Abstract
Free full text

Emergence of Methicillin-Resistant Staphylococcus aureus of Animal Origin in Humans
Abstract
In 2003 in the Netherlands, a new methicillin-resistant Staphylococcus aureus (MRSA) strain emerged that could not be typed with Sma1 pulsed-field gel electrophoresis (NT-MRSA). The association of NT-MRSA in humans with a reservoir in animals was investigated. The frequency of NT-MRSA increased from 0% in 2002 to >21% after intensified surveillance was implemented in July 2006. Geographically, NT-MRSA clustered with pig farming. A case–control study showed that carriers of NT-MRSA were more often pig or cattle farmers (pig farmers odds ratio [OR] 12.2, 95% confidence interval [CI] 3.1–48.6; cattle farmers OR 19.7, 95% CI 2.3–169.5). Molecular typing showed that the NT-MRSA strains belonged to a new clonal complex, ST 398. This study shows that MRSA from an animal reservoir has recently entered the human population and is now responsible for >20% of all MRSA in the Netherlands.
Methicillin-resistant Staphylococcus aureus (MRSA) has traditionally been considered a nosocomial pathogen. However, for several years the number of reports of so-called community-onset MRSA (CO-MRSA) has been rapidly increasing (1). CO-MRSA has no relation to healthcare and is usually associated with the presence of Panton-Valentine leukocidin toxin (PVL) and SCCmec types IV and V (2,3). In 2004 and 2005, some unexpected cases of MRSA were found in patients who were associated with pig farms (4,5). Genotyping showed that these MRSA isolates were nontypable by pulsed-field gel electrophoresis (PFGE) and belonged to 1 spa type (t108). The aims of this study were to determine if nontypable MRSA (NT-MRSA) isolates are associated with pig farming and to compare the phenotypic, genotypic, and epidemiologic features of NT-MRSA with those of typable MRSA strains.
Methods
National MRSA Database
The National Institute for Public Health and the Environment (RIVM) is the national reference center for MRSA in the Netherlands (www.rivm.nl/mrsa). According to national guidelines, all microbiology laboratories send the first isolate of newly identified carriers of MRSA to RIVM. Strains are confirmed to be MRSA by a Martineau PCR and by mecA PCR assay (6,7). Since 2002, all strains are typed by using PFGE (8), and the presence of PVL genes is determined (9).
Selection of Cases and Controls
Cases and controls were selected from the national MRSA database at RIVM. Case-patients were defined as persons carrying NT-MRSA who provided the first isolate from a cluster of 1 particular referring laboratory (index-patient) in the period January 2003 to September 2005. Cases were considered to be secondary to an index case when the strain was isolated within 3 months after the previous isolate with the same PFGE typing result. Controls were persons who carried MRSA that was typable with PFGE and who also fulfilled the index-patient definition. Controls were derived from the laboratories that provided cases and were selected at random. Twice as many controls as case-patients were selected.
Collection of Epidemiologic Background Information
Data were collected by questionnaires that were sent to the referring laboratories. The questionnaire contained items about patient characteristics (birth date, sex, postal code, presence or absence of infection, hospital admission dates, profession, profession of partner, profession of parents, and contact with animals, e.g., pigs, cows, horses, chickens, cats, or dogs) and microbiologic data (isolation date, source of culture, medical specialty). All data were collected and entered into the database without our knowing whether it concerned a case or control.
Initially, 41 cases and 82 controls from 26 different laboratories were selected from the national database. The response rate was 98% (40 cases and 81 controls). During workup, 5 cases and 5 controls were excluded for the following reasons: the confirmation test of the isolate indicated that it was not methicillin resistant (1 case), or the case did not fulfill the case definition because it was not the first case from a cluster (4 cases). Since 2 of these cases were from laboratories that had no other case in the study, the accompanying controls were excluded (n = 3). Two controls were identified outside the study period. Finally, 35 cases and 76 controls from 24 different laboratories were included in the analysis.
Molecular Typing and Susceptibility Testing
All MRSA isolates were typed by PFGE (8). All isolates from case-patients and 74 isolates from controls were typed by spa typing (10). Multilocus sequence typing (MLST) was performed on all case isolates, as well as on 1 strain of each spa type of the control isolates (n = 37) (11). PCR of the staphylococcal chromosome cassette (SCCmec) was performed according to Zhang et al. on all isolates from case-patients and 74 isolates from controls (12). The presence or absence of PVL genes (lukS-PV/lukF-PV) was determined in all case isolates and in 71 control isolates. The PVL genes were detected by PCR according to the method of Lina et al. (13). The susceptibility to antimicrobial agents was tested for 32 case isolates and 74 control isolates, according to CLSI guidelines that used Mueller-Hinton agar and multipoint inoculation (14).
Statistical Analysis
Data were entered into an Excel database (Microsoft Windows version 97 SR-2, Redmond, WA, USA) and further analyzed by using SAS (version 9.1) software package (SAS Institute Inc., Cary, NC, USA). Chi-square test and Fisher exact test for ordinal variables and Student t test for continuous variables were used for univariate analysis. Variables associated with both case-control status and the exposure (i.e., contact with pigs or cattle, respectively) with a p value <0.2 were included in the multivariate logistic regression model. If such variables changed the risk estimate for >10%, they were left in the model. All statistical tests were 2-sided, and a p value <0.05 was considered statistically significant.
Results
Epidemic Curve
The first NT-MRSA isolate was found in February 2003. In subsequent years, an increasing number of NT-MRSA isolates were found. The percentage of NT-MRSA relative to the total number of MRSA isolates in the Netherlands that were unique or first from a cluster rose from 0% in 2002 to 5.5% in the first half of 2006 and to >21% in the second half of 2006, after the introduction of intensified surveillance in July 2006.
Geographic Distribution
Figure 1 shows the geographic distribution of NT-MRSA and typable MRSA isolates, plotted over the density of the pig and human populations, respectively. The density of NT-MRSA isolates corresponds to the density of pig farming, whereas the density of typable strains corresponds to the density of the human population. The density of cattle farms is more or less identical to the density of pig farms.
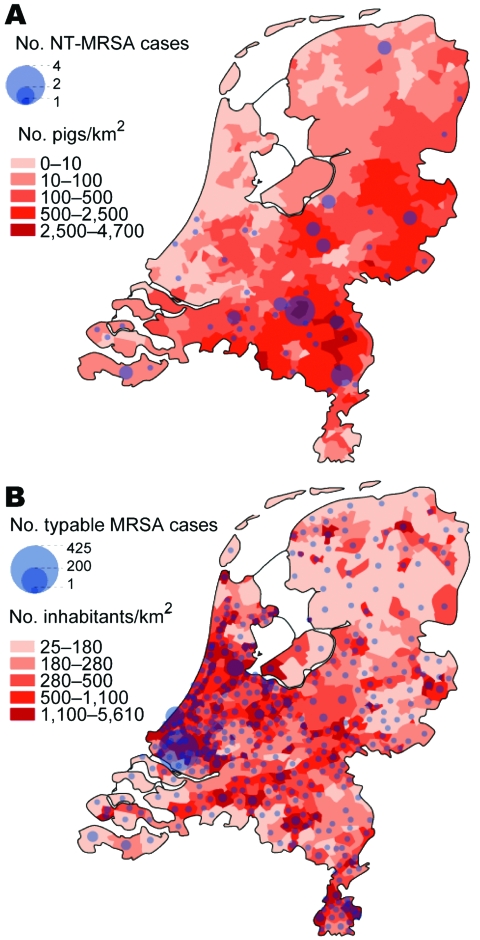
A) Number of nontypable methicillin-resistant Staphylococcus aureus (NT-MRSA) isolates per municipality received at the National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands, January 2003–June 2005. The background color represents the density of pigs per km2 in 2003. B) Number of typable MRSA per municipality received at the RIVM January 2003–June 2005. The background color represents the population density per km2 (source: CBS Statline).
Epidemiologic Data
Results of the univariate analysis are shown in Table 1. Comparable values were observed for the baseline characteristics of sex and age. Case-patients more often lived in rural areas and indicated more frequent contact with pigs or cattle than did controls. Controls were more often associated with healthcare facilities.
Table 1
| Variable | Cases | Controls | Odds ratio (95% CI)† | p value | |||
|---|---|---|---|---|---|---|---|
| No. | No. (%) or mean ± SD with variable | No. | No. (%) or mean ± SD with variable | ||||
| Gender (male) | 35 | 20 (57) | 76 | 36 (47) | 1.5 (0.7–3.3) | 0.34 | |
| Age, y | 35 | 42.7 ± 25.3 | 76 | 47.3 ± 24.7 | 0.37 | ||
| Residence | 35 | 75 | |||||
| Rural area | 14 (40) | 6 (8) | 7.7 (2.6–22.7)§ | <0.01 | |||
| Urban area | 20 (57) | 66 (85) | |||||
| Foreign country |
| 1 (3) |
|
| 3 (4) |
|
|
| Contact with pigs | 29 | 11 (38) | 63 | 3 (5) | 12.2 (3.1–48.6) | <0.01 | |
| Contact with cattle | 29 | 7 (24) | 63 | 1 (2) | 19.7 (2.3–169.5) | <0.01 | |
| Unexpected MRSA | 35 | 27 (77) |
| 76 | 34 (45) | 4.2 (1.7–10.4) | <0.01 |
| Probable source | 35 | 76 | |||||
| Healthcare | 5 (14) | 39 (51) | 0.01 | ||||
| Foreign country | 3 (9) | 5 (7) | |||||
| Other | 12 (34) | 10 (13) | |||||
| Unknown |
| 15 (43) |
|
| 22 (29) |
|
|
| Active infection | 35 | 19 (54) | 76 | 29 (38) | 1.9 (0.9–4.3) | 0.11 | |
| Skin/soft tissue | 10 (56) | 24 (83) | 0.3 (0.1–1.0) | 0.05 | |||
| Airways | 3 (17) | 0 | |||||
| Other | 6 (28) | 5 (17) | |||||
| Hospital admission | 35 | 17 (49) | 76 | 24 (32) | 2.0 (0.9–4.6) | 0.08 | |
| Hospital stay, d | 16 | 18.9 ± 20.2 | 22 | 23.5 ± 30.9 | 0.60 | ||
*SD, standard deviation; CI, confidence interval; No., number of cases or controls for whom data are available.
†Odds ratio was determined for rural area relative to urban area.
Among case-patients, MRSA was more frequently found in clinical samples (an unexpected finding) compared with controls, whose MRSA was found more often by targeted screening in nose, throat, and perineum. Among persons infected by MRSA, respiratory tract infections were more frequent in case-patients, whereas skin and soft tissue infections predominated in controls.
Multivariate analysis that used a model with the variables describing type of residence (rural vs. other) and contact with pigs, cattle, cats, and dogs (yes, no, or unknown) showed that contact with pigs and contact with cattle were independent statistically significant variables. The adjusted odds ratios (OR) for pigs and cattle were 9.4 (95% confidence interval [CI] 1.8–47.7) and 13.5 (95% CI 1.0–179.3), respectively.
Molecular Typing
Thirty-two of 35 case-patients had MLST sequence type (ST) 398; 1 had ST 9; and the remaining 2 had ST 752 and 753, closely related to 398 (Figure 2). Among case-patients, the most frequent spa types were t108, t011, and t034 (Table 2). These MLST and spa types were not found among the controls. Twenty-two different STs and 37 different spa types were found in the controls (Table 2 and Figure 2).

Genetic relatedness of methicillin-resistant Staphylococcus aureus from cases and controls, represented as a minimum spanning tree based on multilocus sequence typing (MLST) profiles. Each circle represents a sequence type, and numbers in the circles denote the sequence type. The size of the circle indicates the number of isolates with this sequence type. The number under and right of the lines connecting types denotes the number of differences in MLST profiles. The halos surrounding the circles indicate complexes of sequence types that differ by <3 loci.
Table 2
| Type | Cases, no. (%) | Controls, no. (%) | p value |
|---|---|---|---|
| spa | |||
| t108 | 14 (40) | 0 | <0.01 |
| t011 | 8 (23) | 0 | |
| t034 | 6 (17) | 0 | |
| t571 | 3 (9) | 0 | |
| t567 | 2 (6) | 0 | |
| t337 | 1 (3) | 0 | |
| t898 | 1 (3) | 0 |
|
| SCCmec | |||
| I | 0 | 4 (9) | <0.01 |
| II | 0 | 7 (16) | |
| III | 4 (17) | 6 (14) | |
| IV | 2 (8) | 21 (49) | |
| V | 18 (75) | 5 (12) |
Panton-Valentine leukocidin
3 (9)
10 (14)
0.21
SCCmec typing showed that in isolates from cases SCCmec types III, IV, and V were found, whereas in isolates from controls all SCCmec types were found (Table 2). For 11 cases and 33 controls, the SCCmec type could not be determined. There was no difference in the presence of the PVL genes (Table 2).
Antimicrobial Agent Susceptibility
Table 3 shows the percentage of strains that were resistant to various antimicrobial agents. Isolates from case-patients were significantly more often resistant to doxycycline and clindamycin than were isolates from controls.
Table 3
| Agent | Cases, no. (%) | Controls, no. (%) | p value |
|---|---|---|---|
| Doxycycline | 25 (78) | 10 (14) | <0.01 |
| Ciprofloxacin | 1 (3) | 36 (49) | <0.01 |
| Tobramycin | 4 (13) | 25 (34) | 0.02 |
| Gentamicin | 2 (6) | 12(16) | 0.14 |
| Clindamycin | 12 (38) | 15 (20) | 0.05 |
| Erythromycin | 15 (46) | 29 (39) | 0.35 |
| Cotrimoxazole | 0 | 7 (10) | 0.07 |
| Rifampin | 0 | 6 (8) | 0.11 |
| Mupirocin | 0 | 5 (7) | 0.15 |
| Vancomycin | 0 | 0 |
*MRSA, methicillin-resistant Staphylococcus aureus.
Discussion
A new type of MRSA recently emerged in the Netherlands. The first isolate was found in 2003, and since then it has been found with increasing frequency. The geographic origin of NT-MRSA correlates with the density of pig populations. This association was confirmed by the results from this case-control study, which show that NT-MRSA is significantly related to contact with pigs. In addition, a significant association was found with cattle. After multivariate analysis, contact with pigs and cattle were the only 2 significant independent variables. Screening of a representative sample of pigs in the Netherlands was recently performed and showed that nearly 40% of the pigs were colonized with a comparable strain of MRSA (MLST 398) and that ≈80% of the pig farms were affected (15). The association between NT-MRSA and cattle was not expected when this study was initiated and needs further evaluation.
On the basis of the above-mentioned findings, we conclude that this new MRSA strain is of animal origin (pigs and probably cows). Transmission of MRSA between animals and humans has previously been described, e.g., associated with colonized companion animals, horses, and persons who take care of them (16–19). However, the MRSA clones in these reports were known human clones, suggesting human-to-animal transmission in origin. Baptiste et al. found specific PFGE clones in horses that were never observed before (20). Until now, transmission of these clones to humans has not been reported.
We assume that this problem is not limited to the Netherlands. First, widespread dissemination in pigs in the Netherlands has been found. When one considers the intensive international transport of pigs, it is unlikely that this situation is limited to the Netherlands. Second, 3 of the case-patients came from abroad, 1 tourist and 2 adopted children from Asia. Also, MLST 398 was recently found in animals (pig, dog, and foal) and in humans in Germany (21). Finally, in Hong Kong Special Administrative Region, People’s Republic of China, MRSA with MLST 398 has been found in 2 patients with bacteremia (22).
The origin of the current NT-MRSA situation is difficult to elucidate. One earlier study can be found on carriage of S. aureus in pig farmers and pigs in France (23). It reported an increased carriage rate in pig farmers caused by transmission of S. aureus from pigs that also carried MLST ST 9 and 398. Further typing of the French ST 398 isolates at RIVM showed homology with the Dutch isolates. However, in the French study most of the MLST 398 strains were susceptible to β-lactam antimicrobial agents. The most likely explanation for the current findings is that MLST 398 is a commensal strain in pigs, which originally was methicillin susceptible. As most NT-MRSA isolates were resistant to doxycycline, the spread is facilitated by the abundant use of tetracyclines in pig and cattle farming (15).
What are the implications of these findings? Persons working or living in close contact with pigs or cows are at increased risk of becoming colonized and infected with MRSA. Infections can be severe, as is indicated by the hospital admission rate. Also, a case of endocarditis has been reported recently (24). At present, whether this strain is spreading further in the community is not clear. Before final recommendations for control can be made, the current size of the reservoir in farm animals and in humans has to be determined at an international level.
Acknowledgments
We thank Ing. H. Giesbers for constructing Figure 2; R. Bosboom, A. Demeulemeester, B. v. Dijke, B. Hendrickx, R. Hendrix, C. Hol, E. IJzerman, S. Kuijpers, A. Lampe, M.K.E. Nohlmans-Paulssen, N. Meessen, A. Möller, M. Peeters, D. Potters, P. Rietra, P. Schneeberger, F. Sebens, L. Spanjaard, A. Troelstra, C. Vandebroucke-Grauls, C. Verduin, and M. Wulf for answering the questionnaires; M. van Santen-Verheuvel and E. Spalburg for performing spa, MLST, and SCCmec typing and susceptibility testing; C. Klaassen for SCCmec typing; and L. Schouls for analysis of the MLST data.
Biography
Dr van Loo is a medical microbiologist at the University Hospital Maastricht. The research described in this manuscript was performed as part of her training as a medical microbiologist.
Footnotes
Suggested citation for this article: van Loo I, Huijsdens X, Tiemersma E, de Neeling A, van de Sande-Bruinsma N, Beaujean D, et al. Emergence of methicillin-resistant Staphylococcus aureus of animal origin in humans. Emerg Infect Dis [serial on the Internet]. 2007 Dec [date cited]. Available from http://www.cdc.gov/EID/content/13/12/zzz.htm
References
Articles from Emerging Infectious Diseases are provided here courtesy of Centers for Disease Control and Prevention
Full text links
Read article at publisher's site: https://doi.org/10.3201/eid1312.070384
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2876750?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.3201/eid1312.070384
Article citations
Isolation of community-associated methicillin-resistant Staphylococcus aureus (MRSA) sequence type (ST) 30 from house rats (Rattus tanezumi) in Hong Kong.
One Health, 19:100861, 18 Jul 2024
Cited by: 0 articles | PMID: 39157653 | PMCID: PMC11327950
Virulence attributes of successful methicillin-resistant Staphylococcus aureus lineages.
Clin Microbiol Rev, 36(4):e0014822, 20 Nov 2023
Cited by: 1 article | PMID: 37982596
Review
Effect of veterinary feed directive rule changes on tetracycline-resistant and erythromycin-resistant bacteria (Salmonella, Escherichia, and Campylobacter) in retail meats in the United States.
PLoS One, 18(8):e0289208, 03 Aug 2023
Cited by: 4 articles | PMID: 37535600 | PMCID: PMC10399851
The Relationship between Reticuloruminal Temperature, Reticuloruminal pH, Cow Activity, and Clinical Mastitis in Dairy Cows.
Animals (Basel), 13(13):2134, 28 Jun 2023
Cited by: 0 articles | PMID: 37443932
Biosecurity and Hygiene Procedures in Pig Farms: Effects of a Tailor-Made Approach as Monitored by Environmental Samples.
Animals (Basel), 13(7):1262, 05 Apr 2023
Cited by: 4 articles | PMID: 37048519 | PMCID: PMC10093544
Go to all (232) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Increase in a Dutch hospital of methicillin-resistant Staphylococcus aureus related to animal farming.
Clin Infect Dis, 46(2):261-263, 01 Jan 2008
Cited by: 103 articles | PMID: 18171259
A Livestock-Associated, Multidrug-Resistant, Methicillin-Resistant Staphylococcus aureus Clonal Complex 97 Lineage Spreading in Dairy Cattle and Pigs in Italy.
Appl Environ Microbiol, 82(3):816-821, 20 Nov 2015
Cited by: 53 articles | PMID: 26590279 | PMCID: PMC4725266
Community-acquired MRSA and pig-farming.
Ann Clin Microbiol Antimicrob, 5:26, 10 Nov 2006
Cited by: 198 articles | PMID: 17096847 | PMCID: PMC1654169
Methicillin resistance in Staphylococcus isolates: the "mec alphabet" with specific consideration of mecC, a mec homolog associated with zoonotic S. aureus lineages.
Int J Med Microbiol, 304(7):794-804, 28 Jun 2014
Cited by: 73 articles | PMID: 25034857
Review

 §¶
§¶



