inflammation.
Materials and methods
Mice
All studies were approved by The Scripps Research Institute Animal Care and Use Committee and comply with National Institutes of Health guidelines. Low TF mice expressing very low levels of human (h) TF (approximately 1% relative to mouse [m] TF) and completely lacking murine TF (mTF−/−/hTF+) and heterozygous littermate controls (mTF+/−/hTF+) were generated as described previously.32 The generation of PAR-1−/− and PAR-2−/− mice has been described.33,34 All genetically modified mice were backcrossed for at least 5 generations with C57BL/6 mice. Mice were used for experiments between 2 and 3 months of age.
Model of endotoxemia
We employed a mouse model of endotoxemia that consists of intraperitoneal injection of a high dose of LPS (10 mg/kg) (E coli serotype O111:B4; Sigma Chemical, St Louis, MO).35 Littermate controls were used in all experiments. In the bone marrow transplantation experiments, we used a lower dose of LPS (5 mg/kg) due to increased sensitivity of the mice to LPS.
Hirudin treatment
Hirudin specifically blocks thrombin activity through competitive inhibition of its catalytic site.36 We used recombinant hirudin (lepirudin; Hoechst Marion Roussel, Kansas City, MO). The dose and frequency of injections was based on Ehrlich et al’s previous experience with the use of hirudin in rabbits and measuring the activated partial thromboplastin time (APTT) of plasma samples from mice injected with hirudin.37 APTTs were measured using the Automated APTT reagent (bioMérieux, Durham, NC) and a Start 4 clot analyzer (Diagnostica Stago, Parsippany, NJ). After a single intraperitoneal injection of recombinant hirudin (1 mg/kg), the APTT was increased from a baseline of 26.4 ± 1.4 seconds (mean ± SD; n = 4) to 56.1 ± 7.0 seconds and 52.0 ± 8.1 seconds at 30 and 60 minutes, respectively, before returning to baseline levels at 4 hours. For the experiments, 4 injections of hirudin were administered 30 minutes before injection of LPS and 30 minutes, 2 hours, and 4 hours after injection of LPS. Mice in the control group received saline.
Ancrod treatment
Ancrod cleaves only the A-chains of fibrinogen, producing soluble degradation products that are removed from the circulation.38 The dose of ancrod was based on our experience with rabbits and a previous study with mice.37,39 Mice received ancrod (Sigma Chemical) in 4 bolus injections (1 unit in 0.2 mL of saline each) via the retro-orbital sinus, starting 16 hours before LPS challenge (−16 hours, −8 hours, 0 hours, and +8 hours). Control mice were injected with saline.
Measurement of thrombin–antithrombin-III (TAT) and fibrinogen
Blood was drawn from the inferior vena cava (IVC) into syringes containing 3.2% sodium citrate (final ratio, 10:1). Levels of thrombin–antithrombin-III (TAT) complex in the plasma were determined using a commercial enzyme-linked immunosorbent assay (ELISA) (Enzygnost TAT micro; Dade Behring, Marburg, Germany). Fibrinogen levels were determined by the Clauss method using the Sigma Diagnostics Fibrinogen Kit (Sigma Chemical).
Measurement of cytokines
Blood samples (0.1 mL) were collected from the retro-orbital sinus. Plasma concentrations of TNF-α and IL-6 were measured using commercial ELISA kits (R&D Systems, Minneapolis, MN).
Bone marrow transplantation
Bone marrow of recipient mice was ablated with a single dose of radiation (13 Gy [1300 rad]) from a cesium 137 irradiator (Gammacell 40; Atomic Energy of Canada, Mississauga, ON, Canada). Recipient mice were injected with 2 × 106 bone marrow cells via the retro-orbital sinus. Mice were allowed to recover for 6 weeks after transplantation before LPS injection. Blood samples were collected from WT mice receiving bone marrow from either low TF mice or littermate mTF+/−/hTF+ control mice and used to determine platelet counts, red cell counts, hematocrit, and hemoglobin levels (Laboratory Corporation of America, San Diego, CA).
Genotyping of DNA from bone marrow recipient mice
Blood was collected from the IVC. The WT mouse TF allele was detected by polymerase chain reaction (PCR) using a forward primer located 1097 to 1070 base pair (bp) upstream of exon 1 and a reverse primer located 563 to 539 bp upstream of exon 1 (numbering from 787).
Immunohistochemistry and Western blotting
Mice were perfused through the left ventricle with phosphate-buffered saline (PBS) to remove fibrinogen from the blood vessels. Tissues were rapidly removed, fixed in 10% zinc formalin solution, and embedded in paraffin. Tissue sections were incubated overnight with a rabbit antimouse fibrinogen antibody (1:5000) (Dako, Carpinteria, CA), which was detected using the Rabbit Vectastatin Elite ABC kit (Vector Laboratories, Burlingame, CA). Antigen-antibody complexes were visualized using 3′, 3′-diamino benzidine (Vector Laboratories). The amount of cross-linked fibrin present in livers was determined by Western blotting using a mouse antihuman β-chain monoclonal antibody (59D8) (1:500) that binds to fibrin but not fibrinogen.40 A pan-anticadherin antibody (Sigma Chemical) was used to monitor protein loading.
Data analysis
Cytokine, TAT, and fibrinogen levels are presented as mean ± standard error (SE). Statistical analysis was performed using an unpaired Student t test. The survival rate of the groups was analyzed by log-rank test. Differences were determined to be statistically significant at P values less than .05.
Results
Low TF mice exhibit decreased LPS-induced coagulation, inflammation, and mortality
In this study, we determined whether a genetic deficiency in TF that dramatically reduces TF expression in all tissues would reduce mortality of the mice in a model of endotoxemia. We measured LPS-induced activation of coagulation by monitoring levels of TAT complex. Administration of LPS increased TAT levels in WT mice in a time-dependent manner from a baseline of 3.5 ± 1.2 ng/mL (mean ± SD; n = 4) to 11.2 ± 3.2 ng/mL at 2 hours, 21.2 ± 6.9 ng/mL at 4 hours, 45.5 ± 9.2 ng/mL at 8 hours, and 31.5 ± 12.1 ng/mL at 12 hours. We found that LPS induction of TAT complex levels was significantly reduced in low TF mice compared with littermate mTF+/−/hTF+ control mice (Figure 1A), indicating that activation of coagulation is significantly attenuated in these mice. Importantly, low TF mice exhibited prolonged survival and decreased mortality compared with control mice (Figure 1B). We measured LPS induction of TNF-α and IL-6 to determine whether TF deficiency was associated with reduced inflammation. TF deficiency did not affect LPS-induced TNF-α expression but was associated with a significant reduction in IL-6 expression at later times (Figure 1C–D). These results indicate that TF deficiency in all tissues reduces coagulation and inflammation and decreases mortality in this model of endotoxemia.
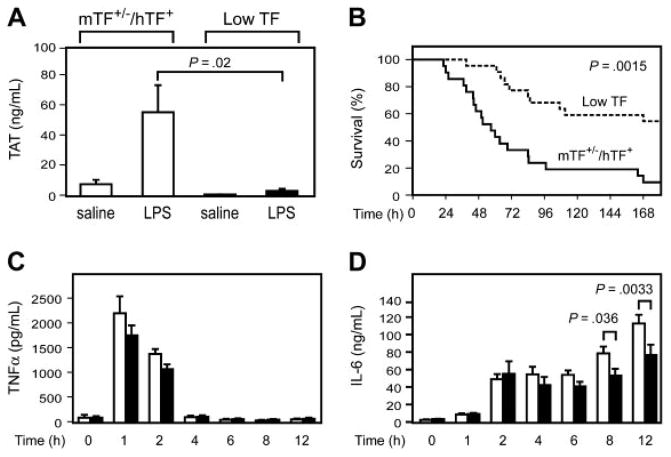
(A) TAT complex levels were measured at 6 hours in mTF+/−/hTF+ and low TF mice injected with either saline or LPS (10 mg/kg). Data are shown as mean ± SE (n = 5 per group). (B) Kaplan-Meier plot of the survival of mTF+/−/hTF+ mice (solid line; n = 22) and low TF mice (dashed line; n = 21) after administration of LPS (10 mg/kg). (C–D) □ indicates TNF-α and IL-6 levels in mTF+/−/hTF+ mice, and ■ indicates levels in low TF mice (mean ± SE; n = at least 4 per group).
Role of hematopoietic cell TF in LPS-induced lethality
We analyzed the role of hematopoietic cell–derived TF expression in LPS-induced lethality by transplanting, into WT mice, bone marrow from either low TF mice or littermate mTF+/−hTF+ control mice. PCR analysis of DNA from peripheral blood cells showed that irradiated recipient mice reconstituted with bone marrow from low TF mice had no detectable levels of the WT mTF allele (Figure 2A). A deficiency in TF expression by hematopoietic cells did not affect the composition of the peripheral blood cell population (not shown). LPS-induced TAT complex levels were significantly reduced in WT mice receiving bone marrow from low TF mice (Figure 2B). Importantly, WT mice reconstituted with bone marrow from low TF mice had significantly reduced mortality after LPS injection compared with WT reconstituted with bone marrow from control mice (Figure 2C). Finally, a reduction in TF expression by hematopoietic cells resulted in reduced levels of IL-6 expression compared with WT controls (Figure 2D), although these differences did not achieve statistical significance. These results indicate that hematopoietic cells are the major site of pathologic TF expression that contributes to LPS-induced coagulation, inflammation, and lethality.
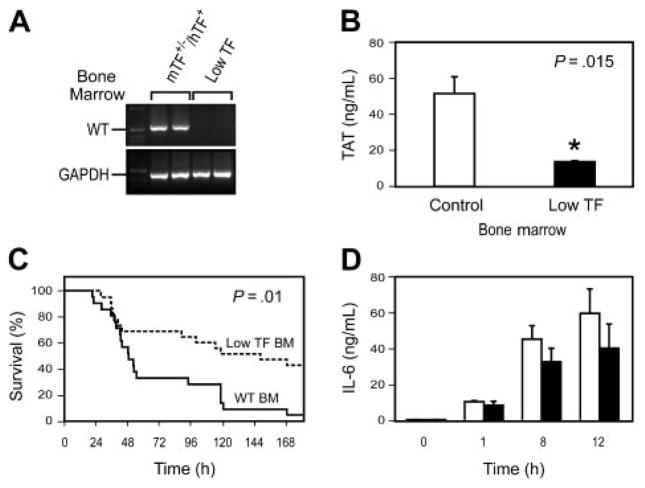
WT mice were irradiated and reconstituted with bone marrow from either low TF mice or littermate mTF+/−/hTF+ control mice. (A) PCR analysis of DNA from peripheral blood cells from WT mice that underwent transplantation with bone marrow from either mTF+/−/hTF+ or low TF mice was used to demonstrate reconstitution of the donor mice with recipient bone marrow 6 weeks after the transplantation. PCR was performed for the WT TF (WT) allele. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. First lanes contain molecular weight standards. (B) LPS-induced TAT complex levels (6 hours) (mean ± SE; n = 4 to 5 per group). (C) Survival of endotoxemic mice (5 mg/kg LPS intraperitoneally) reconstituted with bone marrow from either mTF+/−/hTF+ mice (solid line; n = 20) or low TF mice (dashed line; n = 22). For survival studies, we used mice that underwent transplantation with bone marrow derived from 3 independent donors for each genotype. (D) LPS-induced IL-6 expression in WT mice receiving bone marrow from mTF+/−/hTF+ (□) or low TF (■) mice (mean ± SE; n = 4 per group). Differences between the 2 groups were not statistically significant.
Role of thrombin and fibrin in LPS-induced lethality
We used the selective thrombin inhibitor, hirudin, and the defibrinogenating agent, ancrod, to analyze the role of thrombin and fibrin deposition in this endotoxemia model.
Administration of hirudin reduced LPS induction of TAT complex levels compared with mice receiving saline (Figure 3A). Levels of TAT complex observed in endotoxemic mice treated with hirudin were similar to those observed in endotoxemic low TF mice. Hirudin significantly prolonged the survival time of mice (Figure 3B). However, hirudin treatment did not affect LPS induction of TNF-α and IL-6 expression (Figure 3C–D). Next, we analyzed the effect of hirudin on LPS-induced fibrin deposition. Hirudin treatment reduced LPS-induced fibrin deposition in the liver as determined by both immunohistochemical analysis and Western blotting (Figure 3E–F). To further examine the role of thrombin-dependent fibrin deposition in LPS-induced lethality, we used ancrod to deplete circulating fibrinogen in the mice. Administration of ancrod decreased fibrinogen levels from 5.9 ± 0.5 μM (1.99 ± 0.16 g/L) (mean ± SD; n = 3) to undetectable levels (less than 0.015 μM [0.05 g/L]; n = 3). Ancrod treatment significantly prolonged the survival of mice compared with that of control mice (Figure 3G). This result is similar to those observed using hirudin and strongly suggests that thrombin-dependent intravascular fibrin deposition contributes to death in this model of endotoxemia.
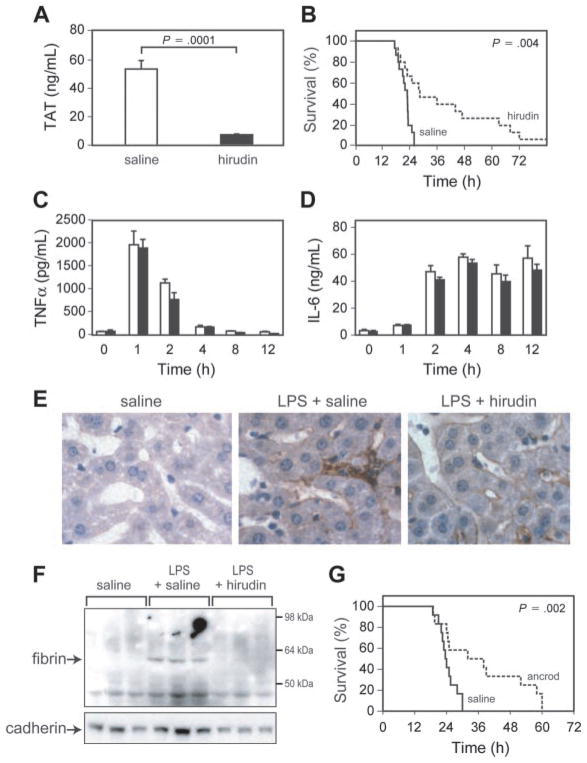
(A) TAT complex levels were measured at 6 hours in endotoxemic mice treated with saline or hirudin. Data are shown as mean ± SE (more than 4 mice per group). (B) Survival of WT mice injected with LPS and treated with either saline (solid line; n = 15) or hirudin (dashed line; n = 15). (C–D) TNF-α and IL-6 levels in saline-treated (□) and hirudin-treated (■) endotoxemic mice are shown. Data are shown as mean ± SE (more than 4 mice per group). (E) Immunohistochemical analysis of fibrin deposition in the liver. Mice were injected with saline, LPS and saline, or LPS and hirudin, and livers were collected at 8 hours. Fibrin(ogen) (brown color) was detected using a rabbit antifibrin(ogen) polyclonal antibody. Original magnification, × 400. Representative photomicrographs from 1 of 3 mice per group are shown. (F) Fibrin deposition in the liver. Mice (3 per group) were injected with saline, LPS and saline, or LPS and hirudin. Fibrin was detected in livers at 8 hours by Western blotting using an antifibrin monoclonal antibody. A pancadherin antibody was used to monitor protein loading. (G) Survival of wild-type mice injected with LPS and treated with either saline (solid line; n = 12) or ancrod (dashed line; n = 12). P values are shown.
Role of PARs in LPS-induced inflammation and lethality
We used PAR-1−/− and PAR-2−/− mice to determine whether either PAR-1 or PAR-2 mediates the crosstalk between coagulation and inflammation and contributes to LPS-induced lethality. We found no difference in the survival of PAR-2−/− and PAR-2+/+ mice and no difference in the expression of TNF-α and IL-6 (Figure 4A–C). Similarly, we found that PAR-1 deficiency did not affect either survival or LPS-induced TNF-α and IL-6 expression (Figure 4D–F).
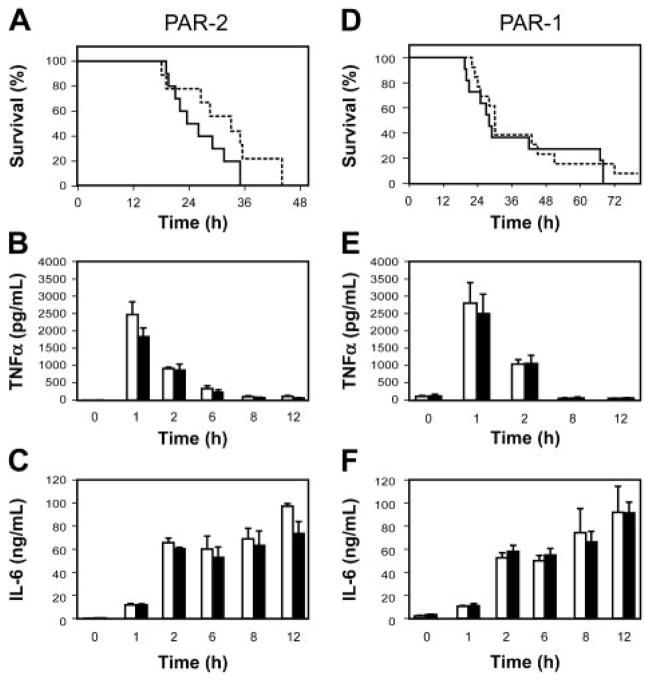
(A) Survival of endotoxemic PAR-2+/+ (solid line; n = 10) and PAR-2−/− (dashed line; n = 9) mice. (B–C) LPS induction of TNF-α and IL-6 expression in PAR-2+/+ (□) and PAR-2−/− (■). Data are shown as mean ± SE (3 to 6 mice per group). (D) Survival of endotoxemic PAR-1+/+ (solid line; n = 11) and PAR-1−/− (dashed line; n = 13). (E–F) LPS induction of TNF-α and IL-6 expression in PAR-1+/+ (□) and PAR-1−/− (■). Data are shown as mean ± SE (3 to 6 mice per group). No statistically significant differences were observed.
We have shown that neither thrombin inhibition nor PAR-1 or PAR-2 deficiency alone affects LPS induction of IL-6 expression. We hypothesized that multiple PARs may mediate the crosstalk between coagulation and inflammation. Therefore, deficiency of a single PAR or hirudin inhibition of thrombin–PAR-1 and thrombin–PAR-4 signaling may not significantly affect inflammation. To test this hypothesis, we examined the effect of combining thrombin inhibition, which would reduce fibrin deposition and thrombin-dependent signaling, with PAR-2 deficiency. PAR-2−/− mice treated with hirudin showed a significantly prolonged survival time when compared with PAR-2+/+ mice treated with hirudin (Figure 5A). Importantly, in PAR-2−/− mice, hirudin treatment significantly reduced IL-6 expression compared with that in hirudin-treated PAR-2+/+ mice (Figure 5B). As seen previously in WT mice, there was no difference in either survival or IL-6 expression in endotoxemic PAR-2+/+ mice treated with saline or hirudin (Figure 5). These data suggest that multiple PARs mediate the crosstalk between coagulation and inflammation during endotoxemia.
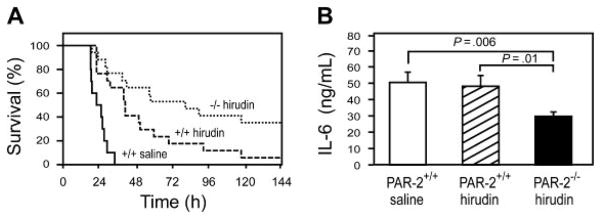
(A) Survival of LPS-treated PAR-2+/+ mice with saline (solid line; n = 10), LPS-treated PAR-2+/+ mice with hirudin (dashed line; n = 17), and LPS-treated PAR-2−/− mice with hirudin (dotted line; n = 17). Hirudin statistically increased the survival time of PAR-2+/+ mice (P =.0001) and PAR-2−/− mice (P %.0001) compared with saline-treated PAR-2+/+ mice. The difference in survival between PAR-2+/+ and PAR-2−/− mice treated with hirudin is also statistically significant (P =.037). (B) LPS-induced IL-6 expression (8 hours) in PAR-2+/+ and PAR-2−/− mice treated with either saline or hirudin. Data are shown as mean ± SE (more than 6 mice per group).
Discussion
In this study, we showed in a model of endotoxemia that low TF mice had reduced coagulation, reduced expression of IL-6, and decreased mortality compared with littermate mTF+/−/hTF+ control mice. These results are consistent with studies showing that inhibition of the TF-FVIIa complex in septic baboons reduced the expression of IL-6 and IL-8 and reduced mortality.3,17,18 Thus, the TF-FVIIa complex contributes to LPS-induced coagulation, inflammation, and mortality in different animal models of endotoxemia and sepsis. The TF-FVIIa complex can be inhibited by either TFPI-1, FVIIai, or anti-TF antibodies (Figure 6). The failure of the TFPI-1 phase 3 clinical trial for sepsis is disappointing.41 However, TFPI-1 is the least effective inhibitor of the 3 inhibitors of the TF-FVIIa complex because it requires the generation of FXa for the formation of the inhibitory quaternary complex.3 In contrast, FVIIai acts as a competitive inhibitor of FVII/FVIIa binding to TF, and the anti-TF antibodies used in these studies bind to the TF-FVIIa complex and inhibit substrate binding.42–44 Therefore, FVIIai and anti-TF antibodies may prove to be more effective drugs than TFPI-1 in the treatment of inflammatory diseases where there is crosstalk between coagulation and inflammation. In the model shown in Figure 6, inhibition of the TF-FVIIa complex would reduce fibrin deposition, platelet activation, and all PAR-dependent signaling.
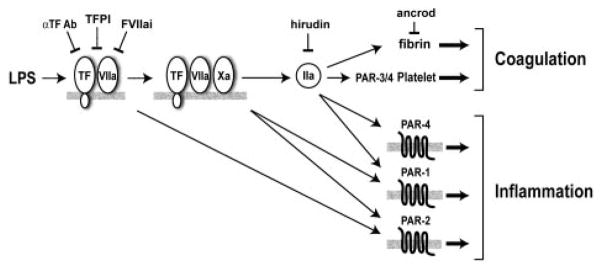
FVIIa and FXa activation of PAR-2 and thrombin (IIa) activation of PAR-1 and PAR-4 may enhance inflammation. Thrombin also cleaves fibrinogen to fibrin and activates platelets by cleavage of PAR-3/4. Inhibitors of the TF-FVIIa complex are shown: α F Ab (anti-TF antibody), FVIIai (active site-inhibited FVIIa), and TFPI-1. Hirudin inhibits thrombin, and ancrod depletes fibrinogen.
Monocytes are the primary hematopoietic cells that express TF in endotoxemic animals and humans.11 We found that TF deficiency in hematopoietic cells reduced LPS-induced activation of coagulation, IL-6 expression, and decreased mortality in a similar manner to that observed in low TF mice. However, the levels of TAT in WT mice with low TF bone marrow were not as low as those observed in endotoxemic low TF mice, and the reduction in LPSinduced IL-6 did not achieve statistical significance. This suggests that other vascular cells may contribute to the generation of thrombin and IL-6 expression during endotoxemia. Nevertheless, our results demonstrate that hematopoietic cells, presumably the monocyte population, are the major pathologic site of TF expression during endotoxemia. In endotoxemic animals there is a rapid decrease in the number of circulating leukocytes, which suggests that the majority of TF-positive monocytes will be present adhered to the endothelium.3,18 At this site, TF expression may contribute to both fibrin deposition and inflammation.
We found that hirudin inhibition of thrombin prolonged the survival of endotoxemic mice. This protection may be due to a reduction in thrombin-dependent fibrin deposition and platelet activation as well as a reduction in PAR-1 and PAR-4 signaling (Figure 6). However, neither thrombin inhibition nor PAR-1 deficiency reduced inflammation. In contrast, we showed that hirudin significantly inhibited LPS-induced activation of coagulation and fibrin deposition. In addition, we found that ancrod prolonged the survival of endotoxemic mice. These results suggest that hirudin protected endotoxemic mice primarily by reducing fibrin deposition, although we cannot exclude the possibility that hirudin inhibition of platelet activation as well as PAR-1 and PAR-4 signaling also contributed to the protection.
PAR-1 deficiency did not affect the survival of endotoxemic mice and the expression of IL-6. However, loss of PAR-1 may affect both protective and pathologic pathways. For instance, in endotoxemic rats, the endothelial cell protein C receptor was induced in a thrombin-dependent manner, presumably by activation of PAR-1 on endothelial cells.45 In addition, APC activation of PAR-1 may be protective in endotoxemia.46
What mediates the crosstalk between coagulation and inflammation? We found that PAR-1 or PAR-2 deficiency did not affect LPS-induced IL-6 expression or survival. In addition, inhibition of thrombin-dependent signaling did not reduce inflammation. These data suggested that there may be redundancy in the PAR signaling pathways. PAR-1, PAR-2, and PAR-4 may all contribute to the crosstalk. Therefore, we examined the combined effects of inhibition of thrombin-dependent signaling and PAR-2 deficiency to simultaneously inhibit multiple PAR-dependent pathways. Hirudintreated PAR-2−/− mice had significantly reduced IL-6 expression and showed additional protection in survival compared with hirudin-treated PAR-2+/+ mice. These data suggest that PAR-2 signaling as well as thrombin-dependent signaling, most likely via PAR-1 and/or PAR-4, contribute to LPS-induced IL-6 expression in endotoxemic mice. Therefore, inhibition of PAR-dependent signaling in combination with anticoagulants may be an effective strategy in the treatment of human sepsis.47,48
Acknowledgments
We thank M. Riewald for helpful discussions, M. Szeto for technical assistance, and C. Johnson for preparing the manuscript.
Supported by grant HL48872 from the National Institutes of Health.
References
Full text links
Read article at publisher's site: https://doi.org/10.1182/blood-2003-09-3051
Read article for free, from open access legal sources, via Unpaywall:
http://www.bloodjournal.org/content/bloodjournal/103/4/1342.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
The mechanisms behind heatstroke-induced intestinal damage.
Cell Death Discov, 10(1):455, 28 Oct 2024
Cited by: 0 articles | PMID: 39468029 | PMCID: PMC11519599
Review Free full text in Europe PMC
Interpretable Machine Learning Models for Predicting Critical Outcomes in Patients with Suspected Urinary Tract Infection with Positive Urine Culture.
Diagnostics (Basel), 14(17):1974, 06 Sep 2024
Cited by: 0 articles | PMID: 39272758 | PMCID: PMC11394224
Sepsis-Induced Coagulopathy: A Comprehensive Narrative Review of Pathophysiology, Clinical Presentation, Diagnosis, and Management Strategies.
Anesth Analg, 138(4):696-711, 07 Feb 2024
Cited by: 3 articles | PMID: 38324297 | PMCID: PMC10916756
Review Free full text in Europe PMC
Evaluation of the ability of commercial enzyme-linked immunosorbent assays to measure mouse tissue factor.
Res Pract Thromb Haemost, 8(1):102325, 26 Jan 2024
Cited by: 1 article | PMID: 38404939 | PMCID: PMC10883813
Insights into pathophysiology and therapeutic strategies for heat stroke: Lessons from a baboon model.
Exp Physiol, 109(4):484-501, 20 Dec 2023
Cited by: 2 articles | PMID: 38124439 | PMCID: PMC10988686
Review Free full text in Europe PMC
Go to all (190) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Tissue factor, coagulation proteases, and protease-activated receptors in endotoxemia and sepsis.
Crit Care Med, 32(5 suppl):S293-7, 01 May 2004
Cited by: 62 articles | PMID: 15118533
Review
Essential role of platelet activation via protease activated receptor 4 in tissue factor-initiated inflammation.
Arthritis Res Ther, 10(2):R42, 15 Apr 2008
Cited by: 22 articles | PMID: 18412955 | PMCID: PMC2453761
Chronological expression of PAR isoforms in acute liver injury and its amelioration by PAR2 blockade in a rat model of sepsis.
Thromb Haemost, 96(6):830-838, 01 Dec 2006
Cited by: 14 articles | PMID: 17139380
Tissue factor-deficiency and protease activated receptor-1-deficiency reduce inflammation elicited by diet-induced steatohepatitis in mice.
Am J Pathol, 176(1):177-186, 11 Dec 2009
Cited by: 38 articles | PMID: 20008134 | PMCID: PMC2797880
Funding
Funders who supported this work.
NHLBI NIH HHS (4)
Grant ID: HL48872
Grant ID: R01 HL048872
Grant ID: R01 HL048872-04
Grant ID: R01 HL048872-11




