Abstract
Free full text

Molecular mechanism in the formation of a human ring chromosome 21.
Abstract
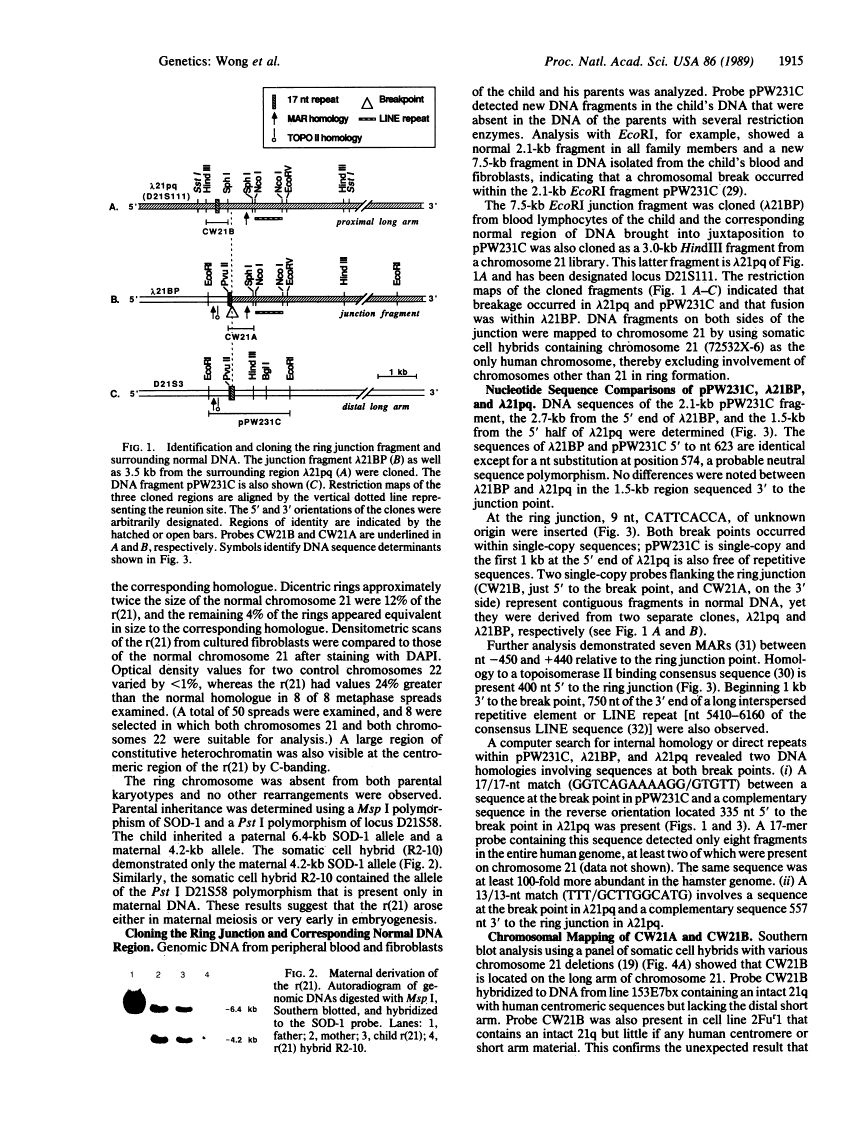
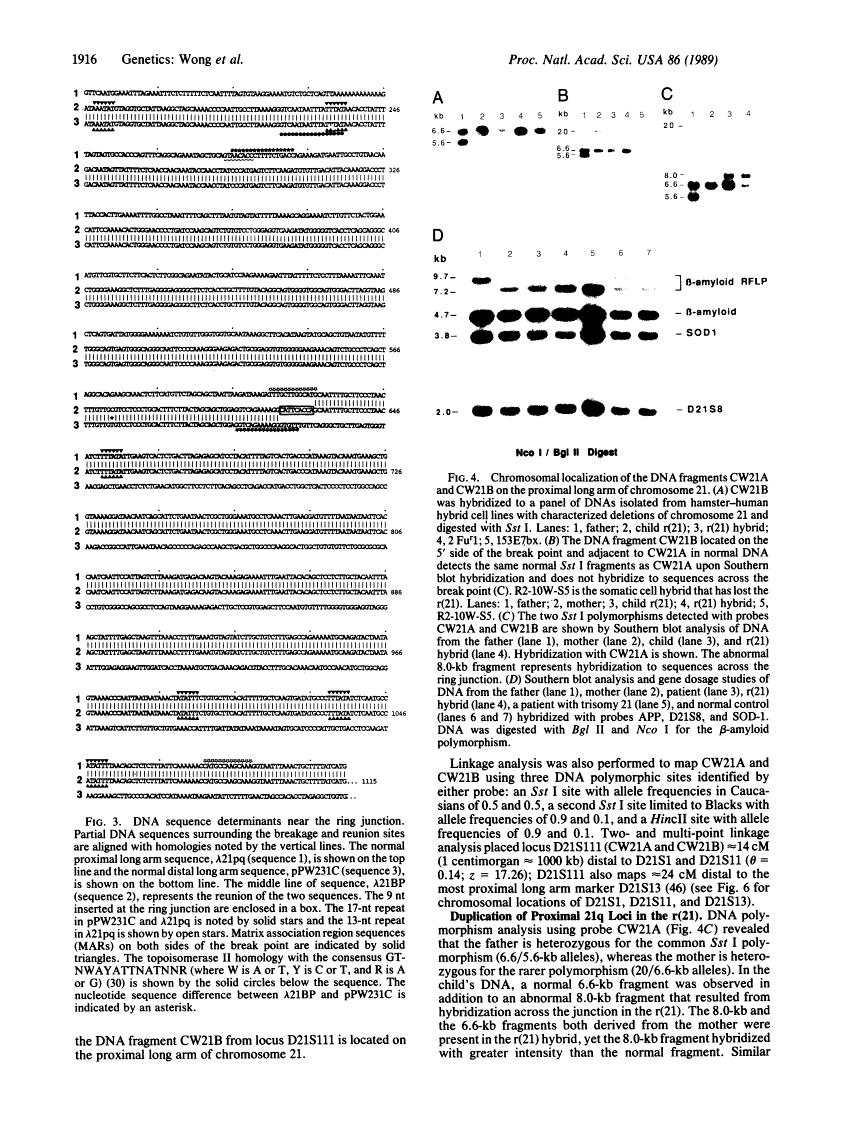
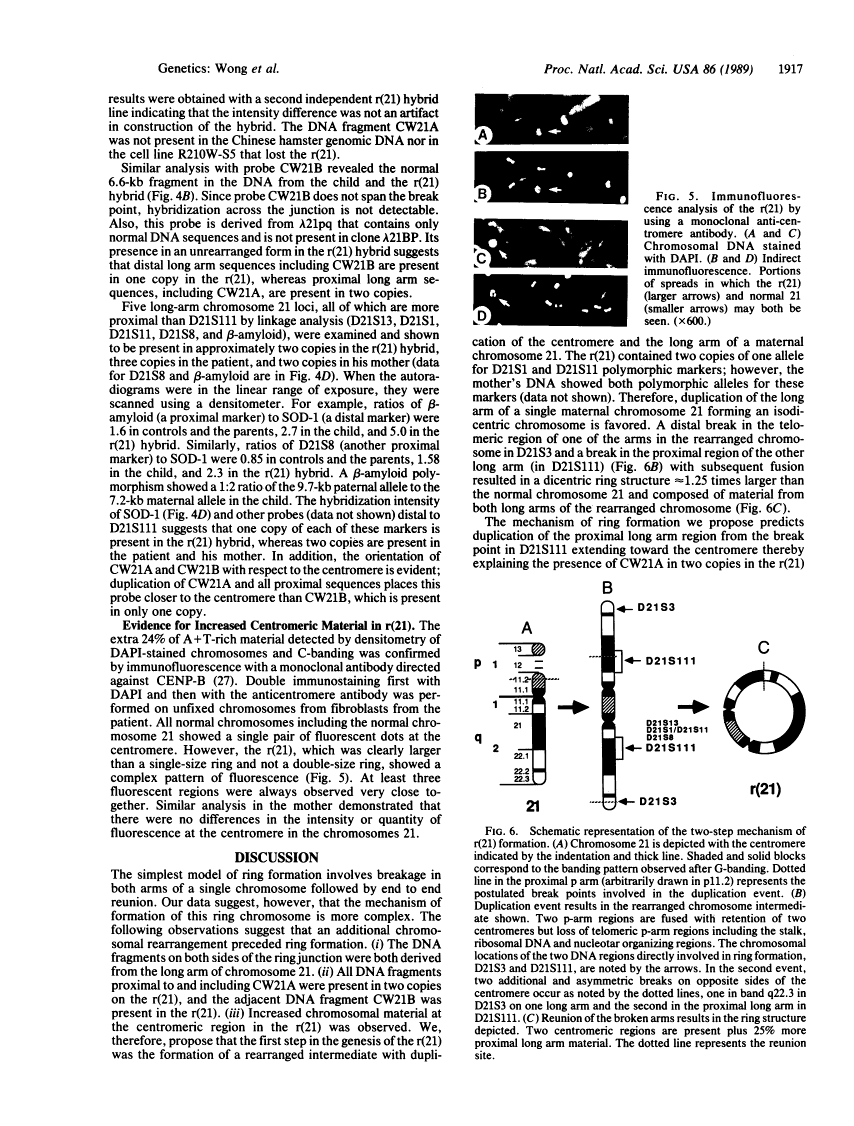
We have characterized the structural rearrangements of a chromosome 21 that led to the de novo formation of a human ring chromosome 21 [r(21)]. Molecular cloning and chromosomal localization of the DNA regions flanking the ring junction provide evidence for a long arm to long arm fusion in formation of the r(21). In addition, the centromere and proximal long arm region of a maternal chromosome 21 are duplicated in the r(21). Therefore, the mechanism in formation of the r(21) was complex involving two sequential chromosomal rearrangements. (i) Duplication of the centromere and long arm of one maternal chromosome 21 occurred forming a rearranged intermediate. (ii) Chromosomal breaks in both the proximal and telomeric long arm regions on opposite arms of this rearranged chromosome occurred with subsequent reunion producing the r(21).
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.3M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Zdansky R, Bühler EM, Vest M, Bühler UK, Stalder G. Familiäres Mosaik mit G-Ring. Humangenetik. 1969;7(4):275–286. [Abstract] [Google Scholar]
- Palmer CG, Hodes ME, Reed T, Kojetin J. Four new cases of ring 21 and 22 including familial transmission of ring 21. J Med Genet. 1977 Feb;14(1):54–60. [Europe PMC free article] [Abstract] [Google Scholar]
- Matsubara T, Nakagome Y, Ogasawara N, Oka S, Yokochi T. Maternally transmitted extra ring (21) chromosome in a boy with Down's syndrome. Hum Genet. 1982;60(1):78–79. [Abstract] [Google Scholar]
- Schmid W, Tenconi R, Baccichetti C, Caufin D, Schinzel A. Ring chromosome 21 in phenotypically apparently normal persons: report of two families from Switzerland and Italy. Am J Med Genet. 1983 Nov;16(3):323–329. [Abstract] [Google Scholar]
- Jacobs PA. Mutation rates of structural chromosome rearrangements in man. Am J Hum Genet. 1981 Jan;33(1):44–54. [Europe PMC free article] [Abstract] [Google Scholar]
- Lejeune J. De la duplication de structures circulaires. Ann Genet. 1968 Jun;11(2):71–77. [Abstract] [Google Scholar]
- Niebuhr E. Reexamination of a family with a t(13q14q) and a ring D(13) child. Ann Genet. 1973 Sep;16(3):199–202. [Abstract] [Google Scholar]
- Orye E, Craen M. A t(21q21q) ring chromosome. Hum Hered. 1974;24(3):253–258. [Abstract] [Google Scholar]
- Ieshima A, Ogasawara N, Yamamoto Y, Kuroki Y. A case of r(21) with stigmata of atypical Down syndrome. Hum Genet. 1980;55(1):65–69. [Abstract] [Google Scholar]
- Dallapiccola B, Bianco I, Brinchi V, Santulli B, Scarano G, Sicolo A, Stabile M, Ventruto V. t(21q21q)/r[t(21q21q)] mosaic in two unrelated patients with mild stigmata of Down's syndrome. Ann Genet. 1982;25(1):56–58. [Abstract] [Google Scholar]
- de Almeida JC, Llerena JC, Jr, Gomes DM, Martins RR, Pereira ET. Ring 13 in an adult male with a 13:13 translocation mother. Ann Genet. 1983;26(2):112–115. [Abstract] [Google Scholar]
- Neri G, Ricci R, Pelino A, Bova R, Tedeschi B, Serra A. A boy with ring chromosome 15 derived from a t(15q;15q) Robertsonian translocation in the mother: cytogenetic and biochemical findings. Am J Med Genet. 1983 Feb;14(2):307–314. [Abstract] [Google Scholar]
- Mules EH, Stamberg J, Jabs EW, Leonard CO. Two different structural abnormalities of chromosome 13 in offspring of chromosomally normal parents with two fragile sites. Clin Genet. 1983 May;23(5):380–385. [Abstract] [Google Scholar]
- Kunkel LM, Smith KD, Boyer SH, Borgaonkar DS, Wachtel SS, Miller OJ, Breg WR, Jones HW, Jr, Rary JM. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. [Europe PMC free article] [Abstract] [Google Scholar]
- Watkins PC, Tanzi RE, Gibbons KT, Tricoli JV, Landes G, Eddy R, Shows TB, Gusella JF. Isolation of polymorphic DNA segments from human chromosome 21. Nucleic Acids Res. 1985 Sep 11;13(17):6075–6088. [Europe PMC free article] [Abstract] [Google Scholar]
- Van Keuren ML, Watkins PC, Drabkin HA, Jabs EW, Gusella JF, Patterson D. Regional localization of DNA sequences on chromosome 21 using somatic cell hybrids. Am J Hum Genet. 1986 Jun;38(6):793–804. [Europe PMC free article] [Abstract] [Google Scholar]
- Lieman-Hurwitz J, Dafni N, Lavie V, Groner Y. Human cytoplasmic superoxide dismutase cDNA clone: a probe for studying the molecular biology of Down syndrome. Proc Natl Acad Sci U S A. 1982 May;79(9):2808–2811. [Europe PMC free article] [Abstract] [Google Scholar]
- Robakis NK, Ramakrishna N, Wolfe G, Wisniewski HM. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4190–4194. [Europe PMC free article] [Abstract] [Google Scholar]
- Kao FT, Puck TT. Induction and isolation of auxotrophic mutants in mammalian cells. Methods Cell Biol. 1974;8(0):23–39. [Abstract] [Google Scholar]
- Benton WD, Davis RW. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. [Abstract] [Google Scholar]
- Williamson DH, Fennell DJ. The use of fluorescent DNA-binding agent for detecting and separating yeast mitochondrial DNA. Methods Cell Biol. 1975;12:335–351. [Abstract] [Google Scholar]
- Earnshaw WC, Migeon BR. Three related centromere proteins are absent from the inactive centromere of a stable isodicentric chromosome. Chromosoma. 1985;92(4):290–296. [Abstract] [Google Scholar]
- Earnshaw WC, Sullivan KF, Machlin PS, Cooke CA, Kaiser DA, Pollard TD, Rothfield NF, Cleveland DW. Molecular cloning of cDNA for CENP-B, the major human centromere autoantigen. J Cell Biol. 1987 Apr;104(4):817–829. [Europe PMC free article] [Abstract] [Google Scholar]
- Stetten G, Sroka B, Corson VL, Boehm CD. Prenatal detection of an unstable ring 21 chromosome. Hum Genet. 1984;68(4):310–313. [Abstract] [Google Scholar]
- Kazazian HH, Jr, Antonarakis SE, Wong C, Trusko SP, Stetten G, Oliver M, Potter MJ, Gusella JF, Watkins PC. Ring chromosome 21: characterization of DNA sequences at sites of breakage and reunion. Ann N Y Acad Sci. 1985;450:33–42. [Abstract] [Google Scholar]
- Sander M, Hsieh TS. Drosophila topoisomerase II double-strand DNA cleavage: analysis of DNA sequence homology at the cleavage site. Nucleic Acids Res. 1985 Feb 25;13(4):1057–1072. [Europe PMC free article] [Abstract] [Google Scholar]
- Cockerill PN, Garrard WT. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986 Jan 31;44(2):273–282. [Abstract] [Google Scholar]
- Scott AF, Schmeckpeper BJ, Abdelrazik M, Comey CT, O'Hara B, Rossiter JP, Cooley T, Heath P, Smith KD, Margolet L. Origin of the human L1 elements: proposed progenitor genes deduced from a consensus DNA sequence. Genomics. 1987 Oct;1(2):113–125. [Europe PMC free article] [Abstract] [Google Scholar]
- Fryns JP, Kleczkowska A. Ring chromosome 21 in the mother and 21/21 translocation in the fetus: karyotype: 45,XX,-21,-21,+t(21;21)(p11;q11). Ann Genet. 1987;30(2):109–110. [Abstract] [Google Scholar]
- Fryns JP, Van den Berghe H. Ring chromosome 22 in a mentally retarded child and mosaic 45,XX,-15,-22,+t(15;22)(p11;q11)/46,XX,r(22)/46,XX karyotype in the mother. Hum Genet. 1979 Mar 12;47(2):213–216. [Abstract] [Google Scholar]
- Piccoli SP, Caimi PG, Cole MD. A conserved sequence at c-myc oncogene chromosomal translocation breakpoints in plasmacytomas. Nature. 310(5975):327–330. [Abstract] [Google Scholar]
- Tsujimoto Y, Gorham J, Cossman J, Jaffe E, Croce CM. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985 Sep 27;229(4720):1390–1393. [Abstract] [Google Scholar]
- Mager DL, Henthorn PS, Smithies O. A Chinese G gamma + (A gamma delta beta)zero thalassemia deletion: comparison to other deletions in the human beta-globin gene cluster and sequence analysis of the breakpoints. Nucleic Acids Res. 1985 Sep 25;13(18):6559–6575. [Europe PMC free article] [Abstract] [Google Scholar]
- Henthorn PS, Mager DL, Huisman TH, Smithies O. A gene deletion ending within a complex array of repeated sequences 3' to the human beta-globin gene cluster. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5194–5198. [Europe PMC free article] [Abstract] [Google Scholar]
- Jennings MW, Jones RW, Wood WG, Weatherall DJ. Analysis of an inversion within the human beta globin gene cluster. Nucleic Acids Res. 1985 Apr 25;13(8):2897–2906. [Europe PMC free article] [Abstract] [Google Scholar]
- Berezney R, Coffey DS. Identification of a nuclear protein matrix. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1410–1417. [Abstract] [Google Scholar]
- Earnshaw WC, Heck MM. Localization of topoisomerase II in mitotic chromosomes. J Cell Biol. 1985 May;100(5):1716–1725. [Europe PMC free article] [Abstract] [Google Scholar]
- Ottolenghi S, Giglioni B. The deletion in a type of delta 0-beta 0-thalassaemia begins in an inverted AluI repeat. Nature. 1982 Dec 23;300(5894):770–771. [Abstract] [Google Scholar]
- Jagadeeswaran P, Tuan D, Forget BG, Weissman SM. A gene deletion ending at the midpoint of a repetitive DNA sequence in one form of hereditary persistence of fetal haemoglobin. Nature. 1982 Apr 1;296(5856):469–470. [Abstract] [Google Scholar]
- Chimera JA, Musich PR. The association of the interspersed repetitive KpnI sequences with the nuclear matrix. J Biol Chem. 1985 Aug 5;260(16):9373–9379. [Abstract] [Google Scholar]
- Warren RJ, Rimoin DL. The G deletion syndromes. J Pediatr. 1970 Oct;77(4):658–663. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.86.6.1914
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc286815?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.86.6.1914
Article citations
The past, present, and future for constitutional ring chromosomes: A report of the international consortium for human ring chromosomes.
HGG Adv, 3(4):100139, 10 Sep 2022
Cited by: 2 articles | PMID: 36187226 | PMCID: PMC9519620
Review Free full text in Europe PMC
Thrombocytopenia and Predisposition to Acute Myeloid Leukemia due to Mosaic Ring 21 with Loss of RUNX1: Cytogenetic and Molecular Characterization.
Mol Syndromol, 9(6):306-311, 09 Nov 2018
Cited by: 5 articles | PMID: 30800047 | PMCID: PMC6381886
Unique genomic structure and distinct mitotic behavior of ring chromosome 21 in two unrelated cases.
Cytogenet Genome Res, 136(3):180-187, 07 Mar 2012
Cited by: 8 articles | PMID: 22398511
Stability of monocentric and dicentric ring minichromosomes in Arabidopsis.
Chromosome Res, 19(8):999-1012, 29 Oct 2011
Cited by: 3 articles | PMID: 22038284
Pallister-Killian syndrome caused by mosaicism for a supernumerary ring chromosome 12p.
Am J Med Genet A, 149A(3):505-509, 01 Mar 2009
Cited by: 5 articles | PMID: 19215037
Go to all (29) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Regional assignment of six polymorphic DNA sequences on chromosome 21 by in situ hybridization to normal and rearranged chromosomes.
Am J Hum Genet, 42(4):542-549, 01 Apr 1988
Cited by: 8 articles | PMID: 3348217 | PMCID: PMC1715241
Characterization of a ring chromosome 21 by FISH-technique.
Clin Genet, 48(4):188-191, 01 Oct 1995
Cited by: 6 articles | PMID: 8591669
Mechanisms of ring chromosome formation in 11 cases of human ring chromosome 21.
Am J Hum Genet, 50(1):15-28, 01 Jan 1992
Cited by: 72 articles | PMID: 1346075 | PMCID: PMC1682523
Mapping small DNA sequences by fluorescence in situ hybridization directly on banded metaphase chromosomes.
Proc Natl Acad Sci U S A, 87(16):6223-6227, 01 Aug 1990
Cited by: 71 articles | PMID: 2201023 | PMCID: PMC54505