Abstract
Objective
Familial cold autoinflammatory syndrome (FCAS) is caused by mutations in the CIAS1 gene, leading to excessive secretion of interleukin-1beta (IL-1beta), which is associated with cold-induced fevers, joint pain, and systemic inflammation. This pilot study was conducted to assess the safety and efficacy of rilonacept (IL-1 Trap), a long-acting IL-1 receptor fusion protein, in patients with FCAS.Methods
Five patients with FCAS were studied in an open-label trial. All patients received an initial loading dose of 300 mg of rilonacept by subcutaneous injection, were evaluated 6 and 10 days later for clinical efficacy, and remained off treatment until a clinical flare occurred. At the time of flare, patients were again treated with 300 mg of rilonacept and then given maintenance doses of 100 mg/week. Patients whose FCAS was not completely controlled were allowed a dosage increase to 160 mg/week and then further to 320 mg/week during an intrapatient dosage-escalation phase. Safety, disease activity measures (daily diary reports of rash, joint pain and/or swelling, and fevers), health quality measures (Short Form 36 health survey questionnaire), and serum markers of inflammation (erythrocyte sedimentation rate [ESR], high-sensitivity C-reactive protein [hsCRP], serum amyloid A [SAA], and IL-6) were determined at 3, 6, 9, 12, and 24 months after initiation of rilonacept and were compared with baseline values.Results
In all patients, clinical symptoms typically induced by cold (rash, fever, and joint pain/swelling) improved within days of rilonacept administration. Markers of inflammation (ESR, hsCRP, and SAA) showed statistically significant reductions (P < 0.01, P < 0.001, and P < 0.001, respectively) at doses of 100 mg. Dosage escalation to 160 mg and 320 mg resulted in subjectively better control of the rash and joint pain. Furthermore, levels of the acute-phase reactants ESR, hsCRP, and SAA were lower at the higher doses; the difference was statistically significant only for the ESR. All patients continued taking the study drug. The drug was well-tolerated. Weight gain in 2 patients was noted. No study drug-related serious adverse events were seen.Conclusion
In this study, we present 2-year safety and efficacy data on rilonacept treatment in 5 patients with FCAS. The dramatic improvement in clinical and laboratory measures of inflammation, the sustained response, and the good tolerability suggest that this drug may be a promising therapeutic option in patients with FCAS, and the data led to the design of a phase III study in this patient population.Free full text

A Pilot Study to Evaluate the Safety and Efficacy of the Long-acting IL-1 Inhibitor, Rilonacept (IL-1 Trap) in Patients with Familial Cold Autoinflammatory Syndrome (FCAS)
Associated Data
Abstract
Objective
Familial cold autoinflammatory syndrome (FCAS) is caused by mutations in CIAS1 leading to excessive secretion of IL-1β, which is associated with cold induced fevers, joint pain and systemic inflammation. This pilot study was conducted to assess the safety and efficacy of the long acting IL-1 receptor fusion protein, rilonacept (IL-1 Trap), in patients with FCAS.
Methods
Five Caucasian patients with FCAS were studied in an open-label trial. All patients received an initial loading dose of 300 mg of rilonacept by subcutaneous injections, were evaluated 6 and 10 days later for clinical efficacy and remained off drug until a clinical flare occurred. At the time of flare patients were retreated with 300 mg of rilonacept and then maintained on weekly doses of 100 mg per week. Patients who were not completely controlled could have their dose increased to 160 mg per week and further to 320 mg per week during an intra-patient dose escalation phase. Safety, disease activity measures, (daily diary reports of rash, joint pain and/or swelling, fevers) and quality of health measures (SF-36 questionnaire) as well as various serum markers of inflammation (ESR, CRP, SAA and IL-6) were measured at 3, 6, 9, 12 and 24 months after initiation of rilonacept administration and compared with baseline values.
Results
In all patients, clinical symptoms typically induced by cold (rash, fever, joint pain and swelling) improved within days of rilonacept administration. Inflammatory markers (ESR, CRP and SAA) showed a statistically significant reduction (p < 0.01 for ESR and p<0.001 for CRP and SAA) at doses of 100 mg. Although dose escalation to 160 mg and 320 mg resulted in subjectively better control of rash and joint pain, the lower measurements at the higher doses differed only significantly for the ESR, and a non-significant trend towards lower CRP and SAA levels was observed. All patients remained on study drug. The drug was well tolerated. Weight gain in two patients was noted. No study drug related serious adverse events were seen.
Conclusion
In this study using the long acting IL-1 inhibitor, rilonacept in five patients with FCAS, we present two-year safety and efficacy data. The dramatic improvement in clinical and laboratory measures of inflammation, the sustained response and good tolerability suggest that this drug may be a promising therapeutic option in patients with FCAS and the data led to the design of a phase 3 study in this patient population ((1)New Hoffman Ref).
INTRODUCTION
Familial cold autoinflammatory syndrome (FCAS) is a rare autosomal dominant disorder caused by mutations in the gene CIAS1 (NLRP3) that encodes a pyrin-related protein called “cryopyrin” (also NALP3, PYPAF1) (2). FCAS (MIM 120100) is characterized by episodes of rash, arthralgia, and fever that are induced after cold exposure (3;4). Symptoms first start within the first 6 months of life and disease flares develop about 2.5 hours after cold exposure and last on average 12 hours. Renal amyloidosis is seen infrequently, in about 2% of patients (5). FCAS is at the less severe end of a spectrum of CIAS1 associated periodic fever syndromes (CAPS) that also includes Muckle Wells Syndrome (MWS) and Neonatal-Onset Multisystem Inflammatory Disease (NOMID) (6;7).
Cryopyrin is expressed in peripheral blood leukocytes (8;9) and has structural similarities to pyrin, which is mutated in patients with familial Mediterranean fever (10;11). Cryopyrin mutations are gain of function mutations leading to constitutive activation of the inflammasome (12;13), a multi-molecular complex with pro-IL-1 beta-processing activity that is involved in the regulation of inflammation (14) and macrophage necrosis (15;16). Models of the structural basis for the activation of the inflammasome have been suggested (17;18) and cryopyrin is essential for the ATP-driven activation of caspase-1 in lipopolysaccharide-stimulated macrophages and for the efficient secretion of the caspase-1-dependent cytokines IL-1beta (19;20), and possibly IL-18 (21) and IL-33 (22). While fine structure mapping of CIAS1 identified an ancestral haplotype carrying the mutation L353P that is common to a large kindred of North American patients with FCAS (23), de novo mutations can also account for new cases of FCAS and more recently patients with clinical FCAS but no mutation in CIAS1 have been identified (24).
Previous clinical studies in patients with CAPS have suggested that most of the disease manifestations of CAPS are associated with IL-1β over-secretion and rapidly respond to blockade of IL-1 beta signaling with the IL-1 receptor antagonist anakinra (25–27). Although effective, anakinra needs to be administered daily and drug withdrawal leads to immediate disease flares (28). In the present study we evaluated efficacy and safety of the long acting IL-1 inhibitor, rilonacept (IL-1 Trap), a fusion molecule comprised of the extracellular component of the IL-1 receptor (IL-1 receptor type 1 and accessory protein) and the Fc portion of IgG1 which binds circulating IL-1β and IL-1α with very high affinity that can be administered once weekly by subcutaneous injection.
PATIENTS AND METHODS
Study patients
Five adult patients with CIAS1 mutation-positive FCAS were screened and enrolled. All patients had classical features of FCAS including cold-induced fevers, rash, joint pain and swelling. All patients had at least one disease flare per week as indicated by self report and documented clinical signs and symptoms on a daily diary score filled out for a minimum of 2 weeks prior to enrollment. All patients had baseline elevation of the acute phase reactants, erythrocyte sedimentation rate (ESR) analyzed by Westergren method, high-sensitivity C-reactive protein (CRP) and serum amyloid A (SAA). Two patients are related; patient 1 is the son of patient 3 and has the same CIAS1 mutation. All patients were naive to disease modifying anti-rheumatic drugs (DMARDs) or prednisone; and no patients had prior exposure to the IL-1β blocking agent anakinra. The Institutional Review Board of the NIAMS/NIDDK at the National Institutes of Health approved this study (clinicaltrials.gov, identifier: NCT00094900), and written informed consent was obtained from all participants. Other medical conditions are listed in Table 1. The patients’ ages ranged from 20 to 64 years at enrollment.
Table 1
Baseline Demographics and Clinical and Laboratory Characteristics
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Mutation* | E627G | M659K (de novo mutation) | E627G | L353P | L353P |
| Familial disease | Son of patient 3 | No family history | Father of patient 1 | Positive family history | Has a son with FCAS |
| Gender | Male | Female | Male | Male | Male |
| Race | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian |
| Age at enrollment | 42 | 20 | 64 | 58 | 40 |
| Attack frequency | 5 times per week | 1–2 times per week; rash only: 4 times per week | 1–2 times per week | Winter time: 2–3 times per month; summer time 1–2 times per month | 1–2 times per week |
| Flare features**, All patients had: Cold induced rash, migratory arthralgias, conjunctivitis | Fatigue, morning stiffness, tinnitus Prodrome: uncomfortable “chill in the bones”, headaches | Headaches, oral ulcers | Chills, arthritis (knees) | Prodrome: difficulty getting warm, chills, joint pain and swelling | |
| Other disease features | Mild bilateral high frequency hearing loss (L> R), uveitis, lymphadenopathy, clubbing, increased fat pads on hands and feet | Anterior uveitis, mild to moderate high frequency sensorineural hearing loss (R>L), clubbing | Injected sclera, corneal opacity in right eye, scleredema over both calves, tissue laxity over both thighs, clubbing, extra fat pads on feet, axillary lymphadenopathy | Mild to moderate bilateral high frequency hearing loss | Recurrent aphthous ulcers, clubbing |
| FCAS/MWS unrelated symptoms | Varicose veins, pitting edema, allergies, hypertension, rosacea, gastroesophageal reflux disease | Subcapsular cataracts, h/o “viral” meningitis, beta thalassemia, keratosis pilaris, depression | Gout, glaucoma, diabetes, hypertension, calcified lung granuloma in RLL, thyroid nodules | Exposure to “agent orange” with h/o basal cell and squamous cell carcinoma, ganglion cyst right wrist, actinic keratosis, mechanical back pain | Congenital fusion of cervical spine C2-3 |
Study Design
After a baseline evaluation, all subjects received an initial loading regimen of 300 mg beginning on Day 1 (100 mg/day for 3 consecutive days). All subjects were observed without further medication until a disease flare occurred. The primary outcomes were drug safety and clinical efficacy (improvement in patient diary score and a change in the acute phase reactants, CRP, SAA and ESR) post drug administration on days 6 and 10. A flare was defined as elevation of acute phase reactants by greater than 50% from the time of maximal efficacy (day 10 for patients 1, 2, 4 and 5 and day 6 for patient 3). At the time of disease flare patients were retreated with 300 mg of rilonacept, followed by weekly subcutaneous injections of 100 mg. If a complete inflammatory remission, defined as CRP < 0.5 mg/dL and/or SAA <10 mg/L and daily diary score of < 0.5, had not occurred, patients were eligible for a dose escalation during the extension phase of the study. The time on a weekly dose of 100 mg of rilonacept varied between 8 and 42 weeks. Two intra-patient dose escalation steps were allowed if remission criteria were not met. The first dose increase to 160 mg per week (dose escalation A), and if remission as defined above had not occurred after four weeks at the higher dose or at any time thereafter, patients were eligible for further dose escalation to 320 mg per week (dose escalation B). Once the dose of 160 mg per week was reached, the time for study continuation was two additional years. Blood samples for safety (chemistry profile) and markers of inflammation (acute phase reactants and complete blood cell count) were scheduled at baseline, days 6 and 10, at the time of disease flare and then monthly for the first year and every two months for the second year of the extension phase of the study.
Assessment of Efficacy
Baseline assessment included a pre-study daily diary score (daily diary data collected under a natural history study between 15 days and 45 days prior to initial study drug administration. The primary efficacy endpoint was the improvement in the acute phase reactants (ESR, CRP and SAA) and clinical daily diary scores (a composite score including fever, rash and arthritis/arthralgia scoring each of the 3 symptoms from 0, no symptom to 4, worst symptom) with a range of daily scores from 0 to 12. On day 10 post drug administration patients returned to clinic for evaluation. Diary data for day 10 efficacy evaluation included a mean of scores collected 5 calendar days prior to and including day 10 data. Patient 3 flared on day 10 and his maximal clinical and laboratory improvement was seen on day 6. For this patient, maximal benefit data was collected for day 6 data and he fulfilled flare criteria on day 10. Flare diary data included means of data collected 3 calendar days prior to and including the flare date. Diary data collected at a dose level of 100 mg per week included means of data collected starting 10 days after the retreatment of 300 mg and after each dose escalation, (this was considered steady state at each dose level). The designated 3 month visit refers to 3 months post dose escalation to 160 mg of rilonacept per week, 6 months visit to 6 months post escalation to 160 mg per week and so on.
Secondary efficacy endpoints included the development of and time to a flare after the first dose of rilonacept. This was aimed at assessing the duration of clinical and biochemical responses following discontinuation of rilonacept treatment. Other endpoints measured included a visual analogue scale (VAS) assessment of patients’ and physician global health assessment, patients’ pain and fatigue, tender and swollen joint count, quality of health assessment (SF-36 questionnaire), health assessment questionnaire (HAQ), and ELISA measurements of IL-6 levels (Rules Based Medicine Inc., Austin, TX).
Assessment of Safety
Adverse events were captured from the daily diaries, history and physical examination, and from results of clinical laboratory tests performed during and between NIH visits.
Statistical Analysis
This is an exploratory pilot study of an investigational agent in the treatment of patients with FCAS. Summary statistics were performed including means and standard deviations and frequency distributions where appropriate. All statistical analyses were done using paired t-tests. The optimal Box-Cox power transformation was used to reach normal distribution of the data. During the initial phase of the study, comparisons were made between baseline and the time of maximal efficacy and the day of maximal efficacy and flare. During the extension phase of the study, comparisons were made between baseline data and the subsequent timepoints at which the parameter was assessed. For secondary endpoint analysis p-values were not adjusted for multiple comparisons.
RESULTS
Baseline disease characteristics
All enrolled patients were CIAS1 mutation positive, had reported cold induced episodes of fever, rash and arthralgia; patients 1 to 3 also had mild to moderate bilateral high frequency hearing loss. All but one patient had a history of familial disease; patient 2 had a de novo mutation that was previously reported in a familial case of FCAS (29) (Table 1). All patients had active disease as indicated in their baseline elevation of the daily diary scores, as well as elevation of the acute phase reactants.
Initial study phase: response to the 300 mg loading regimen of rilonacept
Within hours of administration of the initial dose of rilonacept, all patients reported benefit with a decrease in cold induced attacks and improvement in symptoms (mainly rash, fever and arthralgia) as measured by the reduction in daily diary scores. Although improvement in eye redness and fatigue was also seen, these symptoms were not suitable measures of FCAS disease activity for two of the five patients (patients 2 and 3) had confounding medical conditions. These two symptoms were therefore not included in the calculation of the daily diary primary clinical efficacy score for all subjects, but are described separately in supplementary Figure 1. Maximal clinical improvement occurred in four patients at day 10 and in one patient at day 6 (patient 3). Patient 3 fulfilled flare criteria with recurrence of symptoms and elevation of acute phase reactants at day 10. Interestingly, rilonacept plasma levels measured at day 10 were lowest in the patient who flared and comparable to a plasma drug level at which one other patient experienced return of symptoms (data not shown). The improvement in daily symptoms from a baseline score of 3.48 ± 0.93 by a mean of 3.09± 1.03 (p<0.05, 81% mean reduction) was paralleled by improvement in all three primary inflammatory laboratory parameters measured, the ESR decreased by a mean of 32.20 ± 7.31 mm/hr (p<0.01, 58% mean reduction), the CRP by a mean of 4.22 ± 0.98 mg/dL and the SAA levels by a mean of 216.10 ± 92.14 mg/L (p<0.001, 88% mean reduction for CRP and 95% for SAA). A disease flare occurred at a range of 10 to 28 days after the initial loading regimen. The daily diary scores increased by 1.51± 0.14 (p<0.01), again paralleled by an increase in the acute phase reactants; the ESR increased by a means of 12.20 ± 4.52 mm/hr, the CRP by 2.01± 0.95 mg/dL and the SAA level by a mean of 71.62 ± 39.76 mg/L (all p< 0.01) (Figure 1).
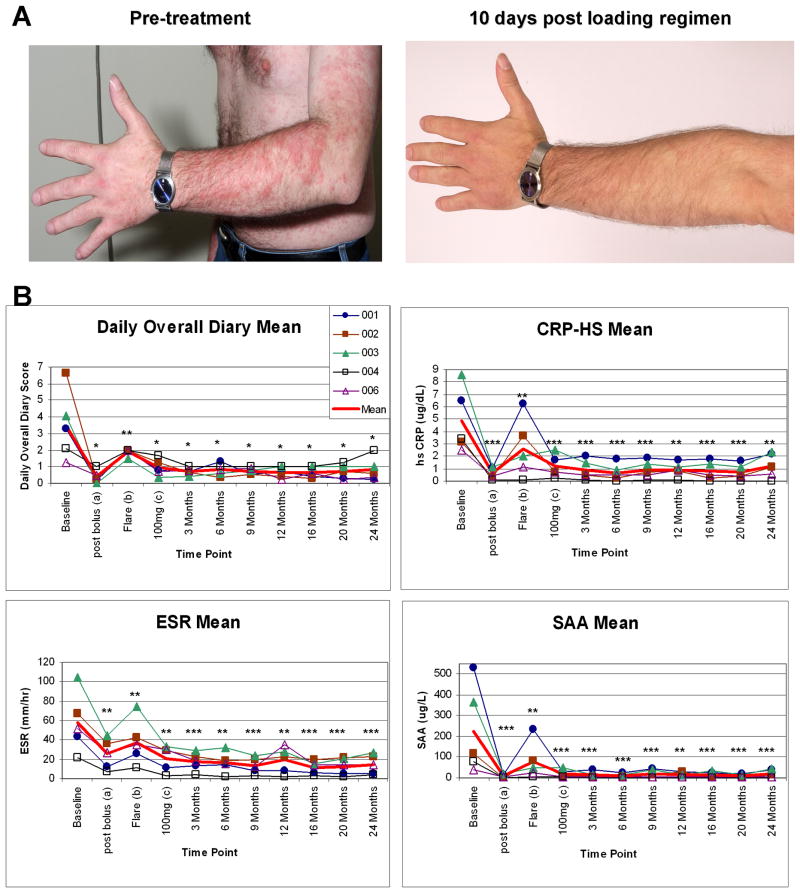
A. Skin rash during an attack induced by a physical exam in an air conditioned exam room (left image) and 10 days post bolus injection, when cold exposure did not trigger an attack (right panel).
B. Change of clinical symptoms as measured by the mean daily diary score, the daily mean of rash, fever and joint pain scored each daily on a scale from 0 (no symptom) to 4 (worst symptom) was generated over the period of treatment. The daily diary scores could range from 0–12.
All statistical analyses were done using paired t-tests. The optimal Box-Cox power transformation was used to reach normal distribution of the data.
*p-value<0.05, **p-value<0.01 and ***p-value<0.001.
aPost loading (post 300 mg of rilonacept administration at baseline) measurements were obtained on day 10 for four patients and on day 6 for one patient.
bComparisons were made between day 10 and flare.
cDatapoints represent a mean of all values obtained on a rilonacept dose of 100 mg/week. The period that patients were on 100 mg/week varied from 8 to 42 weeks.
At 3 months, four patients received 160 mg/week and one patient received 320 mg/week. From month 6 to 24, one patient received 160 mg/week and 4 patients received 320 mg/week.
Changes in other secondary endpoints included significant improvements in the visual analog scale (VAS) measurements of physician global and patient global assessments and pain, and tender joint count (Supplementary Figure 2, Panel A). A non-significant trend was seen in the swollen joint count, however the swollen joint score was not greater than 1 in any patient at baseline and most joints were chronically thickened and often not associated with tenderness or warmth. The baseline health assessment (HAQ) score (range 0–3) was low with a mean of 0.35 and a median of 0.13 and did not significantly change with therapy (data not shown).
Response to rilonacept during the extension phase of the study
All patients entered the extension phase of the study and have completed 24 months of follow up at a dose of 160 mg per week or higher. Clinical and laboratory responses are summarized in Table 2 and indicate a persistent response to weekly rilonacept administration. SAA, CRP and ESR levels remained low in all measurements up to 24 months. This was also seen in the reduction of the overall diary score which indicates a persistent suppression in diseases symptoms (Figure 1). Dose escalation to 160 mg per week was indicated in all patients, as none reached the stringent inflammatory remission criteria of a diary score to equal or less than 0.5 and a CRP of less than 0.5 mg/dL and SAA of less than 10 mg/L. Symptoms and inflammatory markers improved at the higher doses in all patients and the change in the inflammatory markers, at the different dose levels is plotted in Figure 2. At the higher doses a statistically significant improvement was seen in the improvement of the ESR (p<0.05) and a trend in further improvement in CRP (p=0.135) and SAA (p=0.083). The improvement was most pronounced with a dose increase from 100 mg/week to 160 mg/week, but did not significantly change with a further dose increase from 160 mg/week to 320 mg/week (Figure 2). Four out of the five patients did not fulfill remission criteria for CRP and daily diary scores at the 160 mg/week dose, and were subsequently increased to 320 mg/week.
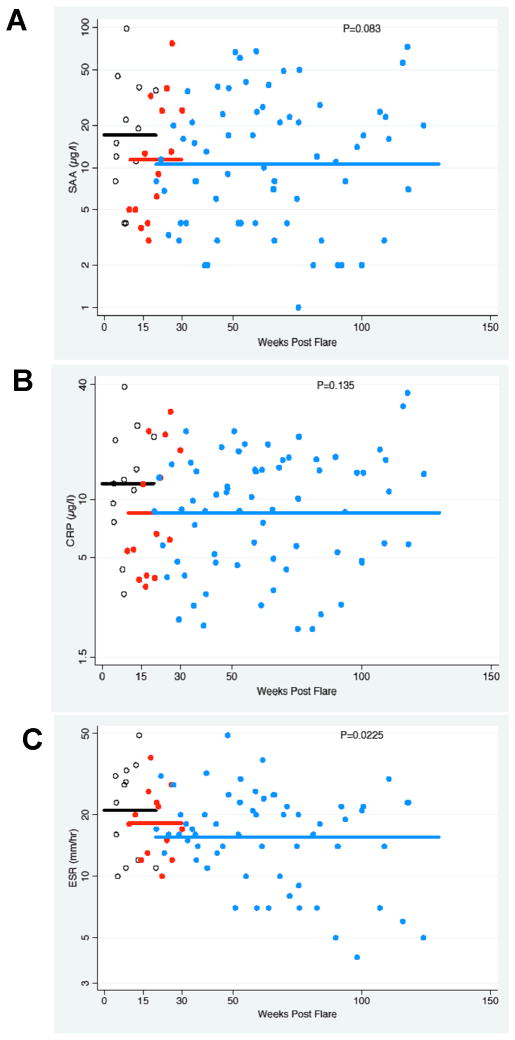
SAA (Panel A), CRP (Panel B) and ESR (Panel C) data is plotted from only the four patients who underwent dose escalation up to 320 mg/week. Values were plotted on a logarithmic scale at the different dose levels of 100mg/week (black open circles), 160mg/week (red closed circles) and 320mg/week (blue closed circles). The mean of all plotted values at each dose level is indicated as solid line.
Table 2
Other Clinical outcomes over 2 years
| Measure | Baseline | Month 3 | Month 6 | Month 9 | Month 12 | Month 16 | Month 20 | Month 24 | |
|---|---|---|---|---|---|---|---|---|---|
| Patient Global VAS | |||||||||
 Mean Mean | 5.29 | Mean Δ | −2.69 | −2.56 | −2.63 | −2.86 | −3.40 | −2.78 | −2.33 |
 Stand. Error Stand. Error | 0.67 | Stand. Error | 1.01 | 1.27 | 1.19 | 1.24 | 0.98 | 1.13 | 1.30 |
 Median Median | 5.20 | Median Δ | −3.02 | −3.50 | −2.65 | −3.60 | −3.60 | −3.60 | −3.55 |
 MIN,MAX MIN,MAX | 3.70, 6.90 | p-value | 0.072595 | 0.091245 | 0.079571 | 0.053641 | 0.020993 | 0.052770 | 0.093472 |
| Physician Global VAS | |||||||||
 Mean Mean | 5.19 | Mean Δ | −4.15 | −4.27 | −4.71 | −4.30 | −4.85 | −4.79 | −4.54 |
 Stand. Error Stand. Error | 0.78 | Stand. Error | 0.89 | 1.01 | 0.75 | 0.90 | 0.95 | 0.66 | 0.99 |
 Median Median | 5.10 | Median Δ | −4.38 | −4.43 | −4.50 | −4.10 | −5.05 | −4.95 | −4.45 |
 MIN,MAX MIN,MAX | 2.95, 6.95 | p-value | 0.011401 | 0.009851 | 0.000562 | 0.006748 | 0.004011 | 0.000568 | 0.007832 |
| Patient Pain VAS | |||||||||
 Mean Mean | 6.25 | Mean Δ | −5.43 | −4.24 | −3.93 | −3.08 | −5.65 | −5.01 | −4.82 |
 Stand. Error Stand. Error | 0.98 | Stand. Error | 1.20 | 1.24 | 1.28 | 2.28 | 1.13 | 1.22 | 1.22 |
 Median Median | 7.55 | Median Δ | −6.58 | −3.25 | −2.70 | −2.80 | −7.20 | −4.80 | −4.15 |
 MIN,MAX MIN,MAX | 3.60, 8.25 | p-value | 0.007494 | 0.024820 | 0.044499 | 0.163955 | 0.003950 | 0.017188 | 0.022074 |
| Patient Fatigue VAS | |||||||||
 Mean Mean | 5.96 | Mean Δ | −3.12 | −2.27 | −2.41 | −2.04 | −3.16 | −2.38 | −3.19 |
 Stand. Error Stand. Error | 0.86 | Stand. Error | 1.04 | 1.15 | 1.10 | 1.17 | 0.67 | 0.89 | 1.63 |
 Median Median | 5.55 | Median Δ | −4.58 | −1.75 | −1.55 | −2.25 | −2.95 | −2.75 | −3.70 |
 MIN,MAX MIN,MAX | 3.25, 8.00 | p-value | 0.066975 | 0.178083 | 0.154269 | 0.235729 | 0.045283 | 0.094328 | 0.120674 |
| Tender Joint Count | |||||||||
 Mean Mean | 9.60 | Mean Δ | −5.70 | −4.30 | −4.80 | −7.40 | −9.60 | −8.40 | −6.80 |
 Stand. Error Stand. Error | 5.71 | Stand. Error | 3.49 | 4.47 | 4.73 | 5.33 | 5.71 | 4.77 | 5.53 |
 Median Median | 6.00 | Median Δ | −3.00 | −1.00 | −1.00 | −4.00 | −6.00 | −5.00 | −2.00 |
 MIN,MAX MIN,MAX | 1.00, 32.00 | p-value | 0.060872 | 0.414900 | 0.382763 | 0.107123 | 0.018259 | 0.004775 | 0.288289 |
| Swollen Joint Count | |||||||||
 Mean Mean | 10.20 | Mean Δ | −7.90 | −2.50 | −2.30 | −6.10 | −9.00 | −9.40 | −4.20 |
 Stand. Error Stand. Error | 4.35 | Stand. Error | 4.64 | 3.66 | 3.33 | 2.82 | 4.09 | 3.59 | 4.45 |
 Median Median | 8.00 | Median Δ | −6.00 | −4.00 | −4.00 | −4.00 | −5.00 | −8.00 | −4.00 |
 MIN,MAX MIN,MAX | 3.00, 27.00 | p-value | 0.087850 | 0.369316 | 0.279739 | 0.018076 | 0.004682 | 0.000462 | 0.180822 |
| SF-36 Physical Component Score | |||||||||
 Mean Mean | 36.60 | Mean Δ | 11.11 | 13.09 | 10.38 | 13.28 | 12.58 | 10.12 | 10.22 |
 Stand. Error Stand. Error | 1.79 | Stand. Error | 3.74 | 3.19 | 4.45 | 3.84 | 3.23 | 5.48 | 4.59 |
 Median Median | 35.20 | Median Δ | 14.00 | 14.40 | 14.20 | 16.80 | 15.20 | 16.50 | 14.90 |
 MIN,MAX MIN,MAX | 33.00, 42.90 | p-value | 0.042432 | 0.015235 | 0.086741 | 0.022931 | 0.016651 | 0.217070 | 0.098381 |
| SF-36 Mental Component Score | |||||||||
 Mean Mean | 40.02 | Mean Δ | 7.56 | 5.95 | 8.80 | 9.76 | 11.74 | 12.98 | 11.46 |
 Stand. Error Stand. Error | 4.64 | Stand. Error | 4.97 | 4.01 | 4.00 | 3.46 | 6.42 | 3.96 | 6.46 |
 Median Median | 36.60 | Median Δ | 6.65 | 5.20 | 5.50 | 9.20 | 21.00 | 16.90 | 17.30 |
 MIN,MAX MIN,MAX | 31.60, 56.50 | p-value | 0.200665 | 0.186839 | 0.089940 | 0.045273 | 0.156900 | 0.035306 | 0.159666 |
Although fevers had disappeared in all patients on the 100 mg/week dose, mild joint pain and mild skin rashes persisted on occasion. The skin rash was difficult to completely suppress in four patients. While patient 4 had almost complete resolution of skin rashes with rash scores ranging from 0.07 to 0.17 on doses of 100 mg/week and rash scores of 0 throughout the study on a dose of 160 mg/week, the other four patients had improvement in their rash from baseline but persistent skin scores of greater than 0. Interestingly, when rash scores were plotted relative to the day of injection there was an increase in rash scores towards the end of the week before the next injection at the 100 mg/week dose which was lost when the dose was increased to 160 mg/week (Figure 3). Fevers and joint pain were well controlled at drug levels of 100 mg/week and no further improvement was seen at the higher doses (Figure 3). One patient with a history of prior gout had no such symptoms during the time of his study participation. Although patients reported subjective improvement at the 320 mg/week dose over the 160mg per week dose, fluctuations of the acute phase reactants were still present although the variability was somewhat reduced.
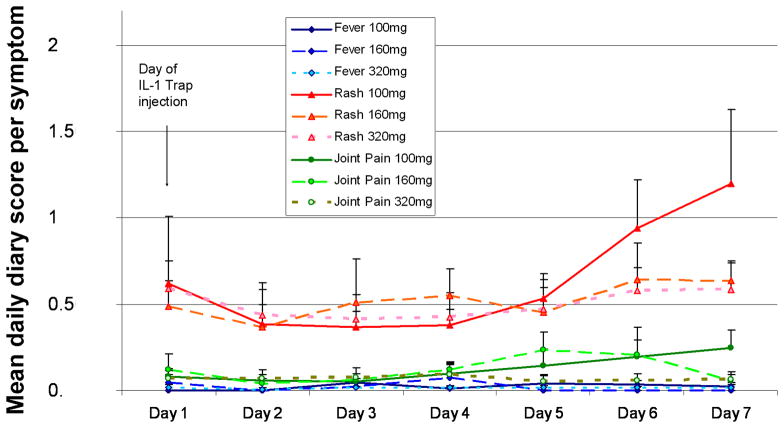
Mean daily diary scores for skin rash (red), joint pain (green) and fever (blue) were plotted for the respective days after the drug injection, with day 1 indicating the day of injection for the 4 patients (pts 1, 2, 3 and 5) who had persistent symptoms on rilonacept doses of 100 mg/week. Solid lines represent mean symptom scores on the diary scores at a rilonacept dose of 100 mg/week. The widely dotted line indicates means at a dose level of 160mg/week and the narrow dotted line means at a dose level of 320 mg/week.
Patient pain and physician global assessment significantly and durably improved on treatment. The global and fatigue assessment improved for all patients temporarily but were influenced by factors unrelated to FCAS and were not robust outcome measures long term (Table 2). Tender and swollen joint counts improved as well but were not statistically significant. On the quality of health assessment (measured by the SF-36), the improvement of the physical component was significant for most timepoints; the improvement in the mental component of the SF-36 was non-significant (Table 2 and Supplementary figure 3). All five patients opted to continue with drug treatment therapy. IL-6 levels measured at baseline and at a dose of 160 mg/week were elevated in three of five patients at baseline (10.6 to 54.7pg/ml) and deceased to undetectable levels (<5pg/ml) in all patients, (data not shown).
Drug safety and tolerability
All five patients had 100 percent treatment compliance on their diaries over the time on study. Treatment with rilonacept was well tolerated. No injection site reactions occurred during the course of the study. All adverse events that were classified as possibly associated with study drug are listed in Table 3. The events were classified as mild or moderate and none required discontinuation of study drug. Infections that occurred on therapy resolved as expected. One patient with preexisting basal cell carcinoma had 2 new lesions removed while on study and one patient with preexisting oral ulcers indicated increased frequency and prolonged healing of these ulcers on study drug. Significant weight gain of 11.3 and 13 kilos was seen in two patients. We could not detect an increase of adverse events at the higher dose levels, but the number of patients treated was very small. No serious adverse events occurred in these five patients.
Table 3
Adverse Events over 2 years
| 1. Cardiovascular System | |
| - Hypercholesterolemia | Pt 1 (mild) at 320 mg dose |
| - Hypertension | Pt 1 (moderate) at 100 mg dose |
| - Weight gain | Pt 1 (moderate) at 100 mg dose and Pt 3 (moderate) at 100mg dose |
| - Dyslipidemia | Pts 3 and 4 (mild) at 100 mg dose |
| 2. Dermatologic System | |
| - Hordeolum in left eye | Pt 1 (mild) at 320 mg dose |
| - Contact dermatitis on foot | Pt 4 (mild) at 100 mg |
| - Worsening aphthous ulcers | Pt 5 (moderate) at 100 mg dose; (mild) at 320 mg dose |
| 3. Endocrine System | |
| - Inhomogeneous thyroid | Pt 3 (mild) at 160 mg dose |
| - Low TSH | Pt 3 (mild) at 320 mg dose |
| - Worsening diabetes | Pt 3 (mild) at 320 mg dose |
| 4. Gastrointestinal System | |
| - Elevated liver function tests | Pt 4 (mild) at 160 mg dose; Pt 5 (mild) 1x at 100 mg dose and 1x elevated AST only at 100 mg dose |
| 5. Infections | |
| - URI | Pt 1 (mild) 3x at 320 mg dose; Pt 3 (mild) at 320 mg dose; Pt 4 (mild) at 100 mg dose |
| - Sinusitis | Pt 1 and Pt 5 (mild) at 160 mg dose |
| - Viral gastroenteritis | Pt 2 (mild to moderate) at 160 mg dose |
| - Bronchitis | Pt 4 (mild) at 100 mg dose |
| - Infection (left first toenail bed) | Pt 4 (mild) at 100 mg dose and 160 mg dose |
| - Infection (tooth) | Pt 4 (moderate) at 160 mg dose |
| - Viral illness | Pt 5 (mild) at 160 mg dose |
| 6. Neurological System | |
| - Xerostomia | Pt 1 (mild) at 100 mg dose |
| - Spongy/metallic taste in mouth | Pt 1 (mild) at 100 mg dose |
| - Intermittent insomnia | Pt 2 (moderate) at 320 mg dose and (severe) at 320 mg dose |
| - Headache | Pt 3 (mild) and Pt 5 (severe) at 320 mg dose |
| - Memory impairment | Pt 5 (mild) at 160 mg dose |
| 7. Respiratory System | |
| - Sore throat | Pt 1 (mild) 1x at 100 mg and 3x at 320 mg; Pt 6 (mild) 2x at 320 mg |
| - Nasal congestion | Pt 4 (mild) at 100 mg dose |
Note: There were no serious adverse events in this study. Other adverse events thought to be unlikely/not related to study drug included: flushed skin, toothache, folliculitis on chest/back, recurring basal cell carcinoma in one patient with pre-existing disease, worsening acne rosacea, diarrhea, intermittent stomachache/gastritis, nausea, hematuria, recurrence of pre-existing oral HSV stomatitis, foot pain, non-cardiac chest pain, lower back pain, neck pain, groin pain, transitory traumatic hearing loss in left ear, allergic sinusitis, hypogonadism, increased urinary urgency, left eye pain and nasal congestion. No events were reported involving the hematologic system.
DISCUSSION
This pilot study shows safety data as well as short and long-term clinical and laboratory responses to treatment with the long acting IL-1 inhibitor, rilonacept (IL-1 Trap) in 5 patients with FCAS who were enrolled in this study. This study allowed us to obtain data on the magnitude of the clinical and laboratory responses at different dose levels and served to help design a larger phase 3 study in patients with FCAS and MWS, a study population that had not previously been treated with rilonacept.
The spectrum of diseases associated with CIAS1 mutations, cryopyrin associated periodic fever syndromes (CAPS), spans from patients who present with less severe disease, FCAS, which presents with urticarial rashes, fevers and joint pain when exposed to cold, to patients who have more persistent attacks without relation to cold exposure and a higher risk for the development of amyloidosis mainly reported in Europe, and are referred to as Muckle Wells syndrome. The most severe phenotype seen in patients with NOMID also presents with aseptic meningitis and increased intracranial pressure, with about 70 % of patients developing bony overgrowth with disabilities from joint contractures and mental retardation. These diseases form a continuous spectrum with clinical overlaps between the classically described syndromes; however the clinical description is important regarding disease prognosis. While patients with FCAS have normal life spans, the mortality is about 20% in children with NOMID before they reach adulthood (30). Many of the disease manifestations of CAPS are caused by excessive secretion of IL-1β and are highly responsive to IL-1 blockade with the inhibitor anakinra (31–33). The disease spectrum of CAPS presents an ideal model to test the effect of other agents that block the IL-1 secretion pathway. The rapid and impressive response to IL-1 blockade in all patients with the disease allowed a study design that only required few patients and close monitoring to obtain data on the efficacy of a new IL-1 blocking agent; the drug withdrawal phase was designed to determine drug serum levels at which patients’ symptoms recurred. All patients enrolled had typical cold induced attacks (FCAS). Among the three patients who also presented with mild high frequency hearing loss, one patient had a history of chronic noise exposure, but in 2 patients the hearing loss was likely associated with the underlying disease, these patients had clinical features consistent with MWS. As expected, all patients experienced clinical benefit within several hours of drug administration and the three patients with hearing loss did not deteriorate during the two years of observation. However a longer observation period is needed to determine if slowly progressive hearing loss continues to occur.
Interestingly, significant improvement of disease control was seen at a dose of 100 mg/week. However, clinical and inflammatory remission as determined by normalization of acute phase reactants and a daily diary score of less than 0.5 was not achieved at that dose. At the 160 mg/week dose clinical and inflammatory remission was seen in one patient, who also had the lowest inflammatory markers at baseline. The four patients with higher inflammatory markers at baseline described further improvement in disease control at 320 mg/week, but this subjective improvement did not result in statistically measurable improvement over the 160 mg/week dose. For these and other reasons the 160 mg/week dose was chosen for a subsequent confirmatory phase 3 study in FCAS/MWS (Hal Hoffman et al. submitted to this journal). Although much improved, the acute phase reactants still continued to fluctuate in individual patients, indicating that minor inflammatory flares can still be seen although clinical symptoms if they recurred on treatment remained mild.
The response to rilonacept was sustained over the trial period of 24 months on doses of at least 160 mg/week. Drug compliance has been high in these patients. Although this pilot study is limited by the small number of patients enrolled, the magnitude and uniformity of the clinical response and the relatively high degree of the baseline inflammation in four out of the five patients allowed us to conclude that continued treatment with rilonacept was effective in acutely suppressing IL-1 mediated inflammation and sustaining the response for up to two years in all patients. Even at doses of 320 mg/week no increase in adverse events was observed over the lower doses. Daily injections of the IL-1 blocking agent anakinra have resulted in significant reduction in inflammation and symptom control in patients with FCAS, however no direct comparisons of efficacy and safety with the 2 drugs are currently available.
This is the first study using rilonacept in patients with FCAS and for a time up to two years. Rilonacept has not been used in patients with NOMID who have CNS inflammation. As our study was designed as a pilot to obtain initial safety and efficacy data on this new long acting IL-1 inhibitor, and no pediatric pharmacokinetic data was available at the time of study design, pediatric patients with NOMID were not eligible to enroll. Additional studies are ongoing in other diseases that have been associated with IL-1 over-stimulation and/or over-secretion such as systemic-onset juvenile idiopathic arthritis, adult onset Still’s disease, gout, and some periodic fever syndromes including FMF, where elevated IL-1 secretion plays a role in causing and maintaining systemic and organ specific inflammation. Results of these investigations may help to determine the benefit of this drug in the wider spectrum of autoinflammatory diseases.
The magnitude of the clinical and laboratory response and the sustained effect in controlling clinical symptoms and inflammatory markers (SAA, CRP and ESR) suggest a potential benefit of using rilonacept in patients with FCAS/MWS and provide a promising option in these patients that led to the conduct of a randomized controlled trial (34).
Acknowledgments
The authors would like to thank Cedric McClinton for his help in preparing this manuscript, and Judith Starling, Pharmaceutical Development Section, NIH for assistance with investigation drug management.
Footnotes
Author Contributions
Drs. Goldbach-Mansky and Wesley had full access to all the data in the study and take full responsibilities for the integrity of the data and the accuracy of the data analysis.Study design: Goldbach-Mansky, Mellis, Pucino, Wesley, Kastner
Acquisition of data: Goldbach-Mansky, Wilson, Shroff, Plehn, Snyder, Barham, Pham, Wesley, Papadopoulos, Weinstein, Mellis
Analysis and interpretation: Goldbach-Mansky, Shroff, Wilson, Snyder, Pham, Pucino, Wesley
Statistical analysis: Goldbach-Mansky, Wesley
Manuscript preparation: Goldbach-Mansky, Shroff, Kastner
Reference List
Full text links
Read article at publisher's site: https://doi.org/10.1002/art.23620
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2875198?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Clinical Utility of Rilonacept for the Treatment of Recurrent Pericarditis: Design, Development, and Place in Therapy.
Drug Des Devel Ther, 18:3939-3950, 04 Sep 2024
Cited by: 0 articles | PMID: 39247795 | PMCID: PMC11380881
Review Free full text in Europe PMC
AA Amyloidosis: A Contemporary View.
Curr Rheumatol Rep, 26(7):248-259, 03 Apr 2024
Cited by: 1 article | PMID: 38568326 | PMCID: PMC11219434
Review Free full text in Europe PMC
Practical Approach to Diagnosis and Management of IL-1-Mediated Autoinflammatory Diseases (CAPS, TRAPS, MKD, and DIRA).
Paediatr Drugs, 26(2):113-126, 20 Feb 2024
Cited by: 1 article | PMID: 38376736
The role of macrophages in rosacea: implications for targeted therapies.
Front Immunol, 14:1211953, 24 Aug 2023
Cited by: 0 articles | PMID: 37691916 | PMCID: PMC10484341
Review Free full text in Europe PMC
The NLRP3 inflammasome and gut dysbiosis as a putative link between HIV-1 infection and ischemic stroke.
Trends Neurosci, 46(8):682-693, 15 Jun 2023
Cited by: 2 articles | PMID: 37330380 | PMCID: PMC10554647
Review Free full text in Europe PMC
Go to all (121) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT00094900
Diseases
- (1 citation) OMIM - 120100
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies.
Arthritis Rheum, 58(8):2443-2452, 01 Aug 2008
Cited by: 334 articles | PMID: 18668535
Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist.
Lancet, 364(9447):1779-1785, 01 Nov 2004
Cited by: 340 articles | PMID: 15541451 | PMCID: PMC4321997
Rilonacept in the treatment of chronic inflammatory disorders.
Drugs Today (Barc), 45(6):423-430, 01 Jun 2009
Cited by: 32 articles | PMID: 19649332
Review
Efficacy and safety of the interleukin-1 antagonist rilonacept in Schnitzler syndrome: an open-label study.
Allergy, 67(7):943-950, 15 May 2012
Cited by: 59 articles | PMID: 22583335
Funding
Funders who supported this work.
Intramural NIH HHS (3)
Grant ID: Z01 AR041138-05
Grant ID: Z01 AR041123-09
Grant ID: Z99 AR999999




