Abstract
Free full text

Cooperation between integrin αvβ3 and VEGFR2 in angiogenesis
Abstract
The cross-talk between receptor tyrosine kinases and integrin receptors are known to be crucial for a number of cellular functions. On endothelial cells, an interaction between integrin αvβ3 and VEGFR2 seems to be particularly important process during vascularization. Importantly, the functional association between VEGFR2 and integrin αvβ3 is of reciprocal nature since each receptor is able to promote activation of its counterpart. This mutually beneficial relationship regulates a number of cellular activities involved in angiogenesis, including endothelial cell migration, survival and tube formation, and hematopoietic cell functions within vasculature. This article discusses several possible mechanisms reported by different labs which mediate formation of the complex between VEGFR-2 and αvβ3 on endothelial cells. The pathological consequences and regulatory events involved in this receptor cross-talk are also presented.
Introduction
Neovascularization in adult organisms or angiogenesis is an essential process in the regulation of various physiological and pathological processes [1]. Modulation of angiogenesis is considered to be a promising target for pharmacological interference in patients with cancer [1–4] and other pathological conditions, including macular degeneration and complications of diabetes [5–7]. Accordingly, the role and mechanisms of angiogenesis have been topics of intensive investigation. From the very early studies of several key investigators in the angiogenesis field [1, 8–10], it has become clear that adhesive receptors and integrins, play very important regulatory roles in neovascularization. Seminal work from Judah Folkman’s lab identified fragments of ECM as modulators of angiogenesis that turned out to be modulators of integrin activity [11]. Results of mechanistic studies allowed development of peptide-based integrin inhibitors and function blocking antibodies which are currently in clinical trials for treatment of cancer [12–14].
Endothelial cells express several integrin heterodimers including αvβ3, α5β1, and αvβ5 [15]. Among these, integrin αvβ3 is expressed at low levels on quiescent endothelial cells in vivo, but is significantly elevated during wound angiogenesis, inflammation [16, 17], and tumor angiogenesis [18–20]. Importantly, expression of this integrin served as a basis for elegant tumor imaging studies [21–23]. Antagonists of integrin αvβ3 inhibit tumor progression by inducing apoptosis of endothelial cells in neo-vasculature without affecting the normal vasculature [18, 19]. Thus, αvβ3 integrin is one of the key regulators of pathological angiogenesis and endothelial functions in general [21, 24, 25]. In this review, we discuss αvβ3 integrin functions in angiogenesis, focusing mainly on the cooperative interactions between this integrin and the receptor for vascular endothelial growth factor (VEGFR).
Integrin αvβ3 in angiogenesis
Studies of numerous groups have demonstrated that antibodies, peptides or peptidomimetics blocking adhesive functions of integrin αvβ3 are able to successfully inhibit angiogenesis in a variety of animal models [12, 26, 27]. However, in apparent disagreement with this, gene ablation of β3 and β3/β5 led to enhanced angiogenesis implanted tumors in mice [28]. Yet, vasculature development seems to be defective in the absence of αvβ3, as evidenced by impaired maturation of coronary capillaries in male β3-null mice [29]. Integrin αv gene knockout in mice resulted in brain hemorrhaging and lethality [30], which is at least in part due to the absence of αvβ8 heterodimer. This notion is supported by the fact that ablation of β8 integrin expression had similar placental and neural vasculature abnormalities [31–33]. The opposite effects of a gene knockout and blocking agents may indicate that there are a plethora of functions for the particular gene, some of which might have opposing effects in a complex biological process such as angiogenesis. An alternative explanation is that β3 knockout in mice might result in compensatory effects during embryogenesis, for instance via up-regulation of other key vascular regulators. In fact, in several models, it was shown that integrin β3-null endothelial cells exhibited enhanced VEGF/VEGFR2 (also known as flk) signaling [28, 29]. Some of these effects in integrin β3-null mice were attributed to increased expression of VEGFR2 on endothelial cells [28]. Some of the compensatory effects can be avoided using knockin mouse models instead of knockouts. Indeed, DiYF-knockin mice expressing defective β3 integrin instead of its normal wild type (two tyrosine residues mutated to phenylalanine: Y747F and Y759F) exhibited impaired angiogenic response, a phenotype similar to that produced by αvβ3-blocking antibodies or peptides [34]. The main lesson from studies on αvβ3 integrin is that this particular receptor regulates not one but several key cellular responses during the multi-stage process of angiogenesis, and interpretation of functional studies is not straight forward. All in vivo data need to be evaluated cautiously considering differences between experimental models. Even within the group of integrin αvβ3 blocking molecules, biological outcomes might differ from one inhibitor to another. In fact, in the field of integrin biology, small molecule inhibitors often produce results distinct from those of antibodies [35–39]. As another example, tumstatin, a naturally occurring proteolytic product of collagen cleavage, seems to block αvβ3 leading to decreased proliferation and increased apoptosis of endothelial cells [40, 41]. A similar function might be attributed to αvβ3-blocking antibodies; however, interactions with divalent antibodies might result in integrin clustering on the cell surface, which in turn, might invoke quite different signaling events [42–44].
Integrin αvβ3–VEGFR2 interactions
Several receptor tyrosine kinases (RTKs) are known to associate with integrins, and this association is essential for the regulation of the kinase activity [25, 45–54]. Initial studies in integrin αvβ3–VEGFR2 interactions performed by Soldi and co-authors [47], showed that phosphorylation of VEGFR2 is enhanced when endothelial cells are plated onto ECM proteins such as vitronectin and fibrinogen, which are ligands for integrin αvβ3 [15]. In contrast, cells in suspension exhibited impaired VEGFR2 phosphorylation in response to VEGF. The anti-integrin αvβ3 antibody BV4, although it did not inhibit cell adhesion to ECM proteins, inhibited phosphorylation of VEGFR2, indicating that αvβ3-augmented VEGFR2 phosphorylation is dependent on the function of integrin αvβ3, not on cell adhesion status. Although at least three key integrins on endothelial cells, αvβ3, αvβ5, and α5β1, were shown to be necessary for an angiogenic response [15], these studies singled out αvβ3 integrin as a regulator of VEGFR2 signaling.
In support of the above findings, another study from Ruoslahti’s lab showed that integrin αvβ3 is able to form complexes with VEGFR2 and PDGFRβ in a CHO cell model system [46]. This study was designed to provide an understanding of the structural aspects of physical interactions between integrin αvβ3 and two receptor tyrosine kinases. This study confirmed that in cells over-expressing integrin subunits and RTKs, integrin β3, but not β1, is able to interact with both VEGFR2 and PDGFRβ. In contrast to the studies using primary endothelial cells and focused on endogenous receptors [47, 55], in this model interactions between RTKs and αvβ3 seemed to be constitutive and did not require either integrin or receptor tyrosine kinase ligation, which might be explained by effects of over-expression. This study demonstrated that integrin αvβ3, in which the β3 cytoplasmic domain was truncated or replaced with integrin β1-cytoplasmic domain, still interacted with VEGFR2 and PDGFRβ, thus indicating that the extracellular domain of β3 is essential for interaction with VEGFR2 or PDGFRβ. Further, this study indicated that while interaction between integrin β3 and VEGFR2 is augmented by the integrin αv subunit, interaction between integrin β3 and PDGFRβ did not require the integrin αv subunit [46]. Importantly, even under conditions of over-expression, VEGFR2 selectively interacted with β3 but not with the β1 integrin subunit, which further emphasized a special regulatory relationship between αvβ3 and VEGF signaling.
Similar to Soldi et al., another study documented a synergistic signaling connection between VEGFR2 and αvβ3 in primary endothelial cells [56]. This cooperation was shown to be required for full phosphorylation of VEGFR2 and the activation of cell motility pathways involving focal adhesion kinase (FAK) and stress-activated protein kinase-2/p38 (SAPK2/p38) in a Hsp90 dependent manner [56]. It was concluded that Hsp90 is associated with VEGFR2, which in turn, is crucial for vinculin recruitment and focal adhesion formation during endothelial cell migration.
The first in vivo evidence of the importance of VEG-FR2-αvβ3 association was reported by our lab [34]. We demonstrated that impaired tumor-induced angiogenesis in DiYF-knockin mice expressing phosphorylation defective β3 integrin is due to the impaired ability of β3 to form a complex with VEGFR2. This study demonstrated that αvβ3 in mouse endothelial cells is able to interact with VEGFR2 when cells are plated on integrin ligand or stimulated by VEGF. Thus, the ligation of either receptor stimulated formation of the complex between β3 and VEGFR2, which in turn, affected VEGFR2 phosphorylation similar to the previous report [47].
Another report from our lab focused on the consequences of the cross-regulation between αvβ3 and VEGFR2 [55]. The study demonstrated that primary endothelial cells (HUVEC) plated on vitronectin, a ligand for integrin αvβ3, exhibited a basal level of VEGFR2 phosphorylation, which was augmented several fold upon VEGF treatment. This increase in VEGFR2 phosphorylation was substrate specific and was not observed when cells were plated on collagen. Further, function blocking antibodies to integrin αv or β3, but not β1 or β5, inhibited VEGFR2 phosphorylation upon VEGF treatment. These data demonstrated functional interconnections between integrin αvβ3 and VEGFR2 that result in up-regulation of the ligand-induced activity of receptor tyrosine kinase by integrin engagement. Although SiRNA mediated knockdown of all three integrins, β1, β3, and β5, in endothelial cells inhibited endothelial tube formation in matrigel, the most profound effect was observed in cells treated with β3 SiRNA, indicating the prominent role of this integrin. Importantly, this study demonstrated that VEGFR2 is associated with an active conformer of αvβ3, which is detected using an engineered Fab fragment of WOW-1 antibody [57] and this association is enhanced upon treatment with VEGF-A or VEGF-DΔNΔC (a truncated variant of VEGF-D that preferentially activated VEGFR2) [58]. Moreover, these interactions between activated αvβ3 and VEGFR2 were observed in vivo. Immunostaining of tumors as well as ischemic tissues revealed co-localization of VEGFR2 with activated αvβ3 in blood vessels.
Further, we investigated the requirements for communication between integrin β3 and VEGFR2 [55]. We showed that SU1498, a specific inhibitor of VEGFR2, inhibited complex formation between VEGFR2 and integrin β3, demonstrating that not only β3 phosphorylation but also VEGFR2 activity is crucial for this interaction [55]. Similarly, SU1498-inhibited activation of αvβ3 was demonstrated, based on WOW-1 binding. Importantly, the cooperation between αvβ3 and VEGFR2 is of a reciprocal nature: activation of each component leads to the augmentation of the other component. While the blockade of integrin β3 inhibited VEGFR2 phosphorylation induced by VEGF, integrin β3-activating antibodies such as AP-7.4, LIBS-1, and CRC54 augmented this process [55]. Overall, these experiments showed that interaction between integrin β3 and VEGFR2 stimulated by interplay between ECM ligands and VEGF is essential for the activation of endothelial cells and stimulation of VEGF-induced angiogenesis both in vitro and in vivo.
Integrin β3-cytoplasmic domain tyrosine residues in αvβ3–VEGFR2 interactions
Integrins have a unique ability to transduce signals in two directions: from outside the cellular environment and from within the cellular compartment by mechanisms known as ‘outside-in’ and ‘inside-out’ signaling, respectively [59]. While outside-in signaling provides the cues from the ECM [60], inside-out signaling, otherwise known as integrin activation, is triggered by stimuli such as growth factors and cytokines via modulation of a structure of the cytoplasmic domain of integrin subunits [61]. Integrin β3 itself is a known substrate for tyrosine phosphorylation [62, 63]. Phosphorylation of tyrosine residues Y747 and Y759 is very prominent upon endothelial adhesion to αvβ3 ligands and is further up-regulated by VEGF treatment [34]. Hence, it appears that at the molecular level, modification of these tyrosine residues is necessary for full-scale VEGFR2 activity. Reciprocally, VEGFR2-induced activation of αvβ3 is also dependent on these modifications. Since the cytoplasmic domain of integrin β3 is necessary for inside-out signaling, a role for integrin β3-cytoplasmic tyrosine residues, involved in recruitment of intracellular signaling molecules such as Shc and Grb-2 [64], becomes apparent. Studies using knockin mice expressing integrin β3 with the two tyrosine residues mutated to phenylalanines (DiYF mice) indicated that VEGF- and FGF-stimulated inside-out activation of αvβ3 are impaired in the absence of these tyrosines [34].
These defects resulted in impaired tumor and wound angiogenesis in vivo [34, 65]. Further analysis revealed that the absence of tyrosine residues within the cytoplasmic domain of integrin β3 prevented VEGF-induced interaction between VEGFR2 and integrin β3. Moreover, these defects in DiYF endothelial cells resulted in impaired phosphorylation of VEGFR2 in response to VEGF. Overall, these studies demonstrated that the cytoplasmic domain of integrin β3, more specifically, the cytoplasmic tyrosine residues, are necessary for the successful functional communication between integrin β3 and VEGFR2. The latter process plays a key regulatory role in tumor induced and wound angiogenesis as well as in the recruitment of bone marrow derived cells into angiogenic sites [34, 65].
Role of Src kinases in integrin αvβ3–VEGFR2 interactions
Outside-in signaling via growth factor receptors and integrins activates a family of tyrosine kinases known as the Src kinases, which are a group of non-receptor tyrosine kinases [66]. Major members of this family include cSrc, Fyn, Lyn, and Yes [67]. Many previous reports have suggested that the Src family of kinases is potential regulators of integrin-growth factor receptor association in vascular cells [68]. Previous studies indicate that interaction between Src with EGFR regulates cell proliferation [76], and association between cSrc and PDGFR induces integrin-dependent cell adhesion and migration [69].
A functional association between Src-family members, integrins, and VEGF receptors is further supported by the analysis of specific knockout mice. Although cSrc null mice develop normal blood vessels, they exhibit impaired vascular permeability in response to VEGF [70, 71] and defects in bone resorption (similar to β3 knockout mice [72, 73]. Interestingly, mice deficient in Yes also exhibit defects in VEGF-induced vascular permeability [74], suggesting non-redundant functions for these two Src kinases in the regulation of vascular permeability. In contrast, Fyn-deficient mice do not exhibit any defect in vascular leakage induced by VEGF [72]. Mice lacking Src kinases cSrc, Yes, and Fyn (SYF) develop blood-filled islands in the embryo, leading to lethality [75]. These reports demonstrate that many of the functions of the Src family of tyrosine kinases are somewhat overlapping. However, together, they are absolutely crucial for the cellular responses to growth factors and vascular development and integrity.
A recent study from our lab has demonstrated the non-redundant function of cSrc in vascular cells in the regulation of integrin αvβ3 and VEGFR2 cross-talk [45]. VEGF stimulation promoted phosphorylation of both Y747 and Y759 in the integrin β3-cytoplasmic domain and complex formation between VEGFR2 and β3. All these responses were further augmented in the presence of the αvβ3 ligand vitronectin [45]. Interestingly, treatment with Src inhibitors completely blocked both phosphorylation of integrin and VEGFR2 as well as VEGF-induced complex formation between VEGFR2 and integrin β3. Analysis of all three key members of the Src family revealed that cSrc, but not Yes or Fyn, is associated with β3 and this association is regulated by VEGF. These data suggested the non-overlapping role of cSrc in the regulation of VEGFR2 and integrin β3 association.
Further analysis was performed to determine the kinase which is recruited to the integrin β3-cytoplasmic domain and which is responsible for the phosphorylation of integrin β3. It was found that VEGFR-mediated recruitment of cSrc but not Fyn or Yes kinase, is responsible for the phosphorylation of Y747 and Y759 within the integrin β3-cytoplasmic domain. This demonstrated the importance of Src kinases in the phosphorylation of integrin β3-cytoplasmic tyrosine residues [45]. Thus physical and functional associations of VEGFR2 and integrin β3 are governed by cSrc, and VEGFR2 kinase activities can be modulated via this interaction. While treatment with Src inhibitor or expression of the dominant negative cSrc inhibited phosphorylation of integrin β3-cytoplasmic tyro-sine residues, expression of the constitutively active form of cSrc resulted in enhanced phosphorylation of both Y747 and Y759. Moreover, SYF(Src, Yes, and Fyn) triple knockout MEF cells exhibited substantially reduced levels of integrin β3 phosphorylation. Most importantly, in vitro phosphorylation assays demonstrated that recombinant cSrc is able to phosphorylate integrin β3. Likewise, a recent study demonstrated that cSrc phosphorylates another β3 integrin, αIIbβ3 in platelets [76]. Specific inactivation of cSrc resulted in inhibition of the complex formation between VEGFR2 and integrin β3, which in turn, caused a reduction in integrin activity. Altogether, these observations point out the direct and specific involvement of cSrc in the regulation of αvβ3-VEGFR2 cross-talk and the resulting integrin and VEGF-dependent cellular responses underlying angiogenesis.
It is possible to argue that both integrin activation and VEGFR2 function are reciprocally linked. The sequence of the molecular events discussed above could be as follows: VEGF treatment induces initial VEGFR2 phosphorylation followed by cSrc recruitment and both events lead to the complex formation between VEGFR2 and β3 integrin (which might involve another yet unknown mediator). All these events promote activation of αvβ3 and result in the increase of ligand binding ability (integrin activation), integrin ligation, and phosphorylation of β3 integrin by cSrc. These events, complex formation in particular, are responsible for prolonged and full activation of VEGFR2 as judged by its phosphorylation status. Thus, there is a clear positive feedback loop mechanism, which makes it difficult to pinpoint the exact sequence of events. It is possible that initial integrin αvβ3 engagement by ECM serves as a prerequisite for interaction with VEGFR2. This model is supported by studies performed using the DiYF-mouse model [34, 45], depicted in Fig. 1.
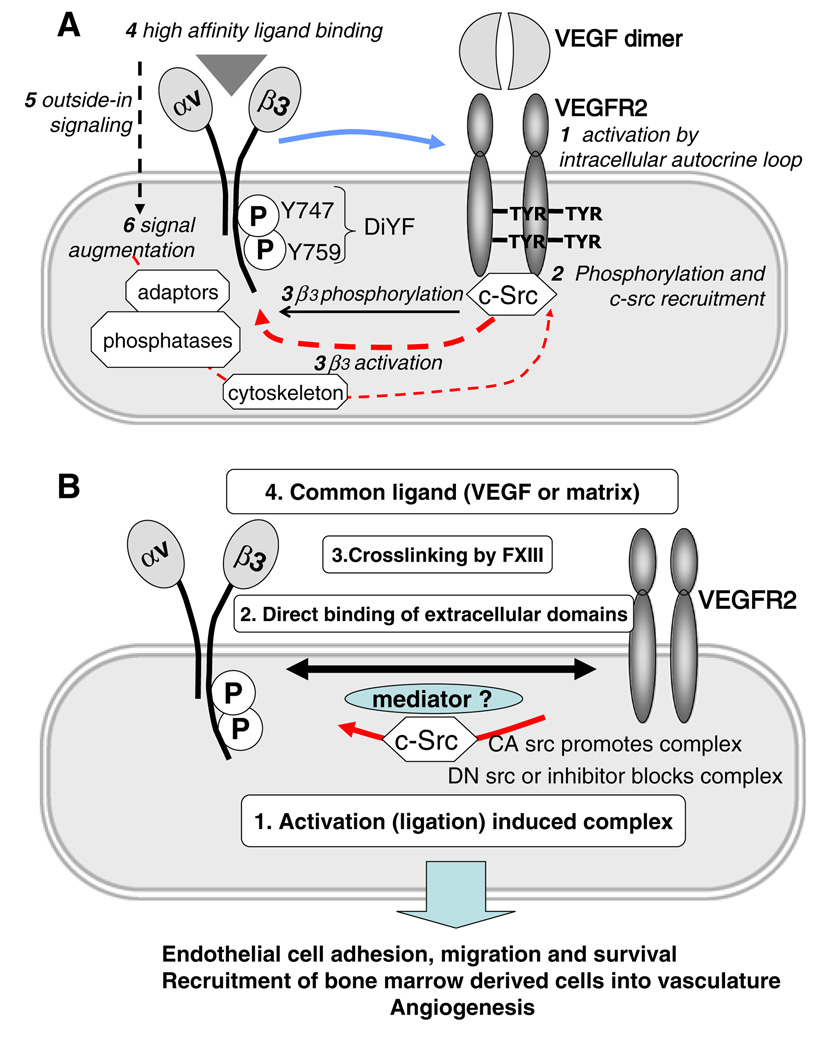
a The cartoon depicts four possible mechanisms responsible for the complex formation between VEGFR2 and αVβ3 on the surface of endothelial cells and the functional consequences of this cross-talk. Activation-induced complex was demonstrated in [48, 55], direct binding of extracellular domains was shown in [47], cross linking by transglutaminases was demonstrated in [87], possible coupling by the common ligands suggested based on [92]. Physiological and pathological consequences of the interplay between these two receptors are discussed in [35, 46, 56]. b The diagram shows possible sequence of molecular events involved in interaction between VEGFR2 and αVβ3. As discussed in the text, the process is likely to be initiated by activation of VEGFR2 by VEGF, possibly, by intracellular-autocrine loop. It followed by the recruitment of c-src to phosphorylated VEGFR2 together with activation of a number of other signaling molecules. C-Src phosphorylates cytoplasmic domain of αVβ3 and in concert with other kinases (e.g. PI3K pathway) promote activation of this integrin, which, in turn, ultimately results in its conformational changes and increase in ligand binding affinity. Ligation of integrin triggers outside-in signaling which further augments cell activation
In fact, the studies using β3-knockout mice also support the model of a close collaboration between VEGFR2 and αvβ3. It was reported by at least two groups that the lack of β3 in endothelial cells leads to over-sensitivity to VEGF [29, 77] and increased expression of VEGFR2. Thus, the imbalance caused by the physical absence of integrin receptor is overcome by expression and over-expression of VEGFR2. The molecular mechanisms of this phenomenon are not yet established. Unoccupied integrin αvβ3 might even, negatively, block VEGFR2 activity. This function is preserved in DiYF animals, in contrast to integrin β3-null mice. Interestingly, many of the abnormalities observed in integrin β3-null mice have no manifestation in DiYF mice, possibly reflecting the basal adhesive function of the integrin is preserved in this mutant. It remains to be tested if other integrins might assume co-signaling functions in integrin β3-null animals, or if other structurally unrelated molecules might fulfill this function as well. It will be of interest to see whether VEGFR2 complexes with other proteins are augmented in the absence of integrin β3, and whether the blocking antibodies against these molecules exert any inhibitory effect on VEGFR2 in the absence of integrin β3. It is possible that the limitations of currently available treatments to block tumor angiogenesis will keep efforts to clarify the current complexity of data proceeding at a fast pace.
Integrin αvβ3–growth factor receptor interactions in outside-in signaling
Involvement of integrin αvβ3 in the activation of tyrosine kinase receptors has been shown by various labs and recently has been reviewed in detail [25, 45–53]. In smooth muscle cells, activation and clustering of integrin β3 promoted the EGF-dependent tyrosine phosphorylation of EGFR [78]. Treatment with integrin αvβ3 function blocking antibody inhibits EGF-stimulated EGFR phosphorylation and cell proliferation [78] Integrin αvβ3 has been reported to interact with PDGFRβ and modulates the PDGFRβ function in fibroblasts [48] and endothelial cells [79]. Moreover, integrin αvβ3 is also known to interact with IRS-1 and regulate the activity of insulin receptor and IGF1-R [49]. Interactions between integrin αvβ3 and HGF receptor ‘Met’ have also been reported earlier in epithelial cells [80]. A more recent study indicates interactions between integrin αvβ3 and HGF receptor in endothelial cells [81]. Overall, these studies show the importance of integrin αvβ3 and its association with various receptor tyrosine kinases in the regulation of cellular functions.
Auto-phosphorylation and activation of VEGFR2, which are responsible for most of the angiogenic activity triggered by VEGF, occur when VEGFR2 interacts with dimeric VEGF [82]. Via its tyrosine phosphorylated cytoplasmic domain, VEGFR2 interacts with an army of intracellular signaling and adapter molecules such as Shc, Grb2, Nck, Ras activating protein, Src kinases, and tyrosine phosphatases SHP-1 and SHP-2 [83–85], and triggers the signaling cascades that include activation of PI3 Kinase-Akt and MAP kinase pathways [86]. Thus, integrin-dependent regulation of VEGFR2 function has consequences for a variety of cellular processes downstream of VEGF.
Yet another possible mechanism for the cross-talk between VEGFR2 and αvβ3 has been demonstrated [87–89]. It was shown that thrombin-activated plasma transglutaminase FXIII is able to cross-link integrin αvβ3 and VEGFR2 on the surface of endothelial cells. Importantly, tissue transglutaminase also appears to have a similar function [89]. It appears that mere complex formation is sufficient to induce VEGFR2 phosphorylation and evokes downstream signaling events even in the absence of exogenous VEGF. However, there is the possibility of a constant and constitutive activation of VEGFR2 by an intracellular autocrine loop mechanism [90]. Such a mechanism explains why it was believed for many years that integrins on endothelial cells are constitutively active [57]. It appears that it is impossible to obtain completely quiescent and unstimulated endothelial cells in vivo or in vitro [34, 91, 92].
Another possibility is the ligation of both αvβ3 and VEGF by the same adhesive ligand. Indeed, common ligands for αvβ3 and VEGF have been reported [93]. It was also shown that αvβ3 is able to bind certain forms of VEGF itself [94]. It will be intriguing to test whether at least a portion of αvβ3/VEGFR2 duplexes is directly linked by VEGF dimers, similar to the neuropilin-1/VEGF/VEGFR2 trimeric complex [95–97].
Other possible components involved in integrin αvβ3–growth factor receptor interactions
The Src homology 2 containing protein phosphatase (SHP-2) is a tyrosine phosphatase whose recruitment to signaling molecules is stimulated by many growth factors [98]. Initial reports related to the role of SHP-2 in the modulation of interaction between growth factors and integrins came from David Clemmons’ laboratory [98–100], where a role for SHP-2 in the regulation of dimerization of IGF-I receptor and αvβ3 integrin in smooth muscle cells was demonstrated. Inhibition of integrin αvβ3 blocked the recruitment of SHP-2 to integrin αvβ3 and its transfer to downstream signaling molecules. In contrast, ligand occupancy of integrin αvβ3 stimulated tyrosine phosphorylation of the integrin β3 subunit, resulting in recruitment of SHP-2, involving an insulin receptor substrate-1 (IRS-1) related protein called DOK-1 [101, 102]. Subsequently, SHP-2 is transferred to another transmembrane protein called SHPS-1, which requires phosphorylation at its two YXXL motifs for interaction with SHP-2 [102]. Once integrin αvβ3 is blocked, SHP-2 is transferred to IGF-I receptor, specifically to the tyrosine residues in the cytoplasmic domain of IGF-1 receptor, resulting in its dephosphorylation and inactivation [103]. Further, augmentation of SHP-2 association with integrin αvβ3 results in complex formation between integrin αvβ3 and IGF-I receptor, followed by transduction of signals to activate intra-cellular pathways such as PI3 kinase-Akt signaling [104] and pro-survival effects mediated by ECM proteins via up-regulation of anti-apoptotic Bcl(xL) [105].
While most of the studies on the role of SHP-2 in regulating interaction between integrin αvβ3 and IGF-I receptor were performed using smooth muscle cells, recent studies using endothelial cells confirmed the importance of SHP-2 in the regulation of interaction between integrin αvβ3 and VEGFR2 [106]. The study from Federico Bussolino’s lab showed that while vitronectin positively regulated VEGFR2 activation, collagen I negatively regulated VEGF-mediated VEGFR2 activation by recruiting SHP-2 to VEGFR2 [106]. The study also showed that stimulation with VEGF in endothelial cells plated on collagen I initiated the complex formation between integrin αvβ3 and VEGFR2 [106]. Conversely, expression of a SHP-2 mutant (SHP-2 C459S) blocked the negative effects of collagen I. Further studies revealed that SHP-2-dependent dephosphorylation of VEGFR2-cytoplasmic domain tyrosine residues results in the internalization of VEGFR2. Expression with SHP-2 C459S mutant inhibited collagen I mediated internalization of VEGFR2. Further analysis utilizing mutations of the tyrosine residues Y1212 and Y1173 on the cytoplasmic domain of VEGFR2 (Y1212F and Y113F, respectively) revealed that residue Y1173 is responsible for the interaction with SHP-2.
Another phosphatase, DEP-1, has been proposed as a positive regulator of VEGFR2 biological effects through dephosphorylation of Src Y529 [107, 108]. Surprisingly, DEP-1 also targeted p-Tyr in the VEGFR2 kinase loop, but the effect seems to be of less functional consequence than up-regulation of Src kinase activity through dephosphorylation of Y529. Increased src activity led to up-regulated Akt-1 activation known to mediate VEGFR2 effects in endothelial cells. Of note, other RTKs reported to function in complexes with integrins, PDGFRβ Met/HGF-R, and EGFR, have been demonstrated to serve as DEP-1 substrates as well.
A recent study indicates that another protein tyrosine phosphatase called T-Cell protein tyrosine phosphatase (TCPTP), also known as PTN2, is highly expressed in human endothelial cells and is involved in VEGFR2 signaling [109]. Authors showed that a TCPTP substrate-trapping mutant interacts with VEGFR2. Additionally, TCPTP dephosphorylates VEGFR2 in a phosphosite-specific manner, inhibiting its kinase activity thus preventing internalization of the VEGFR2 from the cell surface. Activity of TCPTP was induced upon integrin-mediated binding of endothelial cells to collagen matrix. TCPTP activation was also induced by using cell-permeable peptides from the cytoplasmic tail of the collagen-binding integrin α1. Positive regulation of TCPTP activity resulted in inhibition of VEGF-mediated endothelial cell proliferation, angiogenic sprouting, chemokinesis, and chemotaxis, thus implicating that that matrix-controlled TCPTP phosphatase activity inhibits VEGFR2 signaling, migration, and differentiation of human endothelial cells.
VE-cadherin is another protein shown to take part in VEGFR2 signaling and capable of forming complexes with VEGFR2 [110]. Another layer of complexity is added by the finding that VE-cadherin knock-down blocks VEGFR2 signaling [111]. Similar to integrin αvβ3 integrin, SHP2 is recruited to VE-cadherins, which positively influence VEGFR2 signaling [112]. In summary, the role of integrin αvβ3 in VEGFR2 signaling should be considered in the context of all other co-signaling proteins and alternative VEGF receptors (such as neuropilin-1). In sum, additional studies will be needed to address the interplay of all the signaling intermediates between each other, in addition to their effects on VEGFR2.
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s10456-009-9141-9
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2863048
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/100124699
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1007/s10456-009-9141-9
Article citations
Heyde Syndrome Unveiled: A Case Report with Current Literature Review and Molecular Insights.
Int J Mol Sci, 25(20):11041, 14 Oct 2024
Cited by: 0 articles | PMID: 39456826 | PMCID: PMC11507012
Review Free full text in Europe PMC
Integrin αVβ3 antagonist-c(RGDyk) peptide attenuates the progression of ossification of the posterior longitudinal ligament by inhibiting osteogenesis and angiogenesis.
Mol Med, 30(1):57, 02 May 2024
Cited by: 1 article | PMID: 38698308 | PMCID: PMC11067224
Treatment-associated imaging changes in newly diagnosed MGMT promoter-methylated glioblastoma undergoing chemoradiation with or without cilengitide.
Neuro Oncol, 26(5):902-910, 01 May 2024
Cited by: 0 articles | PMID: 38219019 | PMCID: PMC11066942
Prevascularized spongy-like hydrogels maintain their angiogenic potential after prolonged hypothermic storage.
Bioact Mater, 37:253-268, 28 Mar 2024
Cited by: 0 articles | PMID: 38585489 | PMCID: PMC10997873
ID4-dependent secretion of VEGFA enhances the invasion capability of breast cancer cells and activates YAP/TAZ via integrin β3-VEGFR2 interaction.
Cell Death Dis, 15(2):113, 06 Feb 2024
Cited by: 1 article | PMID: 38321003 | PMCID: PMC10847507
Go to all (157) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis.
Circ Res, 101(6):570-580, 19 Jul 2007
Cited by: 189 articles | PMID: 17641225 | PMCID: PMC2723825
Vascular endothelial-cadherin stimulates syndecan-1-coupled insulin-like growth factor-1 receptor and cross-talk between αVβ3 integrin and vascular endothelial growth factor receptor 2 at the onset of endothelial cell dissemination during angiogenesis.
FEBS J, 280(10):2194-2206, 11 Feb 2013
Cited by: 52 articles | PMID: 23331867 | PMCID: PMC3640762
Recombinant RGD-disintegrin DisBa-01 blocks integrin αvβ3 and impairs VEGF signaling in endothelial cells.
Cell Commun Signal, 17(1):27, 20 Mar 2019
Cited by: 20 articles | PMID: 30894182 | PMCID: PMC6425665
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: P01 HL073311
Grant ID: P01 HL073311-01A10004





