Abstract
Free full text

Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats.
Abstract
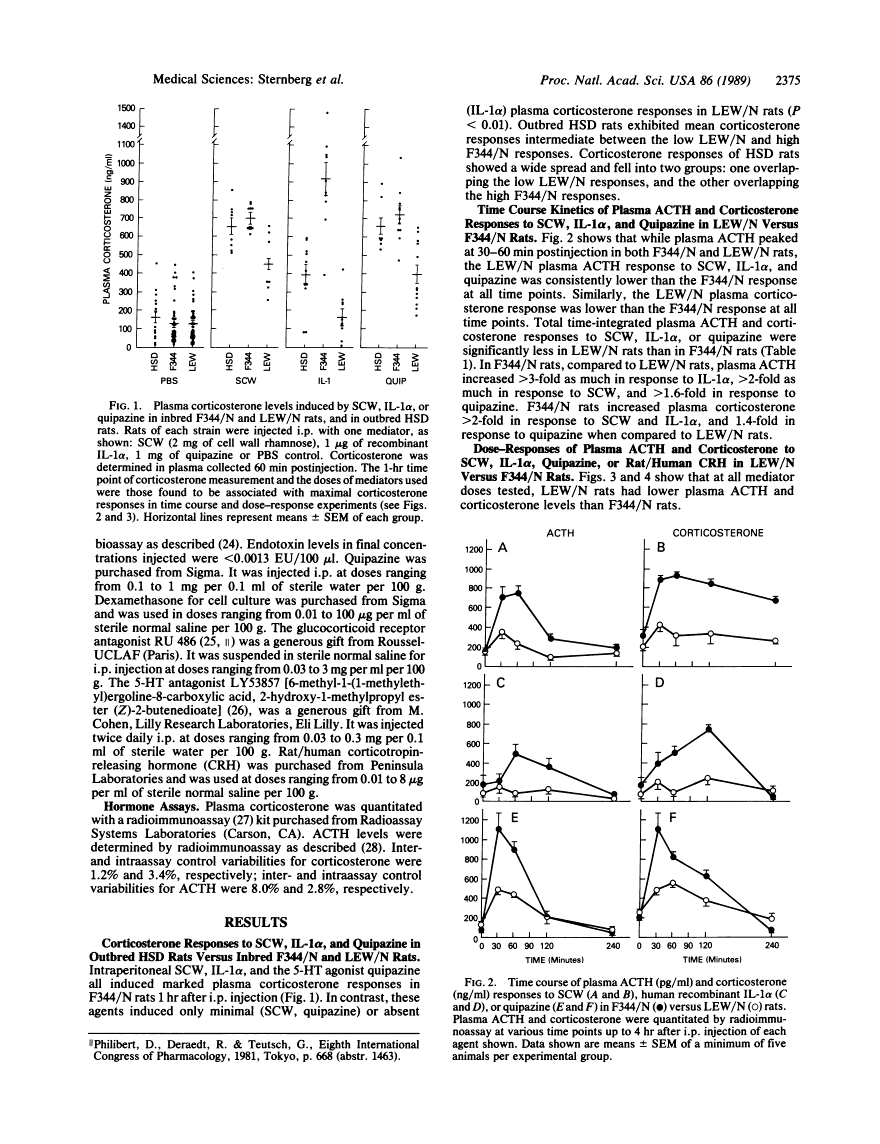
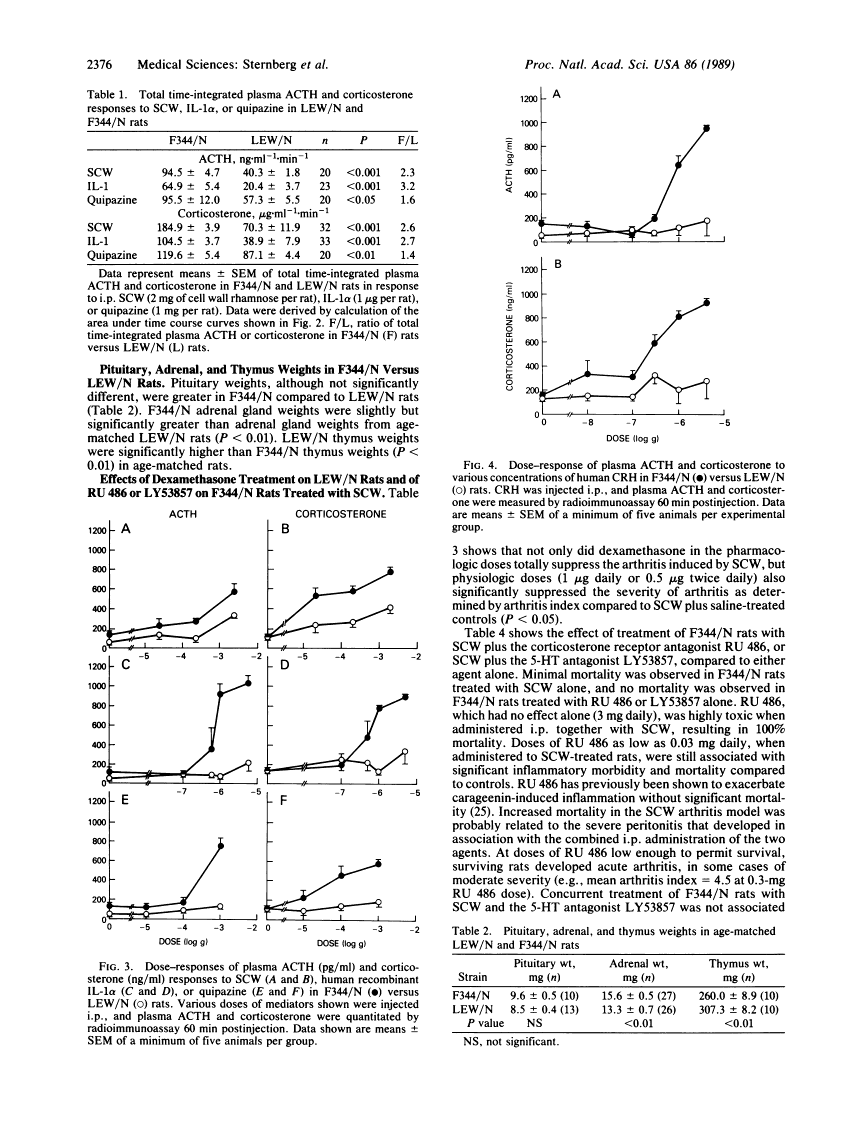
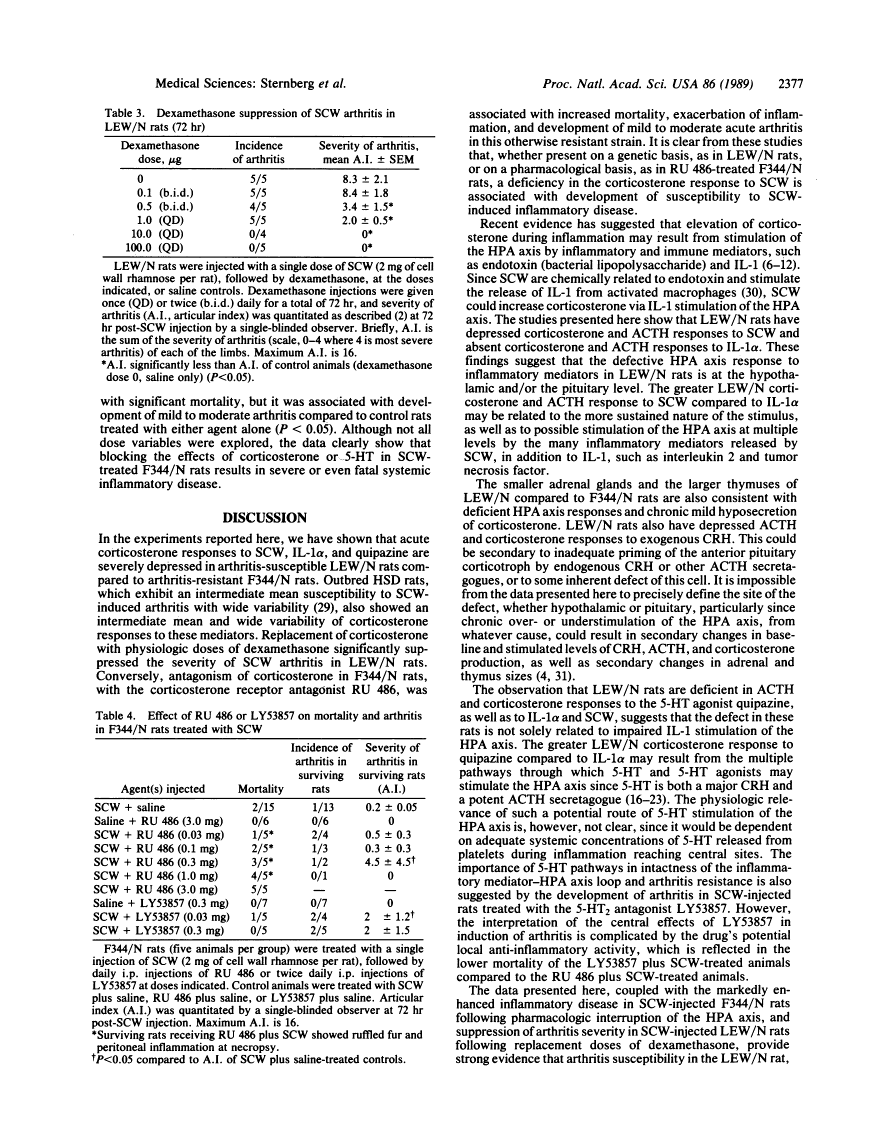
Inbred Lewis (LEW/N) female rats develop an arthritis in response to group A streptococcal cell wall peptidoglycan polysaccharide (SCW), which mimics human rheumatoid arthritis. Histocompatible Fischer (F344/N) rats do not develop arthritis in response to the same SCW stimulus. To evaluate this difference in inflammatory reactivity, we examined the function of the hypothalamic-pituitary-adrenal (HPA) axis and its ability to modulate the development of the inflammatory response in LEW/N and F344/N rats. We have found that, in contrast to F344/N rats, LEW/N rats had markedly impaired plasma corticotropin and corticosterone responses to SCW, recombinant human interleukin 1 alpha, the serotonin agonist quipazine, and synthetic rat/human corticotropin-releasing hormone. LEW/N rats also had smaller adrenal glands and larger thymuses. Replacement doses of dexamethasone decreased the severity of LEW/N rats' SCW-induced arthritis. Conversely, treatment of F344/N rats with the glucocorticoid receptor antagonist RU 486 or the serotonin antagonist LY53857 was associated with development of severe inflammatory disease, including arthritis, in response to SCW. These findings support the concept that susceptibility of LEW/N rats to SCW arthritis is related to defective HPA axis responsiveness to inflammatory and other stress mediators and that resistance of F344/N rats to SCW arthritis is regulated by an intact HPA axis-immune system feedback loop.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1009K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Wilder RL, Allen JB, Hansen C. Thymus-dependent and -independent regulation of Ia antigen expression in situ by cells in the synovium of rats with streptococcal cell wall-induced arthritis. Differences in site and intensity of expression in euthymic, athymic, and cyclosporin A-treated LEW and F344 rats. J Clin Invest. 1987 Apr;79(4):1160–1171. [Europe PMC free article] [Abstract] [Google Scholar]
- Snyder DS, Unanue ER. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J Immunol. 1982 Nov;129(5):1803–1805. [Abstract] [Google Scholar]
- Cupps TR, Fauci AS. Corticosteroid-mediated immunoregulation in man. Immunol Rev. 1982;65:133–155. [Abstract] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984 Winter;5(1):25–44. [Abstract] [Google Scholar]
- Fontana A, Weber E, Dayer JM. Synthesis of interleukin 1/endogenous pyrogen in the brain of endotoxin-treated mice: a step in fever induction? J Immunol. 1984 Oct;133(4):1696–1698. [Abstract] [Google Scholar]
- Woloski BM, Smith EM, Meyer WJ, 3rd, Fuller GM, Blalock JE. Corticotropin-releasing activity of monokines. Science. 1985 Nov 29;230(4729):1035–1037. [Abstract] [Google Scholar]
- Bernton EW, Beach JE, Holaday JW, Smallridge RC, Fein HG. Release of multiple hormones by a direct action of interleukin-1 on pituitary cells. Science. 1987 Oct 23;238(4826):519–521. [Abstract] [Google Scholar]
- Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987 Oct 23;238(4826):522–524. [Abstract] [Google Scholar]
- Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987 Oct 23;238(4826):524–526. [Abstract] [Google Scholar]
- Uehara A, Gottschall PE, Dahl RR, Arimura A. Interleukin-1 stimulates ACTH release by an indirect action which requires endogenous corticotropin releasing factor. Endocrinology. 1987 Oct;121(4):1580–1582. [Abstract] [Google Scholar]
- Pert CB, Ruff MR, Weber RJ, Herkenham M. Neuropeptides and their receptors: a psychosomatic network. J Immunol. 1985 Aug;135(2 Suppl):820s–826s. [Abstract] [Google Scholar]
- Farrar WL, Kilian PL, Ruff MR, Hill JM, Pert CB. Visualization and characterization of interleukin 1 receptors in brain. J Immunol. 1987 Jul 15;139(2):459–463. [Abstract] [Google Scholar]
- Sternberg EM, Trial J, Parker CW. Effect of serotonin on murine macrophages: suppression of Ia expression by serotonin and its reversal by 5-HT2 serotonergic receptor antagonists. J Immunol. 1986 Jul 1;137(1):276–282. [Abstract] [Google Scholar]
- Fuller RW. Serotonergic stimulation of pituitary-adrenocortical function in rats. Neuroendocrinology. 1981 Feb;32(2):118–127. [Abstract] [Google Scholar]
- Cheung A, Hall TR, Harvey S. Serotoninergic regulation of corticosterone secretion in domestic fowl. J Endocrinol. 1987 May;113(2):159–165. [Abstract] [Google Scholar]
- Gibbs DM, Vale W. Effect of the serotonin reuptake inhibitor fluoxetine on corticotropin-releasing factor and vasopressin secretion into hypophysial portal blood. Brain Res. 1983 Nov 28;280(1):176–179. [Abstract] [Google Scholar]
- Holmes MC, Di Renzo G, Beckford U, Gillham B, Jones MT. Role of serotonin in the control of secretion of corticotrophin releasing factor. J Endocrinol. 1982 May;93(2):151–160. [Abstract] [Google Scholar]
- Spinedi E, Negro-Vilar A. Serotonin and adrenocorticotropin (ACTH) release: direct effects at the anterior pituitary level and potentiation of arginine vasopressin-induced ACTH release. Endocrinology. 1983 Apr;112(4):1217–1223. [Abstract] [Google Scholar]
- Larsen GL, Henson PM. Mediators of inflammation. Annu Rev Immunol. 1983;1:335–359. [Abstract] [Google Scholar]
- Kilian PL, Kaffka KL, Stern AS, Woehle D, Benjamin WR, Dechiara TM, Gubler U, Farrar JJ, Mizel SB, Lomedico PT. Interleukin 1 alpha and interleukin 1 beta bind to the same receptor on T cells. J Immunol. 1986 Jun 15;136(12):4509–4514. [Abstract] [Google Scholar]
- Laue L, Kawai S, Brandon DD, Brightwell D, Barnes K, Knazek RA, Loriaux DL, Chrousos GP. Receptor-mediated effects of glucocorticoids on inflammation: enhancement of the inflammatory response with a glucocorticoid antagonist. J Steroid Biochem. 1988 Jun;29(6):591–598. [Abstract] [Google Scholar]
- Cohen ML, Fuller RW, Kurz KD. LY53857, a selective and potent serotonergic (5-HT2) receptor antagonist, does not lower blood pressure in the spontaneously hypertensive rat. J Pharmacol Exp Ther. 1983 Nov;227(2):327–332. [Abstract] [Google Scholar]
- Gross HA, Ruder HJ, Brown KS, Lipsett MB. A radioimmunoassay for plasma corticosterone. Steroids. 1972 Dec;20(6):681–695. [Abstract] [Google Scholar]
- Chrousos GP, Schulte HM, Oldfield EH, Gold PW, Cutler GB, Jr, Loriaux DL. The corticotropin-releasing factor stimulation test. An aid in the evaluation of patients with Cushing's syndrome. N Engl J Med. 1984 Mar 8;310(10):622–626. [Abstract] [Google Scholar]
- Wilder RL, Calandra GB, Garvin AJ, Wright KD, Hansen CT. Strain and sex variation in the susceptibility to streptococcal cell wall-induced polyarthritis in the rat. Arthritis Rheum. 1982 Sep;25(9):1064–1072. [Abstract] [Google Scholar]
- Wilder RL. Proinflammatory microbial products as etiologic agents of inflammatory arthritis. Rheum Dis Clin North Am. 1987 Aug;13(2):293–306. [Abstract] [Google Scholar]
- Chrousos GP, Cutler GB, Jr, Sauer M, Simons SS, Jr, Loriaux DL. Development of glucocorticoid antagonists. Pharmacol Ther. 1983;20(2):263–281. [Abstract] [Google Scholar]
- Kohashi O, Kohashi Y, Takahashi T, Ozawa A, Shigematsu N. Suppressive effect of Escherichia coli on adjuvant-induced arthritis in germ-free rats. Arthritis Rheum. 1986 Apr;29(4):547–553. [Abstract] [Google Scholar]
- Griffiths MM, DeWitt CW. Genetic control of collagen-induced arthritis in rats: the immune response to type II collagen among susceptible and resistant strains and evidence for multiple gene control. J Immunol. 1984 Jun;132(6):2830–2836. [Abstract] [Google Scholar]
- Hill JL, Yu DT. Development of an experimental animal model for reactive arthritis induced by Yersinia enterocolitica infection. Infect Immun. 1987 Mar;55(3):721–726. [Europe PMC free article] [Abstract] [Google Scholar]
- Beraud E, Reshef T, Vandenbark AA, Offner H, Friz R, Chou CH, Bernard D, Cohen IR. Experimental autoimmune encephalomyelitis mediated by T lymphocyte lines: genotype of antigen-presenting cells influences immunodominant epitope of basic protein. J Immunol. 1986 Jan;136(2):511–515. [Abstract] [Google Scholar]
- Caspi RR, Roberge FG, McAllister CG, el-Saied M, Kuwabara T, Gery I, Hanna E, Nussenblatt RB. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol. 1986 Feb 1;136(3):928–933. [Abstract] [Google Scholar]
- Davis JK, Thorp RB, Maddox PA, Brown MB, Cassell GH. Murine respiratory mycoplasmosis in F344 and LEW rats: evolution of lesions and lung lymphoid cell populations. Infect Immun. 1982 May;36(2):720–729. [Europe PMC free article] [Abstract] [Google Scholar]
- Allen JB, Wilder RL. Variable severity and Ia antigen expression in streptococcal-cell-wall-induced hepatic granulomas in rats. Infect Immun. 1987 Mar;55(3):674–679. [Europe PMC free article] [Abstract] [Google Scholar]
- Lehman TJ, Allen JB, Plotz PH, Wilder RL. Lactobacillus casei cell wall-induced arthritis in rats: cell wall fragment distribution and persistence in chronic arthritis-susceptible LEW/N and -resistant F344/N rats. Arthritis Rheum. 1984 Aug;27(8):939–942. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.86.7.2374
Read article for free, from open access legal sources, via Unpaywall:
https://www.pnas.org/content/pnas/86/7/2374.full.pdf
Citations & impact
Impact metrics
Article citations
Diurnal production of cortisol and prediction of treatment response in rheumatoid arthritis: a 6-month, real-life prospective cohort study.
RMD Open, 10(1):e003575, 17 Jan 2024
Cited by: 1 article | PMID: 38233075 | PMCID: PMC10806498
Hypertension contributes to exacerbated osteoarthritis pathophysiology in rats in a sex-dependent manner.
Arthritis Res Ther, 25(1):7, 12 Jan 2023
Cited by: 6 articles | PMID: 36635774 | PMCID: PMC9835335
Differential Hypothalamic-pituitary-adrenal Response to Stress among Rat Strains: Methodological Considerations and Relevance for Neuropsychiatric Research.
Curr Neuropharmacol, 21(9):1906-1923, 01 Jan 2023
Cited by: 2 articles | PMID: 36453492 | PMCID: PMC10514526
Review Free full text in Europe PMC
Autonomic Nervous System Dysregulation and Osteoarthritis Pain: Mechanisms, Measurement, and Future Outlook.
Curr Rheumatol Rep, 24(6):175-183, 14 Apr 2022
Cited by: 11 articles | PMID: 35420372 | PMCID: PMC9189055
Review Free full text in Europe PMC
The immune-neuroendocrine system, a key aspect of poultry welfare and resilience.
Poult Sci, 101(8):101919, 14 Apr 2022
Cited by: 5 articles | PMID: 35704954 | PMCID: PMC9201016
Go to all (384) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A central nervous system defect in biosynthesis of corticotropin-releasing hormone is associated with susceptibility to streptococcal cell wall-induced arthritis in Lewis rats.
Proc Natl Acad Sci U S A, 86(12):4771-4775, 01 Jun 1989
Cited by: 289 articles | PMID: 2786636 | PMCID: PMC287355
Arthritis-susceptible Lewis rats fail to emerge from the stress hyporesponsive period.
Brain Res Dev Brain Res, 65(1):115-118, 01 Jan 1992
Cited by: 25 articles | PMID: 1551226
Neurotransmitter-induced hypothalamic-pituitary-adrenal axis responsiveness is defective in inflammatory disease-susceptible Lewis rats: in vivo and in vitro studies suggesting globally defective hypothalamic secretion of corticotropin-releasing hormone.
Neuroendocrinology, 55(5):600-608, 01 May 1992
Cited by: 72 articles | PMID: 1350069
Streptococcal cell wall-induced polyarthritis in the rat. Mechanisms for chronicity and regulation of susceptibility.
APMIS, 97(10):861-878, 01 Oct 1989
Cited by: 16 articles | PMID: 2679806
Review