Abstract
Free full text

Gene p53 mutations are restricted to poorly differentiated and undifferentiated carcinomas of the thyroid gland.
Abstract
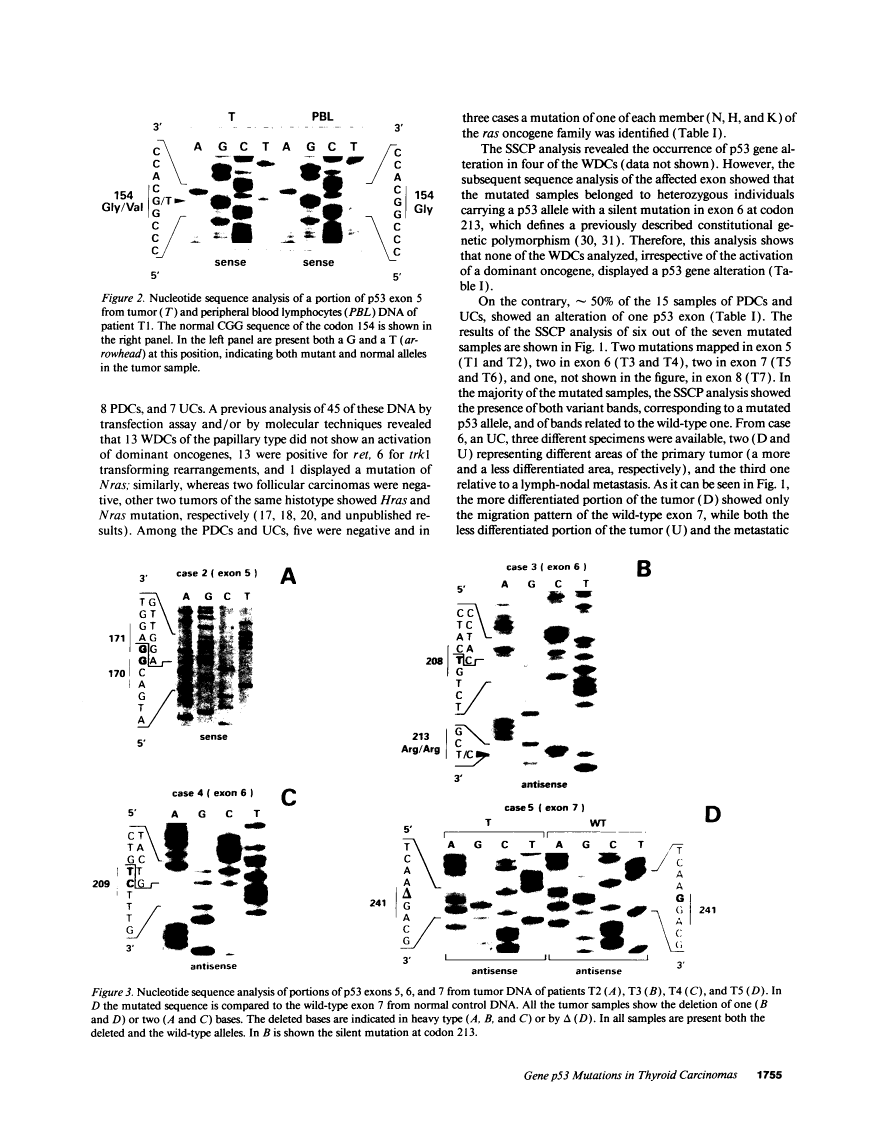
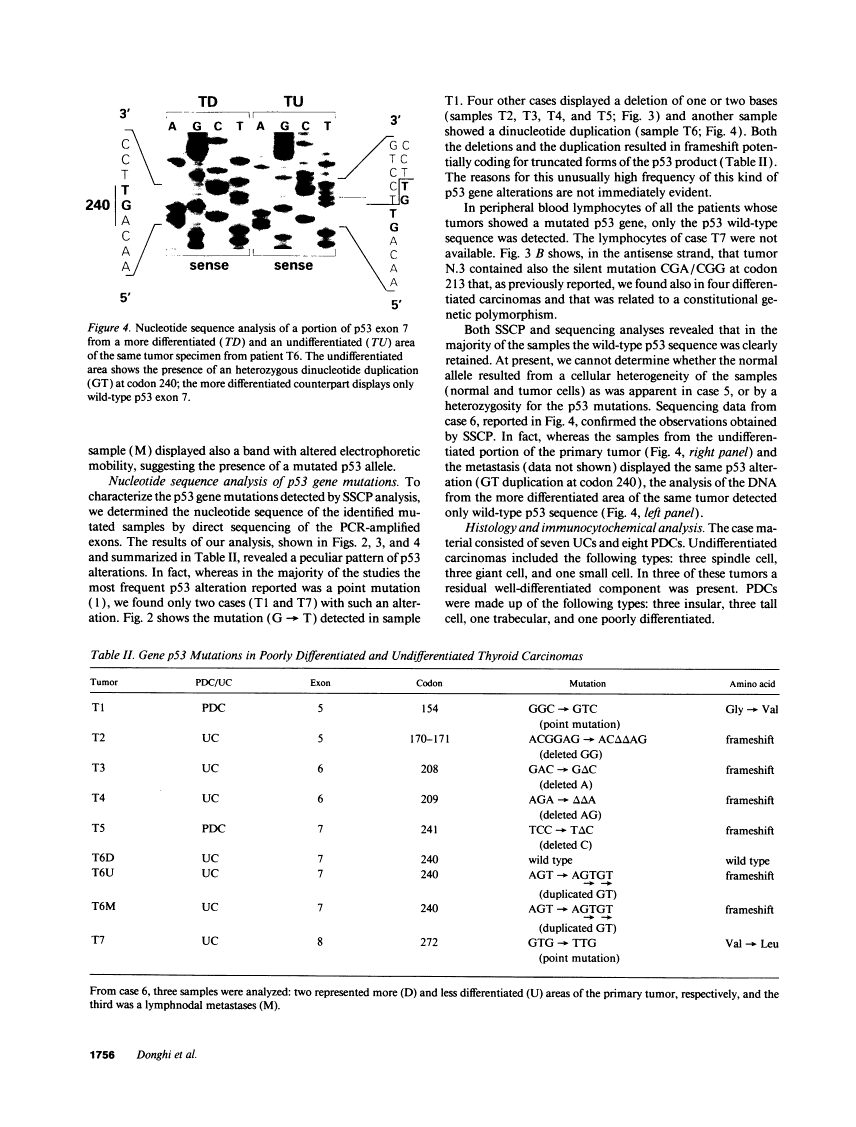
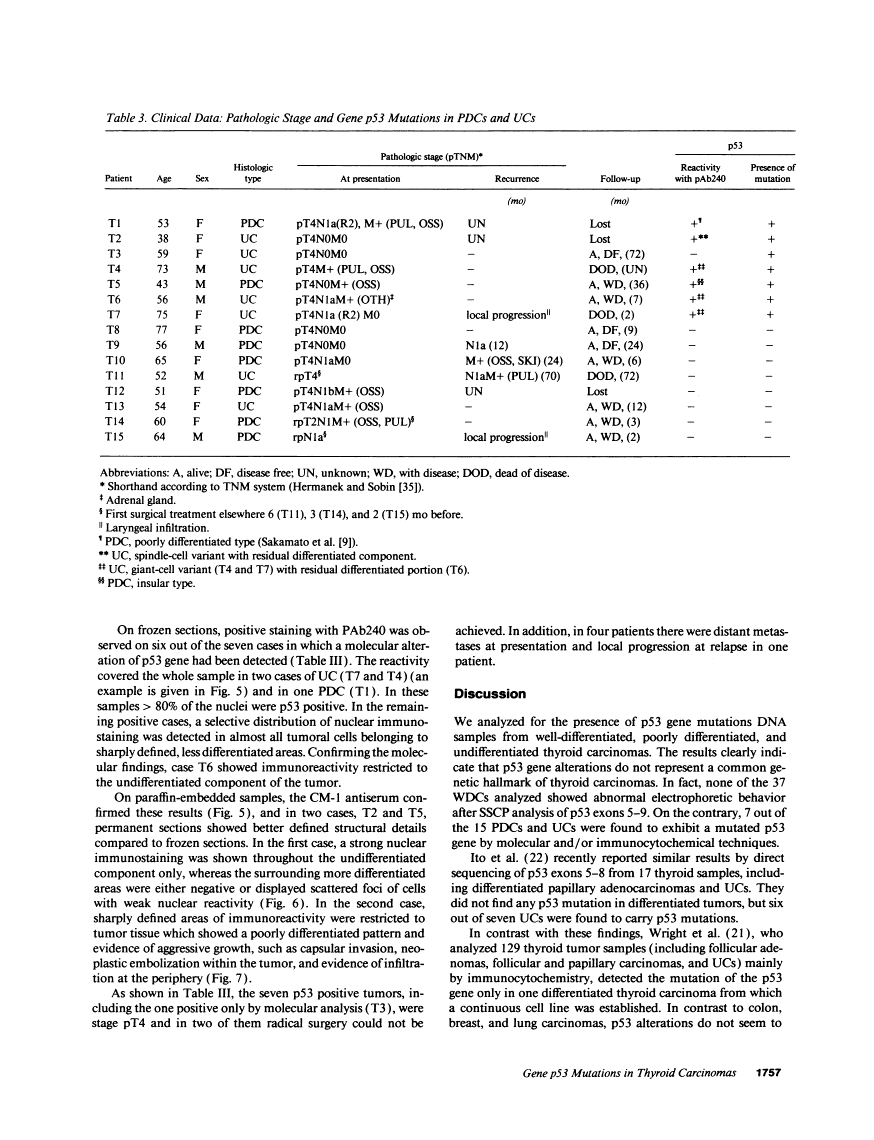
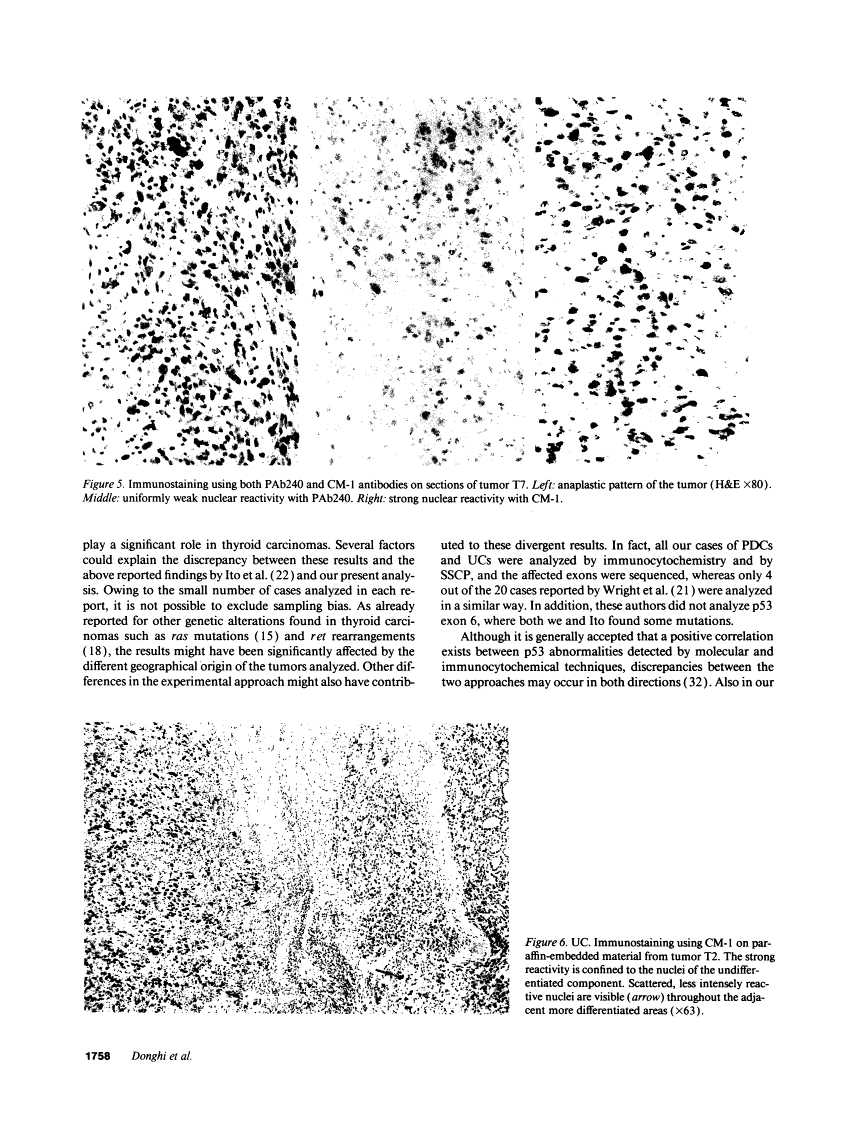
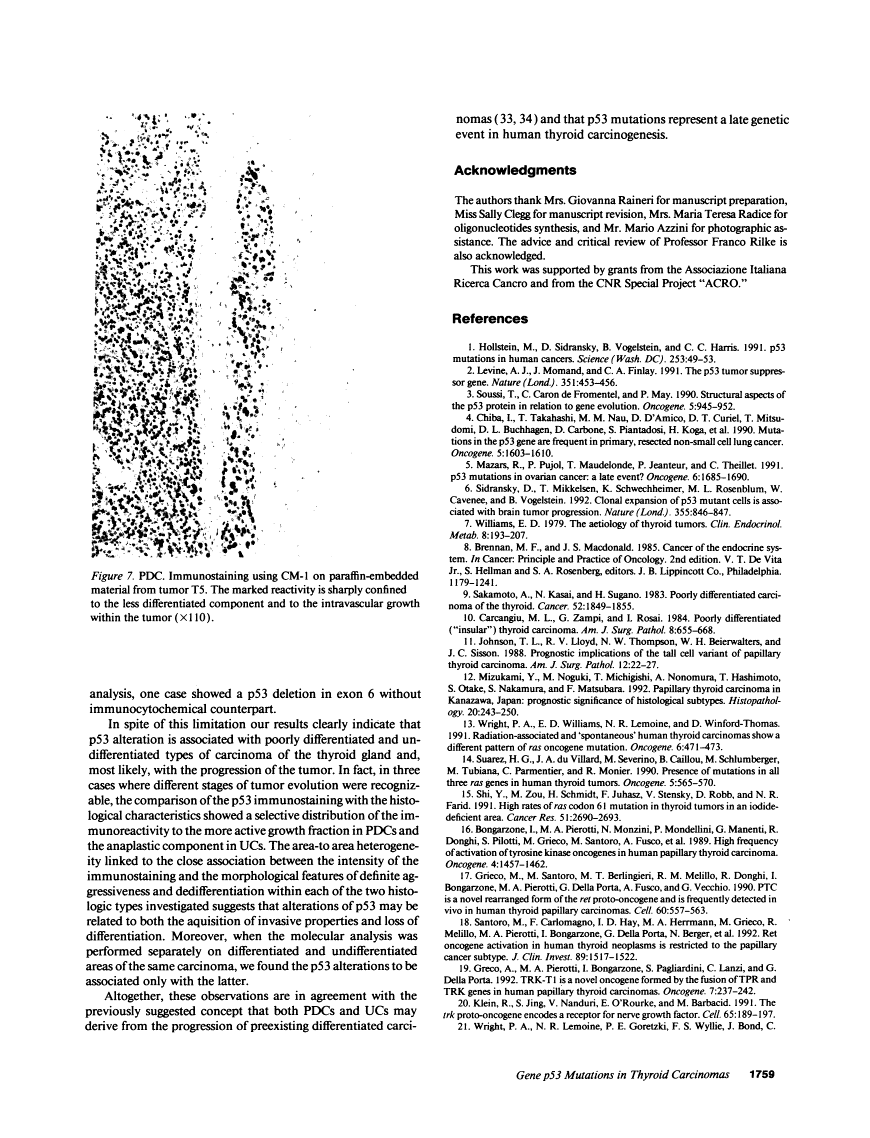
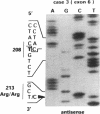
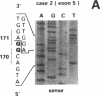
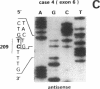
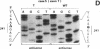
The p53 gene was analyzed in tumor specimens obtained from 52 patients with various types of carcinoma of the thyroid gland by a combined molecular and immunocytochemical approach. The histologic types included 37 well-differentiated papillary and follicular carcinomas, 8 poorly differentiated, and 7 undifferentiated carcinomas. The p53 gene was shown to be unaffected in all differentiated tumors by single-strand conformation polymorphism analysis. However, in two out of eight (25%) of poorly differentiated carcinomas and five out of seven (71%) undifferentiated carcinomas, p53 mutations were identified and subsequently characterized by DNA sequencing. One undifferentiated carcinoma displayed two areas with varying degrees of differentiation. The comparative analysis of the p53 gene, in both the more and the less differentiated area of this tumor, clearly showed that the p53 mutation was confined to the latter component of the tumor specimen. These results indicate that mutations of the p53 gene are associated with the most aggressive histologic types of thyroid tumors, such as the undifferentiated carcinoma and, to a certain extent, the poorly differentiated carcinoma, and that the alterations of this gene represent a late genetic event in human thyroid carcinogenesis.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.5M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. [Abstract] [Google Scholar]
- Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. [Abstract] [Google Scholar]
- Soussi T, Caron de Fromentel C, May P. Structural aspects of the p53 protein in relation to gene evolution. Oncogene. 1990 Jul;5(7):945–952. [Abstract] [Google Scholar]
- Chiba I, Takahashi T, Nau MM, D'Amico D, Curiel DT, Mitsudomi T, Buchhagen DL, Carbone D, Piantadosi S, Koga H, et al. Mutations in the p53 gene are frequent in primary, resected non-small cell lung cancer. Lung Cancer Study Group. Oncogene. 1990 Oct;5(10):1603–1610. [Abstract] [Google Scholar]
- Mazars R, Pujol P, Maudelonde T, Jeanteur P, Theillet C. p53 mutations in ovarian cancer: a late event? Oncogene. 1991 Sep;6(9):1685–1690. [Abstract] [Google Scholar]
- Sidransky D, Mikkelsen T, Schwechheimer K, Rosenblum ML, Cavanee W, Vogelstein B. Clonal expansion of p53 mutant cells is associated with brain tumour progression. Nature. 1992 Feb 27;355(6363):846–847. [Abstract] [Google Scholar]
- Williams ED. The aetiology of thyroid tumours. Clin Endocrinol Metab. 1979 Mar;8(1):193–207. [Abstract] [Google Scholar]
- Sakamoto A, Kasai N, Sugano H. Poorly differentiated carcinoma of the thyroid. A clinicopathologic entity for a high-risk group of papillary and follicular carcinomas. Cancer. 1983 Nov 15;52(10):1849–1855. [Abstract] [Google Scholar]
- Carcangiu ML, Zampi G, Rosai J. Poorly differentiated ("insular") thyroid carcinoma. A reinterpretation of Langhans' "wuchernde Struma". Am J Surg Pathol. 1984 Sep;8(9):655–668. [Abstract] [Google Scholar]
- Johnson TL, Lloyd RV, Thompson NW, Beierwaltes WH, Sisson JC. Prognostic implications of the tall cell variant of papillary thyroid carcinoma. Am J Surg Pathol. 1988 Jan;12(1):22–27. [Abstract] [Google Scholar]
- Mizukami Y, Noguchi M, Michigishi T, Nonomura A, Hashimoto T, Otakes S, Nakamura S, Matsubara F. Papillary thyroid carcinoma in Kanazawa, Japan: prognostic significance of histological subtypes. Histopathology. 1992 Mar;20(3):243–250. [Abstract] [Google Scholar]
- Wright PA, Williams ED, Lemoine NR, Wynford-Thomas D. Radiation-associated and 'spontaneous' human thyroid carcinomas show a different pattern of ras oncogene mutation. Oncogene. 1991 Mar;6(3):471–473. [Abstract] [Google Scholar]
- Suarez HG, du Villard JA, Severino M, Caillou B, Schlumberger M, Tubiana M, Parmentier C, Monier R. Presence of mutations in all three ras genes in human thyroid tumors. Oncogene. 1990 Apr;5(4):565–570. [Abstract] [Google Scholar]
- Shi YF, Zou MJ, Schmidt H, Juhasz F, Stensky V, Robb D, Farid NR. High rates of ras codon 61 mutation in thyroid tumors in an iodide-deficient area. Cancer Res. 1991 May 15;51(10):2690–2693. [Abstract] [Google Scholar]
- Bongarzone I, Pierotti MA, Monzini N, Mondellini P, Manenti G, Donghi R, Pilotti S, Grieco M, Santoro M, Fusco A, et al. High frequency of activation of tyrosine kinase oncogenes in human papillary thyroid carcinoma. Oncogene. 1989 Dec;4(12):1457–1462. [Abstract] [Google Scholar]
- Grieco M, Santoro M, Berlingieri MT, Melillo RM, Donghi R, Bongarzone I, Pierotti MA, Della Porta G, Fusco A, Vecchio G. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell. 1990 Feb 23;60(4):557–563. [Abstract] [Google Scholar]
- Santoro M, Carlomagno F, Hay ID, Herrmann MA, Grieco M, Melillo R, Pierotti MA, Bongarzone I, Della Porta G, Berger N, et al. Ret oncogene activation in human thyroid neoplasms is restricted to the papillary cancer subtype. J Clin Invest. 1992 May;89(5):1517–1522. [Europe PMC free article] [Abstract] [Google Scholar]
- Greco A, Pierotti MA, Bongarzone I, Pagliardini S, Lanzi C, Della Porta G. TRK-T1 is a novel oncogene formed by the fusion of TPR and TRK genes in human papillary thyroid carcinomas. Oncogene. 1992 Feb;7(2):237–242. [Abstract] [Google Scholar]
- Klein R, Jing SQ, Nanduri V, O'Rourke E, Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991 Apr 5;65(1):189–197. [Abstract] [Google Scholar]
- Wright PA, Lemoine NR, Goretzki PE, Wyllie FS, Bond J, Hughes C, Röher HD, Williams ED, Wynford-Thomas D. Mutation of the p53 gene in a differentiated human thyroid carcinoma cell line, but not in primary thyroid tumours. Oncogene. 1991 Sep;6(9):1693–1697. [Abstract] [Google Scholar]
- Ito T, Seyama T, Mizuno T, Tsuyama N, Hayashi T, Hayashi Y, Dohi K, Nakamura N, Akiyama M. Unique association of p53 mutations with undifferentiated but not with differentiated carcinomas of the thyroid gland. Cancer Res. 1992 Mar 1;52(5):1369–1371. [Abstract] [Google Scholar]
- Gaidano G, Ballerini P, Gong JZ, Inghirami G, Neri A, Newcomb EW, Magrath IT, Knowles DM, Dalla-Favera R. p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5413–5417. [Europe PMC free article] [Abstract] [Google Scholar]
- Murray V. Improved double-stranded DNA sequencing using the linear polymerase chain reaction. Nucleic Acids Res. 1989 Nov 11;17(21):8889–8889. [Europe PMC free article] [Abstract] [Google Scholar]
- Frigo B, Scopsi L, Patriarca C, Rilke F. Silver enhancement of nickel-diaminobenzidine as applied to single and double immunoperoxidase staining. Biotech Histochem. 1991;66(3):159–167. [Abstract] [Google Scholar]
- Gannon JV, Greaves R, Iggo R, Lane DP. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. [Europe PMC free article] [Abstract] [Google Scholar]
- Gerdes J, Becker MH, Key G, Cattoretti G. Immunohistological detection of tumour growth fraction (Ki-67 antigen) in formalin-fixed and routinely processed tissues. J Pathol. 1992 Sep;168(1):85–86. [Abstract] [Google Scholar]
- Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991 Jun;39(6):741–748. [Abstract] [Google Scholar]
- Carbone D, Chiba I, Mitsudomi T. Polymorphism at codon 213 within the p53 gene. Oncogene. 1991 Sep;6(9):1691–1692. [Abstract] [Google Scholar]
- Serra A, Gaidano GL, Revello D, Guerrasio A, Ballerini P, Dalla Favera R, Saglio G. A new TaqI polymorphism in the p53 gene. Nucleic Acids Res. 1992 Feb 25;20(4):928–928. [Europe PMC free article] [Abstract] [Google Scholar]
- Wynford-Thomas D. P53 in tumour pathology: can we trust immunocytochemistry? J Pathol. 1992 Apr;166(4):329–330. [Abstract] [Google Scholar]
- Venkatesh YS, Ordonez NG, Schultz PN, Hickey RC, Goepfert H, Samaan NA. Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer. 1990 Jul 15;66(2):321–330. [Abstract] [Google Scholar]
- Ordóez NG, El-Naggar AK, Hickey RC, Samaan NA. Anaplastic thyroid carcinoma. Immunocytochemical study of 32 cases. Am J Clin Pathol. 1991 Jul;96(1):15–24. [Abstract] [Google Scholar]
Associated Data
Articles from The Journal of Clinical Investigation are provided here courtesy of American Society for Clinical Investigation
Full text links
Read article at publisher's site: https://doi.org/10.1172/jci116385
Read article for free, from open access legal sources, via Unpaywall:
http://www.jci.org/articles/view/116385/files/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Risk stratification for radioactive iodine refractoriness using molecular alterations in distant metastatic differentiated thyroid cancer.
Chin J Cancer Res, 36(1):25-35, 01 Feb 2024
Cited by: 1 article | PMID: 38455372
Genetic alterations in thyroid cancer mediating both resistance to BRAF inhibition and anaplastic transformation.
Oncotarget, 15:36-48, 24 Jan 2024
Cited by: 0 articles | PMID: 38275291 | PMCID: PMC10812235
Modulation of EZH2 Activity Induces an Antitumoral Effect and Cell Redifferentiation in Anaplastic Thyroid Cancer.
Int J Mol Sci, 24(9):7872, 26 Apr 2023
Cited by: 5 articles | PMID: 37175580 | PMCID: PMC10178714
Histological and Genetic Diversity in Ovarian Mucinous Carcinomas: A Pilot Study.
Curr Oncol, 30(4):4052-4059, 04 Apr 2023
Cited by: 0 articles | PMID: 37185420 | PMCID: PMC10137024
Recapitulating thyroid cancer histotypes through engineering embryonic stem cells.
Nat Commun, 14(1):1351, 11 Mar 2023
Cited by: 11 articles | PMID: 36906579 | PMCID: PMC10008571
Go to all (222) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Unique association of p53 mutations with undifferentiated but not with differentiated carcinomas of the thyroid gland.
Cancer Res, 52(5):1369-1371, 01 Mar 1992
Cited by: 202 articles | PMID: 1737400
Contribution of p53 gene alterations to development of metastatic forms of follicular thyroid carcinoma.
Diagn Mol Pathol, 4(4):256-260, 01 Dec 1995
Cited by: 5 articles | PMID: 8634781
Prevalence of mutations of ras and p53 in benign and malignant thyroid tumors from children exposed to radiation after the Chernobyl nuclear accident.
Oncogene, 13(4):687-693, 01 Aug 1996
Cited by: 59 articles | PMID: 8761289
[Unique association of p53 mutations with undifferentiated carcinoma of the thyroid].
Nihon Rinsho, 52(4):1069-1074, 01 Apr 1994
Cited by: 0 articles | PMID: 8196165
Review