Abstract
Objective
To determine how childhood overweight, in conjunction with other life course weight characteristics, relates to the development of type 2 diabetes in adulthood.Research design and methods
Among 109,172 women in the Nurses' Health Study II, body fatness at ages 5, 10, and 20 years was assessed by recall using 9-level pictorial diagrams (somatotypes) representing extreme thinness (category 1) to obesity (category 9). Recalled weights at age 18 years and adulthood were used to derive BMI. Self-reported cases of type 2 diabetes were confirmed by supplementary questionnaire.Results
Somatotypes at ages 5 and 10 years were positively associated with diabetes risk (P(trend) < 0.0001). The adjusted relative risk (RR) of women with somatotype >or=6 (vs. 2) at age 5 years was 2.19 (95% CI 1.79-2.67) and at age 10 years was 2.57 (2.20-3.01). Increases in size by somatotype or by weight gain since age 18 were associated with increased risk. Compared with women who were never overweight at any age, women who were overweight as an adult (BMI >25 kg/m(2)) but not previously had an adjusted RR of 8.23 (7.41-9.15). The adjusted RR was 15.10 (13.21-17.26) for women who were also overweight at age 10 (somatotype >or=5) and 18 (BMI >25 kg/m(2)). Increased childhood size was not associated with risk among women who did not continue to be overweight in adulthood.Conclusions
Increased body size starting from childhood is associated with a greater risk of diabetes in adulthood. However, women who become lean in adulthood do not have an increased risk.Free full text

Childhood Size and Life Course Weight Characteristics in Association With the Risk of Incident Type 2 Diabetes
Abstract
OBJECTIVE
To determine how childhood overweight, in conjunction with other life course weight characteristics, relates to the development of type 2 diabetes in adulthood.
RESEARCH DESIGN AND METHODS
Among 109,172 women in the Nurses' Health Study II, body fatness at ages 5, 10, and 20 years was assessed by recall using 9-level pictorial diagrams (somatotypes) representing extreme thinness (category 1) to obesity (category 9). Recalled weights at age 18 years and adulthood were used to derive BMI. Self-reported cases of type 2 diabetes were confirmed by supplementary questionnaire.
RESULTS
Somatotypes at ages 5 and 10 years were positively associated with diabetes risk (Ptrend < 0.0001). The adjusted relative risk (RR) of women with somatotype ≥6 (vs. 2) at age 5 years was 2.19 (95% CI 1.79–2.67) and at age 10 years was 2.57 (2.20–3.01). Increases in size by somatotype or by weight gain since age 18 were associated with increased risk. Compared with women who were never overweight at any age, women who were overweight as an adult (BMI >25 kg/m2) but not previously had an adjusted RR of 8.23 (7.41–9.15). The adjusted RR was 15.10 (13.21–17.26) for women who were also overweight at age 10 (somatotype ≥5) and 18 (BMI >25 kg/m2). Increased childhood size was not associated with risk among women who did not continue to be overweight in adulthood.
CONCLUSIONS
Increased body size starting from childhood is associated with a greater risk of diabetes in adulthood. However, women who become lean in adulthood do not have an increased risk.
Large proportions of children in the U.S. are currently at risk for or are overweight. Immediate and long-term health problems have arisen because of childhood overweight, including poor lipid profile, earlier onset of type 2 diabetes, and other metabolic syndrome traits (1). Although the rise in prevalence of type 2 diabetes in the pediatric population is cause for concern in itself, the risk as these children continue into adulthood will undoubtedly be a greater public health burden.
Despite strong ties between the development of insulin resistance from increased adiposity via multiple biological mechanisms, few studies have looked at the long-term consequences of childhood overweight and the risk of type 2 diabetes in adulthood and findings have been inconsistent (2–8). One study using birth and medical records data from Finland, found that BMI at ages 7–11 years in women was significantly and positively associated with future risk of diabetes (4). However, the study did not investigate the roles of adolescent and adulthood obesity in this association, and the number of type 2 diabetes cases was small (n = 185) (4). In contrast, a more recent study that accounted for life course weight, found that thinness, rather than overweight, from childhood through young adulthood was associated with increased diabetes risk (8). However, these findings were from an older cohort (born 1925–1950) of French women, with a large percentage being extremely lean in childhood, whose early nutritional status might have been affected by World War II (1939–1945). Thus, the objective of this study was to determine the longitudinal association between childhood overweight in combination with other life course weight characteristics and the risk for type 2 diabetes in a more recent birth cohort of young women.
RESEARCH DESIGN AND METHODS
The Nurses' Health Study II (NHSII) is an ongoing prospective study of U.S. female nurses aged 25–42 years. Follow-up is conducted using biennial questionnaires from 1989 to 2005. A total of 109,172 participants remained after exclusion for diagnosis of any type of diabetes, cancer, or cardiovascular disease at baseline (2%) or for missing information on childhood body shape (2.5%), BMI at age 18 years (0.7%), or baseline BMI (0.3%). This study was approved by the institutional review board of the Partners Health Care System (Boston, MA).
Assessment of childhood size and weight characteristics
At baseline, body fatness at ages 5, 10, and 20 years of age was assessed by recall of somatotypes or 9-level pictorial diagrams developed by Stunkard et al. (9) representing sizes ranging from extreme thinness (category 1) to obesity (category 9). The use of recalled somatotypes has been validated in both older (10) and younger (11) women by comparison with childhood records. Somatotypes at ages 5, 10, and 20 years correlated moderately with recorded BMI (r = 0.60, 0.65, and 0.66, respectively) (10). The validity did not differ by adult BMI at the time of report (10). Overweight was defined as somatotype ≥5. (12).
Weight at age 18 and adult height and weight were self-reported. Weight change was the difference between weight at age 18 years and weight at baseline in 1989. The correlation between recalled weight at age 18 and documented weight from college or nursing school records was 0.84, and the correlation between self-reported and technician-measured adult weight was 0.96 (13).
Type 2 diabetes ascertainment
A supplementary questionnaire was mailed to confirm the self-report of diabetes diagnosis and to distinguish different types of diabetes (14). The National Diabetes Data Group diagnostic criteria were used for cases reported through 1997 and required confirmation of at least one of the following: 1) one or more symptoms (weight loss, hunger, thirst, or polyuria) and elevated glucose (fasting ≥7.8 mmol/l [140 mg/dl] or random plasma or 2-h glucose ≥11.1 mmol/l [200 mg/dl]), 2) no symptoms but occurrence of elevated plasma glucose as described above on at least two different occasions, or 3) use of insulin or oral hypoglycemic medication (15). For cases occurring after 1998, the cutoff of fasting plasma glucose was changed to 7.0 mmol/l (126 mg/dl) in accordance with revised criteria (16). We excluded women classified as having only gestational diabetes mellitus or type 1 diabetes. In the Nurses' Health Study, the supplemental questionnaire was highly reliable regarding diabetes diagnosis (14). In a random sample of 84 women classified as being a case subject, medical records were available for 62 of these women and an endocrinologist confirmed the diagnosis in 61 women (98%) (14).
Assessment of covariates
Age was calculated as months from the reported birth date to date of questionnaire return. Race, smoking status, birth weight, prematurity, multiple gestation birth, age of menarche, being breast-fed, alcohol consumption, and family history of diabetes were self-reported at baseline or in 1991. Parity and age at first birth were measured biennially. Physical activity, in MET units, was derived from the average time spent in certain activities (e.g., jogging or running) in 1989, 1991, 1997, and 2001.
Statistical analysis
Differences in participant characteristics by childhood somatotype at age 10 years were compared using χ2 or linear regression. Person-years were calculated based on date of return to date of diagnosis, death, or 1 July 2005, whichever came first. Multivariate Cox proportional hazard models were used to estimate the relative risk (RR) for the associations of somatotypes at ages 5, 10, and 20 years and BMI at age 18 years with type 2 diabetes risk. Somatotypes were categorized by 1 to ≥6. We used category 2 as the reference because most women had reported this category at both ages 5 and 10 years. We collapsed the uppermost categories because few women reported those categories.
In one multivariate model, estimates were adjusted for age (continuous), race (African American, Hispanic, Asian, or white), parity (0, 1–2, or ≥3) in combination with age at first birth (>24 years), family history of diabetes (maternal, paternal, or both), smoking (current, past, or never), and physical activity (quintiles). In a second model among participants not missing birth weight information (n = 87,349, case subjects = 2,771), estimates were adjusted for characteristics influencing childhood size including age, race, family history of diabetes, birth weight (<5.5, 5.5–6.9, 7.0–8.4, 8.5–9.9, or ≥10 pounds), prematurity or multiple gestation birth status of the woman (yes or no), and age of menarche (<12, 12, 13, 14, or >14 years). To test for significant trends, linear models were fitted using the median values of each category of exposure (e.g., 17.0, 19.0, 21.0, 23.5, 28.0, and 35.0 for BMI at age 18 years).
The differences in somatotype categories (between ages 5 and 10 and between ages 10 and 20) or in weight (between age 18 years and baseline) were used to assess whether change in size was associated with diabetes development. Models were additionally adjusted for starting size (e.g., adjustment for age 5 somatotype in evaluating the difference between ages 5 and 10), because where a woman began in size may reflect weight gain or loss (i.e., heavier women have the possibility to lose more weight).
The cumulative effect of overweight across the life course before reported diabetes was evaluated using the combination of somatotype ≥5 at age 10, BMI ≥25 kg/m2 at age 18, and BMI ≥25 kg/m2 at baseline (i.e., to represent adulthood). The reference group comprised women who reported no overweight at any of those time points. Analyses were performed using SAS (version 8.2; SAS Institute, Cary, NC).
RESULTS
There were 3,307 incident cases of type 2 diabetes over 16 years of follow-up, for an incidence rate of 197 cases per 100,000 person-years. Table 1 shows the characteristics for all women and by reported childhood somatotype at age 10 years categorized from 1 to ≥6. Women with somatotype 1 at age 10 years were more likely to be of minority race, to have birth weight <5.5 pounds, to have been born prematurely, and to have had a later age at menarche; whereas women with somatotype ≥6 were more likely to have birth weight >10 pounds, to actively smoke, to be nulliparous, and to have had a positive parental history of diabetes. Similar associations were found at age 5 years. Somatotypes at ages 5, 10, and 20 years were associated with each other. Of the women reporting somatotype ≥6 at age 10 years, 41% reported somatotype ≥6 at age 5 years, and 28% at age 20 years. Weight and BMI were also positively associated with somatotype; a 1–2 kg/m2 increase in adolescent or adult BMI was observed per increase in somatotype category.
Table 1
Baseline characteristics of NHSII participants by somatotype at age 10 years
| Total | Childhood somatotype at age 10 years | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ≥6 | ||
| Women (%) | 100 | 18.9 | 30.7 | 22.5 | 15.8 | 9.0 | 3.1 |
| Age (years) | 34.3 ± 5 | 34.7 ± 5 | 34.0 ± 5 | 34.1 ± 5 | 34.4 ± 5 | 34.7 ± 5 | 34.8 ± 5 |
| White (%) | 95.2 | 92.0 | 95.0 | 96.4 | 96.7 | 96.7 | 95.6 |
| Birth weight <5.5 pounds (%)* | 7.9 | 10.2 | 7.8 | 7.1 | 6.9 | 7.4 | 7.2 |
| Birth weight >10 pounds (%)* | 1.3 | 0.9 | 0.9 | 1.3 | 1.5 | 1.9 | 3.1 |
| Premature (%)* | 6.5 | 7.1 | 6.4 | 6.2 | 6.3 | 6.5 | 6.4 |
| Multiple gestation (%)* | 1.4 | 1.5 | 1.5 | 1.3 | 1.2 | 0.9 | 0.7 |
| Breast-fed (%)* | 34.0 | 33.0 | 34.2 | 35.5 | 33.4 | 33.1 | 31.9 |
| Menarche at age 12 years (%)* | 30.2 | 27.1 | 29.8 | 32.5 | 31.4 | 30.7 | 29.6 |
| Menarche at age ≥15 years (%)* | 7.6 | 12.1 | 8.7 | 5.7 | 4.7 | 4.6 | 4.9 |
| Nulliparous (%) | 30.4 | 29.6 | 29.3 | 29.3 | 31.5 | 34.4 | 37.9 |
| Age at first birth >24 years (%) | 41.0 | 40.5 | 42.1 | 42.3 | 40.6 | 37.3 | 34.7 |
| Parental history of diabetes (%) | 15.2 | 15.0 | 13.6 | 14.4 | 16.6 | 18.7 | 22.1 |
| Active smoker (%) | 13.4 | 13.4 | 11.6 | 12.4 | 14.4 | 17.8 | 20.8 |
| Nondrinker (%) | 35.0 | 33.4 | 34.5 | 36.0 | 35.9 | 35.3 | 36.2 |
| Physical activity (METs/week) | 24.9 ± 37 | 26.3 ± 40 | 25.0 ± 37 | 24.3 ± 36 | 24.1 ± 34 | 24.4 ± 35 | 26.0 ± 41 |
| Somatotype ≥6 at 5 years (%) | 1.6 | 0.1 | 0.1 | 0.1 | 0.4 | 2.3 | 41.0 |
| Somatotype ≥6 at 20 years (%) | 3.3 | 0.3 | 0.5 | 1.5 | 4.4 | 13.9 | 27.7 |
| BMI at age 18 (kg/m2) | 21.3 ± 3 | 19.3 ± 2 | 20.2 ± 2 | 21.6 ± 3 | 22.8 ± 3 | 23.9 ± 4 | 25.6 ± 6 |
| Adult weight (kg) | 65 ± 14 | 59.9 ± 10 | 61.8 ± 11 | 66.3 ± 14 | 70 ± 17 | 73.2 ± 18 | 76.5 ± 19 |
| Adult height (m) | 1.65 ± 0.07 | 1.65 ± 0.07 | 1.65 ± 0.07 | 1.65 ± 0.07 | 1.65 ± 0.07 | 1.65 ± 0.07 | 1.66 ± 0.07 |
| Adult BMI (kg/m2) | 24.1 ± 5 | 22.0 ± 3 | 22.8 ± 4 | 24 ± 5 | 26.1 ± 6 | 26.9 ± 6 | 27.9 ± 7 |
| Weight change since age 18 (kg) | 7.61 ± 11 | 7.31 ± 8 | 6.94 ± 9 | 7.92 ± 11 | 8.74 ± 13 | 8.26 ± 14 | 6.30 ± 16 |
Means ± SD presented unless indicated. n = 109,172.
*Reported in 1991 questionnaire (rather than at baseline in 1989 for all other covariates).
Somatotypes at ages 5, 10, and 20 years and BMI at age 18 years (Table 2) were all significantly and positively associated with diabetes risk (all Ptrend < 0.0001). Compared with having a somatotype of 2, the RR of diabetes associated with a somatotype ≥6 at age 5 was 2.19 (95% CI 1.79–2.67), at age 10 years was 2.57 (2.20–3.01), and at age 20 years was 5.67 (4.92–6.54). The RR among women who reported a BMI at age 18 >30 kg/m2 was 8.72 (7.58–10.02) compared with women with a BMI of 18–19 kg/m2. Among a subgroup not missing birth weight information (n = 87,175), adjustment for childhood factors including birth weight did not substantially change these estimates.
Table 2
Childhood and adolescent size and RR (95% CI) of type 2 diabetes in adulthood in NHSII
| PY | Cases of diabetes | Age-adjusted | Model 2* | Model 3† | |
|---|---|---|---|---|---|
| Age 5 somatotype | |||||
    1 1 | 417,996 | 756 | 1.13 (1.02–1.25) | 1.05 (0.95–1.16) | 1.01 (0.90–1.13) |
    2 2 | 533,990 | 807 | 1.00 | 1.00 | 1.00 |
    3 3 | 396,742 | 774 | 1.28 (1.15–1.41) | 1.25 (1.14–1.38) | 1.20 (1.08–1.34) |
    4 4 | 212,080 | 568 | 1.72 (1.54–1.91) | 1.65 (1.48–1.84) | 1.63 (1.45–1.84) |
    5 5 | 88,885 | 289 | 2.04 (1.78–2.33) | 1.85 (1.62–2.12) | 1.80 (1.55–2.09) |
    ≥6 ≥6 | 26,619 | 113 | 2.61 (2.14–3.18) | 2.19 (1.79–2.67) | 2.00 (1.59–2.51) |
    Ptrend Ptrend | <0.0001 | <0.0001 | <0.0001 | ||
| Age 10 somatotype | |||||
    1 1 | 317,663 | 441 | 1.04 (0.92–1.17) | 0.98 (0.87–1.11) | 0.96 (0.83–1.10) |
    2 2 | 516,959 | 649 | 1.00 | 1.00 | 1.00 |
    3 3 | 377,995 | 716 | 1.50 (1.35–1.67) | 1.49 (1.34–1.66) | 1.49 (1.32–1.67) |
    4 4 | 262,795 | 765 | 2.27 (2.05–2.52) | 2.17 (1.95–2.41) | 2.15 (1.92–2.42) |
    5 5 | 149,808 | 525 | 2.68 (2.39–3.01) | 2.45 (2.18–2.75) | 2.52 (2.21–2.86) |
    ≥6 ≥6 | 51,091 | 211 | 3.09 (2.64–3.61) | 2.57 (2.20–3.01) | 2.41 (2.02–2.88) |
    Ptrend Ptrend | <0.0001 | <0.0001 | <0.0001 | ||
| Age 20 somatotype | |||||
    1 1 | 75,871 | 83 | 0.97 (0.77–1.23) | 0.91 (0.72–1.15) | 0.96 (0.73–1.25) |
    2 2 | 443,762 | 443 | 1.00 | 1.00 | 1.00 |
    3 3 | 633,117 | 928 | 1.54 (1.37–1.72) | 1.52 (1.36–1.70) | 1.55 (1.36–1.76) |
    4 4 | 346,718 | 924 | 2.83 (2.53–3.17) | 2.71 (2.42–3.04) | 2.78 (2.44–3.16) |
    5 5 | 122,949 | 564 | 4.85 (4.28–5.49) | 4.42 (3.90–5.01) | 4.79 (4.16–5.51) |
    ≥6 ≥6 | 53,897 | 365 | 7.24 (6.30–8.32) | 5.67 (4.92–6.54) | 6.17 (5.26–7.23) |
    Ptrend Ptrend | <0.0001 | <0.0001 | <0.0001 | ||
| BMI at age 18 | |||||
    <18 kg/m2 <18 kg/m2 | 158,206 | 164 | 1.13 (0.94–1.35) | 1.06 (0.88–1.26) | 1.06 (0.86–1.30) |
    18–<19 kg/m2 18–<19 kg/m2 | 507,641 | 458 | 1.00 | 1.00 | 1.00 |
    20–<22 kg/m2 20–<22 kg/m2 | 530,083 | 727 | 1.52 (1.35–1.71) | 1.49 (1.32–1.67) | 1.51 (1.32–1.72) |
    22–<25 kg/m2 22–<25 kg/m2 | 312,876 | 848 | 3.02 (2.69–3.38) | 2.79 (2.49–3.13) | 2.86 (2.51–3.25) |
    25–<30 kg/m2 25–<30 kg/m2 | 127,826 | 721 | 6.32 (5.62–7.11) | 5.32 (4.72–5.99) | 6.10 (5.35–6.97) |
    ≥30 kg/m2 ≥30 kg/m2 | 39,682 | 389 | 11.55 (10.08–13.23) | 8.72 (7.58–10.02) | 9.26 (7.92–10.82) |
    Ptrend Ptrend | <0.0001 | <0.0001 | <0.0001 |
Data are RR (95% CI) unless otherwise indicated. n = 109,172.
*Model 2 adjusted for age, race, smoking status, parental history of diabetes, parity, age at first birth, and adult physical activity.
†Model 3 adjusted for age, race, parental history of diabetes, birth weight, multiple birth, prematurity, age of menarche (n = 87,175, case subjects = 2,681 in subgroup not missing birth weight information). PY, person-years.
Type 2 diabetes risk increased among women who reported increases in size whether by somatotype between ages 5 and 10 and between ages 10 and 20 or by weight gain since age 18 (Table 3). Compared with women reporting no change in somatotype, one or more unit increases in somatotype at these ages were associated with approximately twice the risk, whereas decreases in somatotype at these ages were associated with reduced risk. These associations were strengthened after adjustment for earlier somatotype. Weight gain since age 18, which is the difference between baseline weight and weight at age 18, was also significantly associated with risk of diabetes. Compared with women who had little change in weight (i.e., ±4.9 pounds), even a weight gain of 5–8 pounds doubled risks, whereas weight gain of >25 pounds increased risk by >20 times. Weight loss was associated with increased risk until after adjustment for BMI at age 18 to account for greater weight loss being associated with larger adolescent body size. In analyses stratified by BMI at age 18 years, weight loss of ≥10 pounds was significantly associated with a reduced risk of diabetes among women who were overweight (RR 0.45 [95% CI 0.22–0.91]) or obese (0.45 [0.28–0.72]) in adolescence but not among those who were lean (1.72 [0.76–3.90]).
Table 3
Changes in size and RR (95% CI) of type 2 diabetes in NHSII
| PY | Cases of diabetes | Age-adjusted | Model 2* | Model 3† | |
|---|---|---|---|---|---|
| Age 5–10 years | |||||
    Decrease Decrease | 19,572 | 29 | 0.93 (0.65–1.35) | 0.95 (0.65–1.37) | 0.63 (0.43–0.91) |
    No change No change | 1,235,621 | 1,943 | 1.00 | 1.00 | 1.00 |
    +1 +1 | 310,086 | 950 | 2.00 (1.85–2.16) | 1.86 (1.72–2.01) | 1.89 (1.75–2.05) |
    ≥2 ≥2 | 111,033 | 385 | 2.16 (1.93–2.41) | 1.95 (1.75–2.18) | 2.19 (1.95–2.45) |
| Age 10–20 years | |||||
    Decrease Decrease | 109,956 | 196 | 1.04 (0.89–1.21) | 1.00 (0.86–1.17) | 0.53 (0.45–0.62) |
    No change No change | 758,071 | 1,294 | 1.00 | 1.00 | 1.00 |
    +1 +1 | 621,736 | 1,274 | 1.27 (1.18–1.37) | 1.22 (1.12–1.31) | 1.86 (1.71–2.02) |
    ≥2 ≥2 | 186,550 | 543 | 1.89 (1.70–2.08) | 1.72 (1.55–1.90) | 2.87 (2.58–3.20) |
| Weight change since age 18 | |||||
    Loss ≥10 pounds Loss ≥10 pounds | 31,322 | 61 | 5.44 (4.11–7.20) | 4.48 (3.38–5.94) | 1.26 (0.93–1.71) |
    Loss 5–<10 pounds Loss 5–<10 pounds | 58,170 | 41 | 2.02 (1.45–2.82) | 1.84 (1.32–2.56) | 1.17 (0.84–1.63) |
    No change (±5 pounds) No change (±5 pounds) | 714,230 | 249 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
    Gain 5–<8 pounds Gain 5–<8 pounds | 226,283 | 169 | 2.05 (1.69–2.49) | 1.99 (1.64–2.42) | 2.10 (1.73–2.55) |
    Gain 8–<11 pounds Gain 8–<11 pounds | 182,828 | 219 | 3.24 (2.70–3.88) | 3.05 (2.54–3.66) | 3.10 (2.58–3.72) |
    Gain 11–<15 pounds Gain 11–<15 pounds | 177,159 | 437 | 6.54 (5.60–7.65) | 5.99 (5.12–7.00) | 5.78 (4.94–6.77) |
    Gain 15–<20 pounds Gain 15–<20 pounds | 114,008 | 477 | 10.92 (9.36–12.74) | 9.67 (8.28–11.30) | 8.74 (7.48–10.20) |
    Gain 20–<25 pounds Gain 20–<25 pounds | 76,539 | 464 | 15.72 (13.46–18.36) | 13.42 (11.48–15.70) | 11.11 (9.49–13.00) |
    Gain ≥25 pounds Gain ≥25 pounds | 95,774 | 1,190 | 32.28 (28.11–37.06) | 27.13 (23.57–31.23) | 20.41 (17.70–23.52) |
Data are RR (95% CI) unless otherwise indicated. n = 109,172.
*Model 2 adjusted for age, race, smoking status, parental history of diabetes, parity, age at first birth, and adult physical activity.
†Model 2 variables with additional adjustment for earlier size, i.e., somatotype at age 5 and 10 years and BMI at age 18 years. PY, person-years.
The prevalences of overweight by somatotype ≥5 at ages 5, 10, and 20 years were 7, 12, and 11%, respectively. Most of the cases (83%) in the cohort occurred among women who were overweight (BMI ≥25 kg/m2) at baseline with an incidence rate of 562 per 100,000 person-years. Among these women, the prevalences of overweight by somatotype ≥5 were 12, 21, and 23%, respectively.
To evaluate the cumulative effect of being overweight across the life course, women were jointly categorized as being overweight using somatotype at age 10, BMI at age 18 years, and BMI at baseline (with mean age of 34 years) (Fig. 1). Women who were overweight only as an adult (BMI >25 kg/m2) had an adjusted RR of 8.23 (95% CI 7.41–9.15) compared with women who were never overweight at any age. The adjusted RR increased to 15.10 (13.21–17.26) for women who were also overweight at age 10 (somatotype ≥5) and age 18 (BMI >25 kg/m2). However, women who were overweight at age 10 but came off the trajectory and became lean in adulthood (i.e., not being overweight in adulthood) did not have a significantly increased risk of diabetes (1.02, 0.74–1.40).
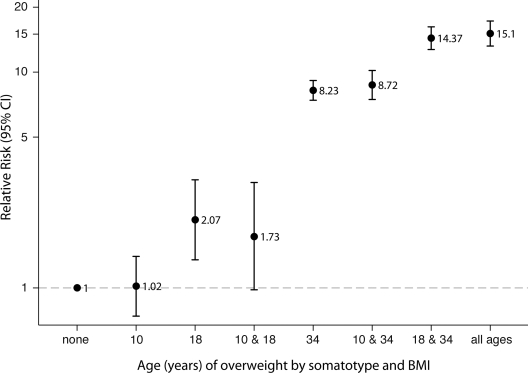
Adjusted RRs (95% CI) of type 2 diabetes by somatotype ≥5 at age 10, BMI ≥25 kg/m2 at age 18 years, and BMI ≥25 kg/m2 at baseline (average age of 34 years), after adjustment for age, race, smoking status, parental history of diabetes, parity, age at first birth, and physical activity. Number of cases of type 2 diabetes for each category: 503 for none; 50 for 10 years; 21 for 18 years; 10 for 10 and 18 years; 1,469 for 34 years; 260 for 10 and 34 years; 664 for 18 and 34 years; and 441 for all ages.
CONCLUSIONS
Among 109,172 women followed for >16 years, somatotypes at ages 5 and 10 years were positively associated with the risk of incident type 2 diabetes after adjustment for major risk factors. However, women who were overweight at age 10 but were lean in adulthood did not have an increased risk of diabetes associated with childhood overweight, underscoring the importance of continued efforts to control adiposity among overweight children.
Limited studies have investigated the long-term consequences of childhood overweight and the risk of type 2 diabetes in adulthood, with inferences hindered by small numbers of case subjects and/or lack of differentiation of diabetes type (2–8). In addition, some studies included case subjects with young age at onset (<30 years old) (3,5,7). One study using data based on records (185 cases) showed that the cumulative incidence of diabetes was doubled (from 4.2 to 8.4%) among women in the largest BMI group at age 11 years (>17.4 kg/m2) compared with those in the smallest BMI group (≤15.3 kg/m2) (4). We found a similar increase in risk using recalled somatotypes. In contrast, a study of older French women using recalled body shape, found an association between childhood thinness and diabetes risk (8). However, this birth cohort vastly differed from the NHSII as demonstrated by >60% of their case subjects having reported extreme leanness (somatotype 1) at age 8 years, possibly due to their nutritional status having been affected by World War II (8). Thus, our findings among a more recent birth cohort may be more relevant.
In addition to absolute size, longitudinal changes through different ages are also relevant to the risk of diabetes (17,18). Under normal development, infants lose weight after 6 months of age and continue doing so until ~5 years of age when their adiposity rebounds (18). Although we were unable to assess age at adiposity rebound, earlier rebound has been associated with increased risk of type 2 diabetes (17), and there is some suggestion that age at rebound matters more than the absolute size of the child at any point in time because it corresponds to weight gain. Our findings of increased risk associated with increases in somatotype and weight gain support the importance of change in size in addition to absolute size. These findings offer support for continued weight reduction efforts across the life span.
Moreover, in analyses of the cumulative effect of overweight through childhood, adolescence, and adulthood, the risk for type 2 diabetes was greatest among women reporting overweight by all three measures, even though the difference was marginal when compared with adolescent overweight. However, women who became lean in adulthood did not have an increased risk of diabetes associated with childhood overweight. Similar observations have been made from other studies of youth overweight and type 2 diabetes or related traits, with findings becoming nonsignificant after accounting for adult BMI (6,19,20). Tracking of metabolic risk factors (e.g., HDL and triglycerides) has also been observed after 21 years of follow-up from childhood (21), supporting the fact that cumulative overweight is linked with prolonged exposure to metabolic irregularities, putting children on the path to β-cell dysfunction earlier. It should be noted that although there is tracking of overweight to obesity from childhood to adulthood, the trajectory is not fixed from youth (18,22). It has been shown that ~10–30% of overweight children do not go on to be overweight as adults with conflicting evidence as to whether there is greater tracking in girls than in boys (22). Conversely, the majority of adults who are overweight were not in childhood (22) as confirmed here with only 12–20% of the overweight women reporting childhood overweight.
Our study had some limitations. Although experts have recommended BMI for the measurement of childhood size (18,23), we used recalled somatotype. Misclassification of childhood size is inevitable. However, two previous studies have shown that the accuracy of recall between childhood somatotypes and recorded childhood BMI did not differ by adult BMI (10,11). The misclassification of childhood size is thus more likely to be nondifferential, which cannot explain the observed positive association between childhood size and type 2 diabetes risk. In addition, our findings were in agreement with those of other studies that used childhood measures of weight and height (4,20). The generalizability of our findings may be limited to white women who comprised >95% of our study population. More research is needed in minority populations who have higher rates of overweight and diabetes (1,24). Last, we cannot rule out the possibility of residual confounding by unmeasured confounders because of the observational nature of the study.
As for strengths, the NHSII included a large number of women (>3,000 case subjects) and adjusted for many risk factors. Follow-up for previous studies required recontacting participants in adulthood with low to moderate success rates (20–60%) (5,7,19,20), whereas we evaluated somatotypes at baseline and >90% of the women remained for follow-up.
In summary, our findings demonstrate that the importance of childhood overweight stems largely from adult overweight. Women who do not continue to be overweight in adulthood do not have increased risks. It remains important then to promote lifestyle changes from youth so that the adverse trajectory could be avoided. Multiple interventions to address childhood overweight have been suggested (23), but these remain to be fully tested.
Acknowledgments
This study was funded by research grants CA50385 and DK58845 from the National Institutes of Health. E.H.Y., C.Z., and G.M.B.L. were supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Funding sources had no role in data collection, analysis, interpretation, or article submission, and researchers acted independently from funders.
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 27th annual scientific meeting of The Obesity Society, Washington, D.C., 24–28 October 2009.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
Articles from Diabetes Care are provided here courtesy of American Diabetes Association
Full text links
Read article at publisher's site: https://doi.org/10.2337/dc10-0100
Read article for free, from open access legal sources, via Unpaywall:
https://diabetesjournals.org/care/article-pdf/33/6/1364/606811/zdc00610001364.pdf
Free after 6 months at intl-care.diabetesjournals.org
http://intl-care.diabetesjournals.org/cgi/content/full/33/6/1364
Free to read at intl-care.diabetesjournals.org
http://intl-care.diabetesjournals.org/cgi/content/abstract/33/6/1364
Free after 6 months at intl-care.diabetesjournals.org
http://intl-care.diabetesjournals.org/cgi/reprint/33/6/1364.pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.2337/dc10-0100
Article citations
Child-to-adult body size change and risk of type 2 diabetes and cardiovascular disease.
Diabetologia, 67(5):864-873, 12 Dec 2023
Cited by: 1 article | PMID: 38085289 | PMCID: PMC10954919
Association of birth and childhood weight with risk of chronic diseases and multimorbidity in adulthood.
Commun Med (Lond), 3(1):105, 31 Jul 2023
Cited by: 1 article | PMID: 37524882 | PMCID: PMC10390459
The Reliability and Validity of Recalled Body Shape and the Responsiveness of Obesity Classification Based on Recalled Body Shape Among the Chinese Rural Population.
Front Public Health, 10:792394, 03 May 2022
Cited by: 0 articles | PMID: 35592083 | PMCID: PMC9110696
Body Mass Index Changes and Insulin Resistance at Age 4: A Prospective Cohort Study.
Front Endocrinol (Lausanne), 13:872591, 23 May 2022
Cited by: 2 articles | PMID: 35677718 | PMCID: PMC9169890
Weight tracking in childhood and adolescence and type 2 diabetes risk.
Diabetologia, 63(9):1753-1763, 18 May 2020
Cited by: 8 articles | PMID: 32424540 | PMCID: PMC9519170
Go to all (27) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Life-course weight characteristics and the risk of gestational diabetes.
Diabetologia, 53(4):668-678, 31 Dec 2009
Cited by: 33 articles | PMID: 20043144 | PMCID: PMC2901841
Body fatness throughout the life course and the incidence of premenopausal breast cancer.
Int J Epidemiol, 45(4):1103-1112, 27 Jul 2016
Cited by: 15 articles | PMID: 27466312 | PMCID: PMC5841631
Prospective study of body size throughout the life-course and the incidence of endometrial cancer among premenopausal and postmenopausal women.
Int J Cancer, 137(3):625-637, 05 Feb 2015
Cited by: 44 articles | PMID: 25641700 | PMCID: PMC5241095
Body fatness over the life course and risk of serrated polyps and conventional adenomas.
Int J Cancer, 147(7):1831-1844, 31 Mar 2020
Cited by: 4 articles | PMID: 32150293 | PMCID: PMC7423709
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: CA50385
Grant ID: R01 CA050385
NIDDK NIH HHS (2)
Grant ID: DK58845
Grant ID: R01 DK058845




