Abstract
Background
Meat consumption is inconsistently associated with development of coronary heart disease (CHD), stroke, and diabetes mellitus, limiting quantitative recommendations for consumption levels. Effects of meat intake on these different outcomes, as well as of red versus processed meat, may also vary.Methods and results
We performed a systematic review and meta-analysis of evidence for relationships of red (unprocessed), processed, and total meat consumption with incident CHD, stroke, and diabetes mellitus. We searched for any cohort study, case-control study, or randomized trial that assessed these exposures and outcomes in generally healthy adults. Of 1598 identified abstracts, 20 studies met inclusion criteria, including 17 prospective cohorts and 3 case-control studies. All data were abstracted independently in duplicate. Random-effects generalized least squares models for trend estimation were used to derive pooled dose-response estimates. The 20 studies included 1 218 380 individuals and 23 889 CHD, 2280 stroke, and 10 797 diabetes mellitus cases. Red meat intake was not associated with CHD (n=4 studies; relative risk per 100-g serving per day=1.00; 95% confidence interval, 0.81 to 1.23; P for heterogeneity=0.36) or diabetes mellitus (n=5; relative risk=1.16; 95% confidence interval, 0.92 to 1.46; P=0.25). Conversely, processed meat intake was associated with 42% higher risk of CHD (n=5; relative risk per 50-g serving per day=1.42; 95% confidence interval, 1.07 to 1.89; P=0.04) and 19% higher risk of diabetes mellitus (n=7; relative risk=1.19; 95% confidence interval, 1.11 to 1.27; P<0.001). Associations were intermediate for total meat intake. Consumption of red and processed meat were not associated with stroke, but only 3 studies evaluated these relationships.Conclusions
Consumption of processed meats, but not red meats, is associated with higher incidence of CHD and diabetes mellitus. These results highlight the need for better understanding of potential mechanisms of effects and for particular focus on processed meats for dietary and policy recommendations.Free full text

Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes: A systematic review and meta-analysis
Abstract
Background
Meat consumption is inconsistently associated with development of coronary heart disease (CHD), stroke, and diabetes, limiting quantitative recommendations for consumption levels. Effects of meat intake on these different outcomes, and of red vs. processed meat, may also vary.
Methods and Results
We performed a systematic review and meta-analysis of evidence for relationships of red, processed, and total meat consumption with incident CHD, stroke, and diabetes. We searched for any cohort, case-control study or randomized trial that assessed these exposures and outcomes in generally healthy adults. Of 1,598 identified abstracts, 20 studies met inclusion criteria, including 17 prospective cohorts and 3 case-control studies. All data were abstracted independently in duplicate. Random-effects generalized least squares models for trend estimation were used to derive pooled dose-response estimates. The 20 studies included 1,218,380 individuals and 23,889 CHD, 2,280 stroke, and 10,797 diabetes cases. Red meat intake was not associated with CHD (n=4 studies, RR per 100g serving/day=1.00, 95%CI=0.81–1.23,p-for-heterogeneity=0.36) or diabetes (n=5, RR=1.16, 95%CI=0.92–1.46,p=0.25). Conversely, processed meat intake was associated with 42% higher risk of CHD (n=5, RR per 50g serving/day=1.42, 95%CI=1.07–1.89,p=0.04) and 19% higher risk of diabetes (n=7, RR=1.19, 95%CI=1.11–1.27,p<0.001). Associations were intermediate for total meat intake. Red and processed meat consumption were not associated with stroke, but only 3 studies evaluated these relationships.
Conclusions
Consumption of processed meats, but not red meats, is associated with higher incidence of CHD and diabetes. These results highlight the need for better understanding of potential mechanisms of effects, and for particular focus on processed meats for dietary and policy recommendations.
INTRODUCTION
The 2005 US Dietary Guidelines for Americans recommend that consumption of red and processed meat should be moderated(1). Such recommendations are in large part derived from expected effects of saturated fat in meat on LDL and total cholesterol levels. However, relationships of meat consumption with disease endpoints such as coronary heart disease (CHD), stroke, and type 2 diabetes mellitus are not well-established, with considerably conflicting results in prior studies(2–19). Thus, sufficient evidence for direct relationships with chronic cardiometabolic diseases has been lacking to support more quantitative recommendations about specific consumption levels of meats or potential differences between unprocessed red meat (referred to hereafter as simply “red meat”) vs. processed meats. Red vs. processed meats may have some important nutritional differences, such as in contents of calories, specific fats, sodium, iron, or additives (e.g., nitrites), or differences in their preparation methods (e.g., high temperature commercial cooking), that could produce differing effects on cardiometabolic risk. However, potential differences in effects of red meat vs. processed meat consumption on risk of CHD, stroke, or diabetes have not been systematically evaluated. In the US alone, 1,700,000 new cases of diabetes(20), 600,000 myocardial infarctions (MI), and 780,000 new or recurrent strokes occur each year(21). Documenting and quantifying the effects of meat consumption on these outcomes, as well as potential differences in effects of red vs. processed meat, is of great scientific and public health importance. To address these important questions and elucidate the conflicting results of prior studies, we performed a systematic review and meta-analysis of the evidence for relationships of red meat, processed meat, and red and processed meat combined (referred to hereafter as “total meat”) consumption with risk of CHD, stroke, and diabetes.
METHODS
We followed Meta-Analysis of Observational Studies in Epidemiology (MOOSE)(22) protocols throughout design, implementation, analysis, and reporting.
Search Strategy
We searched for all prospective or case-control studies or randomized control trials (RCTs) that provided effect estimates for potential associations of red, processed, or total meat consumption and incidence of CHD, stroke, total CVD, or diabetes in adults. Searches were performed using MEDLINE (see Supplemental Methods), EMBASE, AGRIS, AMED, HMIC, PsycINFO, Cochrane library, WEB OF KNOWLEDGE, CABI, CINAHL, conference abstracts (ZETOC), Faculty of 1000, grey literature sources (SIGLE), related articles, hand-searching of reference lists, and direct author contact. Key words were “meat”, “meat products”, “beef”, “ham”, other specific unprocessed red and processed meat subtypes, “cardiovascular diseases”, and “diabetes mellitus”, including the earliest available online indexing year through Mar 2009 without language restrictions. “Red meat” was defined as unprocessed meat from beef, hamburgers, lamb, pork, or game, and excluding poultry, fish, or eggs(23); “processed meat” as any meat preserved by smoking, curing or salting, or addition of chemical preservatives, such as bacon, salami, sausages, hot dogs, or processed deli or luncheon meats, and excluding fish or eggs(24); and “total meat” as the total of these two categories. Processed meat was primarily processed red meat, although in some studies deli-meats, a subcategory of processed meats, may also have included some processed poultry meats that could not be separately excluded. We excluded a priori studies focused on comparing only vegetarians vs. non-vegetarians, as such comparisons could likely be strongly modified or biased by other differences in diet and lifestyle behaviors in vegetarians. We also recognized that the lowest intake category in each of the included studies would include a subset of individuals (including likely at least some vegetarians) consuming no red or processed meat. Thus, such individuals were captured in the included studies, but without the higher potential for bias when analyses were restricted only to special vegetarian populations. We also excluded a priori cross-sectional or ecological studies; commentaries, general reviews, or case reports; and studies reporting only crude risk estimates.
Selection of Articles
Of 1,598 identified articles, 1,505 were excluded based upon review of the title and abstract (Figure 1). Full texts of the 95 remaining manuscripts were independently assessed in duplicate by two investigators to determine inclusion/exclusion, with differences resolved by consensus or, if necessary, group consultation among all investigators. Seventy-five studies were excluded because they were reviews (n=16), cross-sectional (n=7), ecological (n=2), conducted in vegetarians (n=10), or repeated publications from the same study (n=6); assessed only overall dietary patterns (n=19), iron intake (n=2), or only animal protein/fat (n=1); included poultry in the meat definition (n=7); did not assess incident CHD, stroke, or diabetes (n=3); reported only crude risk estimates (n=1); or included participants with prevalent disease (n=1) (see Supplemental Methods). Initial inclusion/exclusion adjudications were 97% concordant. For 29 studies, authors were contacted to request missing data or clarify meat definitions used; sufficient responses were received for 23 of 29 studies to characterize the exposure or missing data. For example, several papers appeared to report findings separately for “red meat” vs. “processed meat”, but upon detailed review or direct contact were found to have included processed meat in the red meat category, requiring direct contact to obtain risk estimates for unprocessed red meat alone.
Data Extraction
For each of the 20 final identified studies, data were extracted independently and in duplicate by two investigators, including years the study was performed and reported, study design, sample size, definition(s) of meat intake and disease outcomes, study location, inclusion and exclusion criteria, duration of follow-up, covariates adjusted for, and adjusted risk estimates and confidence intervals. When more than one multivariable model was reported, risk estimates with the greatest control for potential confounders were extracted. If multivariable models were reported with and without additional adjustment for variables that could be either confounders or intermediates (e.g., high cholesterol), the multivariable model without such variables was selected. If the only multivariable model included such variables, this was selected in preference to crude or minimally adjusted models. Accepted standardized quality scores for observational studies are not available. Therefore, quality assessment was performed by evaluating and scoring five design criteria on an integer scale (0 or 1; 1 being better), including appropriateness and reporting of inclusion and exclusion criteria, assessment of exposure, assessment of outcome, control of confounding, and evidence of bias. These scores were summed; quality scores from 0 to 3 were considered lower quality, and 4–5 higher quality. Differences in data extracted or quality assessment scores between investigators were very unusual and were resolved by consensus. Missing data or definitions were resolved by direct contact with authors as described above. To provide some perspective as to why cardiometabolic effects of red vs. processed meats might differ, we evaluated nationally-representative average nutrient and preservative contents of red and processed meats consumed in the US. To estimate average nutrient qualities, we analyzed data from the 2005–06 US National Health and Nutrition Examination Survey (NHANES), accounting for NHANES sampling and weighting strategies to provide nationally representative estimates(25; 26) (see Supplemental Methods). Foods consumed in this US survey were grouped to match our meta-analysis’ definitions for red and processed meat. Preservative contents were obtained from a recent report of published nitrate, nitrite, and nitrosamine contents of foods commonly consumed in the US(26) and applied directly to meats in the NHANES database using similar methods as for nutrients. We recognized that such nutrient data may not be fully generalizable outside the US, but comparable data were not available from Europe, Asia, or Australia.
Statistical Analysis
All included studies were observational and reported either relative risks (RRs; prospective cohorts) or odds-ratios (ORs; case-control studies) across several different categories of meat intake. ORs were assumed to approximate RRs(24); we also performed analyses limited to prospective cohorts only. The midpoint in each category was used to define median intake in that category, with standardization across studies to a serving size of 100 g (3.5 oz) for red and total meat, and 50 g (1.8 oz) for processed meat. For studies with an open-ended highest category that did not report median intake, we assumed that the difference from the lowest range to the median was equivalent to the same difference in the closest adjacent category. To maximize use of the data to calculate pooled dose-response, summary estimates of log-linear dose-response regressions were estimated using random-effects generalized least squares models for trend estimation(27) (GLST in STATA). This method is ideal for meta-analyses of studies having multiple risk estimates per study because it accounts for appropriate variance-covariance relationships between- and within-studies. Covariance was fit using total numbers of cases and of subjects (controls plus cases) for case-control data or person-years for cohort data, at each level of exposure. Evidence for statistical heterogeneity between studies was tested using goodness-of-fit (chi-square). Generalized least squares models for trend take advantage of the multiple data points in all studies simultaneously to provide the best overall pooled estimate of dose-response in a single (one-stage) estimation. To construct funnel plots and evaluate Begg adjusted-rank-correlation test for publication bias(28), explore potential sources of heterogeneity, and visually display the individual study results in Forest plots, we also performed two-stage estimation: separate generalized least squares models for trend were evaluated for each study to derive study-specific log-linear dose-responses (log RR), and then each study-specific log RR was pooled in a second generalized least squares model for trend. Our prespecified primary outcome was based on the one-stage estimation that better estimates the variance-covariance matrix by using all available beta coefficients in each study, rather than the two-stage estimation that first derives a single beta coefficient per study and then estimates the variance-covariance matrix. We performed sensitivity analyses, when data were available, for subgroups of specific processed meats. Prespecified potential sources of heterogeneity explored were study location (US, Asia/Australia, Europe), degree of covariate adjustment (minimal-sociodemographics; adequate-sociodemographics plus either other risk factors or dietary variables; optimal-all three), overall quality score (0–3, 4–5), single vs. repeated dietary assessment methods, and (to address potential publication bias of “positive” findings) whether the reported analysis was prespecified or post-hoc in each paper. Analyses were performed using STATA 10.0 (College Station, TX), with two-tailed alpha<0.05.
RESULTS
The 20 identified investigations included 17 prospective cohorts studies and 3 case-control studies conducted in US (n=11), Europe (n=6), Asia (n=2), and Australia (n=1), and included 1,218,380 unique individuals in whom 23,889 cases of CHD, 2,280 cases of stroke, and 10,797 cases of diabetes, were identified (Table 1). No randomized controlled trials of red, processed, or total meat consumption and incidence of CHD, stroke, or diabetes were identified. Reported categories of meat consumption typically ranged from never or less than once a month (lowest category of intake) to variable highest categories of intake. Averaged across studies, consumption (mean±SD) levels in the lowest vs. highest category of intake were 1.1±1.1 vs. 8.3±2.7 servings/week for red, 0.4±0.8 vs. 5.7±3.9 servings/week for processed, and 1.8±1.7 vs. 10.5±4.2 servings/week for total meat intake, respectively. Most studies used validated multi-item food frequency questionnaires to quantify meat consumption; some used interview-based(5; 10; 29) or fewer-item food frequency(2) questionnaires. Total numbers of participants (n=342 to 322,263) and events (n=51 to 14,221) varied widely between studies. Extent of covariate adjustment also varied, especially for dietary variables that were often not controlled for(3; 4; 6–8; 13–17)(and Kroger,D, unpublished data, 2009). Approximately half of studies included variables that could be confounders or intermediates (e.g., lipid levels) in addition to sociodemographics and/or dietary variables(5–7; 9; 10; 13–15; 17; 29). Four studies reported how red vs. processed meat intake were associated with other dietary and lifestyle factors at baseline(7; 16–18). Relationships with these other risk factors were similar for red vs. processed meat. For example, higher consumption of both red and processed meat tended to be similarly associated with current smoking, higher body mass index, family history of diabetes, hypertension, higher education and income level, and higher intake of total energy, total fat, saturated, monounsaturated, and polyunsaturated fats, dietary cholesterol, and protein. Also, red and processed meat consumption were similarly associated with less physical activity, multivitamin use, prevalence of high cholesterol, glycemic load, and intake of carbohydrate, fiber, and magnesium. For all but 3 of the studies(4; 10; 12), the reported exposure-outcome assessment was a prespecified primary or secondary aim.
Table 1
Identified studies evaluating the consumption of red, processed, or total meat and incidence of coronary heart disease, stroke, or diabetes.
| First author (year) | Country | Type of Meat* | Consumption in the lowest category (median servings/wk) | Consumption in the highest category (median servings/wk) | Disease outcome | Disease ascertainment | Study name | Population | Age | Sample size | Follow-up (y) | No events. | Person- years | Pre-specified analysis | Adjustm ents† | Quality score‡ | Additional information§ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort studies | |||||||||||||||||
| Burke (2007)(5) | Australia | red | 3.00 | 8.00 | CHD (total) | Regional hospital records and death registry | AAC | Australian Aborigines | 15–88 | 514 | 13 | 118 | 4,381 | Yes (primary) | +++ | 3 | Yes |
| processed | 0.53 | 2.13 | CHD (total) | Australian Aborigines | 15–88 | 514 | 13 | 118 | 4,381 | Yes (primary) | |||||||
| Villegas (2006)(18) | China | red | 1.19 | 6.00 | T2DM | Supplementary questionnaire | SWHS | Females in Shanghai | 40–70 | 70,609 | 4.6 | 1,969 | 326,625 | Yes (primary) | +++ | 3 | Yes |
| processed (&subtypes) | 0.00 | 3.89 | T2DM | Females in Shanghai | 40–70 | 70,609 | 4.6 | 1,969 | 326,625 | Yes (primary) | |||||||
| Salonen (1992)(10) | Finland | total meat | 6.07 | 16.49 | CHD (total MI) | Regional MI registry | KIHD | Eastern Finnish men | 42–60 | 1,931 | 3 | 51 | 5,586 | No | ++ | 3 | Yes |
| Kröger (unpublished data, 2009)|| | Germany | red | 0.67 | 4.16 | T2DM | ICD-10 criteria, validated by physician | EPIC-Potsdam | People in Potsdam, Germany | 19–70 | 25,069 | 7 | 844 | 716,277 | Yes (primary) | +++ | 5 | Yes |
| processed | 3.18 | 17.61 | T2DM | People in Potsdam, Germany | 19–70 | 25,069 | 7 | 844 | 716,277 | Yes (primary) | |||||||
| Sauvaget (2003)(11) | Japan | red | 0.00 | 5.50 | Stroke (fatal) | National death registry | HNLSS | Atomic bomb survivors | 34–103 | 37,130 | 16 | 1,224 | 498,651 | Yes (primary) | ++ | 3 | Yes |
| processed | 0.00 | 5.50 | Stroke (fatal) | Atomic bomb survivors | 34–103 | 37,130 | 16 | 958 | 473,404 | Yes (primary) | |||||||
| Whiteman (1999)(2) | UK | red | 0.50 | 5.50 | CHD (fatal) | National death registry | OXCHE CK | Patients in Bedfordshire, UK | 35–64 | 10,522 | 9 | 94 | 93,464 | Yes (primary) | +++ | 3 | No |
| processed | 0.50 | 5.50 | CHD (fatal) | Patients in Bedfordshire, UK | 35–64 | 10,522 | 9 | 91 | 93,429 | Yes (primary) | |||||||
| Ascherio (1994)(4) | US | red (& subtypes) | 1.11 | 10.16 | CHD (total) | Physician review of medical records, autopsy reports, or death certificate | HPFS | Male health professionals | 40–75 | 44,933 | 4 | 386 | 157,010 | No | +++ | 4 | Yes |
| Meyer (2001)(19) | US | processed | 0.00 | 3.50 | T2DM | Self-report | IWHS | Females in Iowa | 55–69 | 35,988 | 11 | 1,890 | 336,204 | Yes (secondary) | ++ | 3 | Yes |
| total meat | 2.00 | 13.50 | T2DM | Females in Iowa | 55–69 | 35,988 | 11 | 1,890 | 336,204 | Yes (secondary) | |||||||
| van Dam (2002)(15) | US | red (& subtypes) | 0.98 | 9.03 | T2DM | WHO diabetes criteria, using validated supplementary questionnaire | HPFS | Male health professionals | 39–78 | 42,504 | 12 | 1,320 | 466,508 | Yes (primary) | +++ | 5 | Yes |
| processed (& subtypes) | 0.00 | 7.00 | T2DM | Male health professionals | 39–78 | 42,504 | 12 | 1,320 | 466,508 | Yes (primary) | |||||||
| He (2003)(13) | US | total meat | 0.50 | 8.00 | Stroke (hemorrhagi c) | Physician review of medical records, autopsy reports, or death certificate | HPFS | Male health professionals | 40–75 | 43,732 | 14 | 125 | 602,693 | Yes (secondary) | +++ | 5 | No |
| total meat | 0.50 | 8.00 | Stroke (ischemic) | Male health professionals | 40–75 | 43,732 | 14 | 455 | 609,623 | Yes (secondary) | |||||||
| Liu (2003)(7) | US | processed | 0.08 | 2.41 | CHD (total) | Physician review of medical records, autopsy reports, or death certificate | NHS1 | Female nurses | 30–55 | 57,031 | 18 | 1,351 | 752,353 | Yes (primary) | +++ | 5 | Yes |
| total meat | 2.21 | 12.31 | CHD (total) | Female nurses | 30–55 | 57,031 | 18 | 1,351 | 752,353 | Yes (primary) | |||||||
| Schulze (2003)(17) | US | red (& subtypes) | 0.00 | 7.49 | T2DM | National Diabetes Data Group criteria, using validated supplementary questionnaire | NHS2 | Female nurses | 26–46 | 91,246 | 8 | 741 | 716,276 | Yes (primary) | +++ | 5 | Yes |
| processed (& subtypes) | 0.25 | 7.00 | T2DM | Female nurses | 26–46 | 91,246 | 8 | 741 | 716,276 | Yes (primary) | |||||||
| Fung (2004)(14) | US | red | 1.47 | 6.72 | T2DM | National Diabetes Data Group criteria, using validated supplementary questionnaire | NHS1 | Female nurses | 38–63 | 69,554 | 14 | 2,475 | 856,539 | Yes (secondary) | +++ | 4 | Yes |
| processed (& subtypes) | 0.28 | 3.85 | T2DM | Female nurses | 38–63 | 69,554 | 14 | 2,475 | 856,539 | Yes (secondary) | |||||||
| total meat | 2.24 | 9.87 | T2DM | Female nurses | 38–63 | 69,554 | 14 | 2,475 | 856,539 | Yes (secondary) | |||||||
| Fung (2004)(12) | US | red | 0.07 | 11.90 | Stroke (ischemic) | Physician review of medical records, autopsy reports, or death certificate | NHS1 | Female nurses | 38–63 | 71,768 | 14 | 476 | 957,988 | No | ++ | 4 | Yes |
| processed (& subtypes) | 0.07 | 11.90 | Stroke (ischemic) | Female nurses | 38–63 | 71,768 | 14 | 476 | 957,988 | No | |||||||
| total meat | 0.07 | 11.90 | Stroke (ischemic) | Female nurses | 38–63 | 71,768 | 14 | 476 | 478,994 | No | |||||||
| Song (2004)(16) | US | red (only subtypes) | T2DM | Self-report | WHS | Female health professionals | ≥ 45 | 37,309 | 8.8 | 1,539–1,555 | 326,876 | Yes (primary) | +++ | 4 | No | ||
| processed (& subtypes) | 0.00 | 3.92 | T2DM | Female health professionals | ≥ 45 | 37,309 | 8.8 | 1,543 | 326,876 | Yes (primary) | |||||||
| total meat | 0.91 | 9.94 | T2DM | Female health professionals | ≥ 45 | 37,309 | 8.8 | 1,558 | 326,876 | Yes (primary) | |||||||
| Kelemen (2005)(8) | US | total meat | 3.92 | 16.80 | CHD (fatal) | National death registry | IWHS | Females in Iowa | 55–69 | 29,017 | 15 | 739 | 475,755 | Yes (secondary) | +++ | 4 | Yes |
| Sinha (2009)(3) | US | total meat | 1.37 | 8.75 | CVD (fatal) | National death registry | NIH-AARP | Male members of AARP | 50–71 | 322,263 | 10 | 14,221 | 236,937 | Yes (primary) | +++ | 3 | No |
| processed | 0.45 | 6.33 | CVD (fatal) | Male members of AARP | 50–71 | 322,263 | 10 | 14,221 | 236,937 | Yes (primary) | |||||||
| total meat | 1.37 | 8.75 | CVD (fatal) | Female members of AARP | 50–71 | 223,390 | 10 | 5,356 | 191,254 | Yes (primary) | |||||||
| processed | 0.45 | 6.33 | CVD (fatal) | Female members of AARP | 50–71 | 223,390 | 10 | 5,356 | 191,254 | Yes (primary) | |||||||
| Case-control studies | |||||||||||||||||
| Kontogiann (2008)(9)i | Greece | total meat | 0.28 | 1.25 | CHD (nonfatal) | Physician diagnosis | CARDIO -2000 | Hospitalized patients, matched controls | 26–86 | 848 cases; 1078 controls | - | 844 | - | Yes (primary) | ++ | 3 | Yes |
| Tavani (2004)(29) | Italy | processed (only subtypes) | CHD (nonfatal MI) | Physician diagnosis | 3ITALC C | Hospitalized patients, matched controls | 17–79 | 558 cases; 1044 controls | - | 558 | - | Yes (primary) | ++ | 2 | Yes | ||
| Martinez- Gonzalez (2002)(6) | Spain | red | 3.50 | 13.30 | CHD (nonfatal MI) | Physician diagnosis | SPAINC C | Hospitalized patients, matched controls | <80 | 171 cases; 171 controls | - | 171 | - | Yes (secondary) | +++ | 4 | Yes |
| processed | 0.88 | 5.25 | CHD (nonfatal MI) | Hospitalized patients, matched controls | <80 | 171 cases; 171 controls | - | 171 | - | Yes (secondary) | |||||||
3ITALCC, 3 Italian case-control studies; AAC, Australian Aboriginal cohort; CARDIO-2000 (Greek case-control study); CVD, cardiovascular disease; CHD, coronary heart disease; EPIC-Potsdam, European Prospective Investigationinto Cancer and Nutrition-Potsdam Study; HNLSS, Hiroshima/Nagasaki Life Span Study; HPFS, Health Professionals Follow-up Study; IWHS, Iowa Women’s Health Study; KIHD, Kuopio Ischemic Heart Disease Risk Factor Study; NHS1, Nurses’ Health Study 1; NHS2, Nurses’ Health Study 2; NIH-AARP, National Institutes of Health-AARP Diet and Health Study; OXCHECK, OXford and Collaborators HEalth ChecK; SPAINCC, Spanish case-control study; SWHS, Shanghai Women’s Health Study; T2DM, type 2 diabetes mellitus; WHS, Women’s Health Study.
Meat Intake and Coronary Heart Disease
Nine studies provided 16 separate estimates for relationships of consumption of red, processed, or total meat and incident CHD (Figure 2).

Risk of incident coronary heart disease (CHD) associated with servings per day of red meat (top panel; 3 cohort studies and 1 case-control study, 56,311 participants, and 769 events), processed meat (mid panel; 4 cohort studies and 1 case-control study, 614,062 participants, and 21,308 events), and total meat (bottom panel; 4 cohort studies and 1 case-control, 635,558 participants, and 22,562 events). *Assessed total cardiovascular (coronary heart disease + stroke) mortality only.
Red meat
Consumption of red meat was not associated with CHD (RR=1.00 per serving/day, 95% CI=0.81–1.23), with no statistically significant between-study heterogeneity (p=0.36) (top panel). Findings were similar in analyses restricted to cohort studies(2; 4; 5) (RR=0.92, 95% CI=0.74–1.15) or studies for which this exposure-outcome assessment was prespecified(2; 5; 6) (RR=0.95, 95% CI=0.66–1.35).
Processed meat
Each serving/day of processed meat was associated with 42% higher risk of CHD (RR=1.42, 95% CI=1.07–1.89) (mid panel). Statistical between-study heterogeneity was present (p=0.04), not accounted for by any of our prespecified sources of heterogeneity. For all included studies this exposure-outcome assessment was prespecified. Restricting the analysis to cohort studies(2; 3; 5; 7) resulted in similar findings, with 44% higher CHD risk per serving/day (RR=1.44, 95% CI=1.07–1.95). Restricting the analysis to US studies(3; 7) resulted in similar findings (RR=1.40, 95% CI=1.03–1.91). Excluding one large US study that evaluated only total CVD mortality(3) (not CHD alone), each serving/day of processed meat consumption was associated with nearly 2-fold higher risk of CHD (RR=1.90, 95% CI=1.00–3.62), with no evidence for between-study heterogeneity (p=0.29).
Total meat
Total meat consumption was associated with a trend toward higher CHD risk (RR=1.27, 95% CI=0.94–1.72) (bottom panel). Between-study heterogeneity was present (p=0.002), observed to be due to extreme findings in the smallest study(9), that was also the only case-control study. Excluding this study, total meat consumption was associated with 25% higher CHD risk (RR=1.25, 95% CI=1.21–1.29). These findings were largely driven by one study that assessed only total CVD mortality(3) (not CHD alone); excluding this study, a significant association was not confirmed between total meat intake and CHD risk (RR=1.96, 95% CI=0.67–5.70), but CI’s were broad. Findings restricted to studies(3; 7–9) with prespecified aims to assess this exposure-outcome relationship were similar to the overall pooled estimate (RR=1.31, 95% CI=0.92–1.85).
Meat Intake and Diabetes
Seven studies provided 15 separate estimates for relationship of red, processed, or total meat consumption and incidence of diabetes (Figure 3).
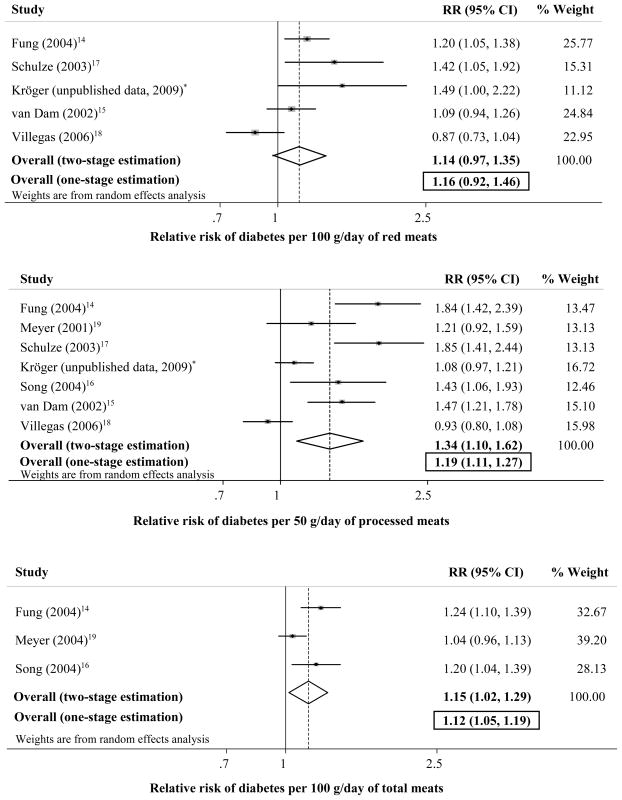
Risk of incident diabetes associated with servings per day of red meat (top panel; 5 cohort studies, 298,982 participants, and 7,349 events), processed meat (mid panel; 7 cohort studies, 372,279 participants, and 10,782 events), and total meat (bottom panel; 3 cohort studies, 142,851 participants, and 5,923 events). *(EPIC)-Potsdam Study, includes most recent results.
Red meat
Consumption of red meat was not significantly associated with incident diabetes (pooled RR=1.16 per serving/day, 95% CI=0.92–1.46) (top panel). Statistical heterogeneity between studies was not evident (p=0.25). All included studies were cohorts, for which the exposure-outcome assessments were prespecified.
Processed meat
Seven studies evaluated relationship of processed meat consumption and incident diabetes (mid panel). All studies were cohorts for which this exposure-outcome assessment was prespecified. In the overall pooled estimate, each serving/day was associated with 19% higher risk (RR=1.19, 95% CI=1.11–1.27). Significant between-study heterogeneity was present (p<0.001), identified in metaregression as related to study location (p=0.03). Excluding one study in Asia/Australia(18), each serving/day was associated with 27% higher risk of diabetes (RR=1.27, 95% CI=1.18–1.37). Restricted to US studies,(14–17; 19) each serving/day was associated with 53% higher risk of diabetes (RR=1.53, 95% CI=1.37–1.71).
Five studies provided estimates for 3 subtypes of processed meat, including (a) bacon (5 estimates, 5 studies)(14–18); (b) hot dogs (4 estimates, 4 studies)(14–17); and (c) other processed meats (4 estimates, 4 studies)(14–17). Each serving (2 slices)/day of bacon was associated with 2-fold higher incidence of diabetes (RR=2.07, 95% CI=1.40–3.04); of hot dogs (each 1/day), with nearly 2-fold higher incidence (RR=1.92, 95% CI=1.33–2.78); and of other processed meats (each 1 piece/day), with 66% higher incidence (RR=1.66, 95% CI=1.13–2.42). Each of these latter analyses were cohort studies and reported as prespecified primary or secondary aims.
Total meat
Each serving/day of total meat was associated with 12% (RR=1.12, 95% CI=1.05–1.19) higher risk of diabetes (bottom panel). Statistical heterogeneity between studies was not evident (p=0.29). All these studies were cohorts for which this exposure-outcome assessment was prespecified.
Meat Intake and Stroke
Only 3 identified studies(11–13), all cohorts, evaluated relationships of red, processed, or total meat consumption and incidence of total stroke or stroke subtypes, including 152,630 individuals and 2,280 stroke events (Figure 4). Generally no two studies evaluated the same meat and stroke subtype, limiting ability to pool results. Two studies(11; 12) evaluated red meat intake and either total ischemic stroke (one study) or total stroke mortality (one study); pooling these studies, the risk estimate was not significant (RR=1.17, 95% CI=0.40–3.43) (top panel). Two studies(11; 12) evaluated processed meat intake and either total ischemic stroke (one study) or total stroke mortality (one study); pooling these studies, the risk estimate was not significant (RR=1.14, 95% CI=0.94–1.39) (mid panel). Two studies(12; 13) evaluated total meat consumption and total ischemic stroke; the pooled risk estimate demonstrated 24% higher risk per daily serving (RR=1.24, 95% CI=1.08–1.43) (bottom panel). Only one study(13) reported an association for total meat consumption and hemorrhagic stroke (RR per daily serving=1.64, 95% CI=0.75–3.60). Evaluation for between-study heterogeneity was limited by the few studies and estimates.
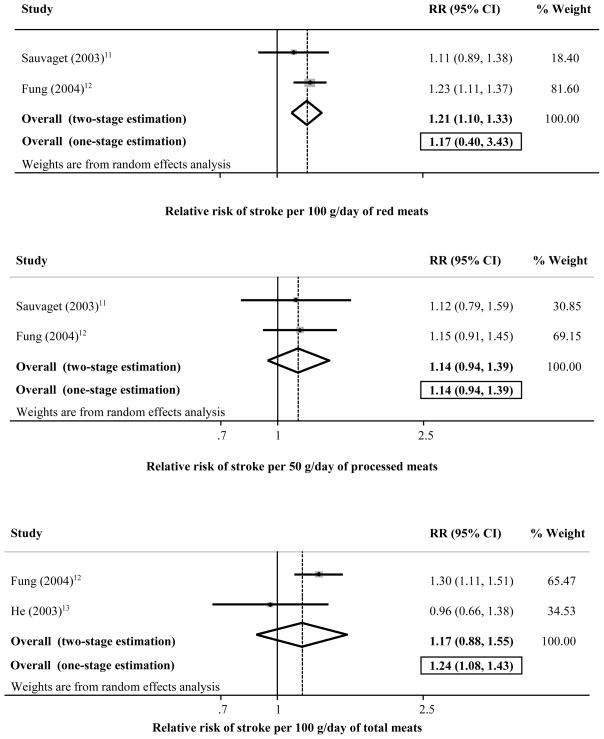
Risk of incident stroke associated with servings per day of red meat (top panel; 2 cohort studies, 108,898 participants, and 1,700 events), processed meat (mid panel; 2 cohort studies, 108,898 participants, and 1,434 events), and total meat (bottom panel; 2 cohort studies, 115,500 participants, and 931 events).
Publication Bias
Evidence for publication bias was not apparent for most of these exposure-outcome relationships based on either visual inspection of the funnel plot or by Begg’s test, a statistical analog of the visual funnel plot (see Supplemental Figure), although such tests have limited statistical power in the setting of relatively few studies. The funnel plot suggested possible publication bias in reporting of studies for processed meat intake and risk of CHD; Begg’s test did not achieve statistical significance (p=0.57), and excluding the smallest study with the most unbalanced results on the funnel plot(5) had little effect on results (RR=1.37, 95% CI=1.05–1.79). The funnel plot also suggested possible publication bias in reporting of studies for red meat intake and diabetes risk, but Begg’s test did not achieve statistical significance (p=0.62); red meat consumption was not significantly associated with diabetes risk in the overall pooled result (Figure 3, top panel); and excluding the 2 smallest studies with the most unbalanced results on the funnel plot(14)(and Kroger) did not appreciably alter these results (RR=1.05, 95% CI=0.73–1.49).
Nutritional Qualities of Red and Processed Meats
Based on nationally-representative data on the types and quantities of meats consumed in the US, both similarities and differences were identified in average nutrient and/or preservative contents of red vs. processed meats (Table 2). Per 50 g serving, processed meats contained modestly higher calories and percent energy from fat, and lower percent energy from protein, compared with 50 g of red meats. Consistent with lower protein content, processed meats also contained less iron. Processed meats contained relatively similar saturated fat and slightly lower cholesterol, the latter perhaps related to some processed meats being derived from pork and/or lower cholesterol deli meats. Relatively small differences were present in contents of monounsaturated fat, polyunsaturated fat, or potassium. Largest differences were seen in levels of sodium, with processed meats containing 4-fold higher levels (622 vs. 155 mg per serving), as well as approximately 50% higher non-salt preservatives including nitrates, nitrites, and nitrosamines.
Table 2
Differences in average nutritional and preservative contents between red meats and processed meats per 50g servings, as consumed in the US.
| Per 50g of meat | Red meats | Processed meats |
|---|---|---|
| mean ± SE (median) | mean ± SE (median) | |
| Energy (kcal) | 123.3 ± 0.7 (124.1) | 138.1 ± 2.0 (150.6) |
| Total fat (% energy) | 49.6 ± 0.3 (54.1) | 57.5 ± 0.6 (69.4) |
| Total fat (g) | 7.1 ± 0.1 (7.7) | 10.2 ± 0.2 (12.3) |
| Saturated fat (% energy) | 18.7 ± 0.1 (20.4) | 19.4 ± 0.3 (22.8) |
| Saturated fat (g) | 2.7 ± 0.0 (2.9) | 3.5 ± 0.1 (4.4) |
| Monounsaturated fat (% energy) | 21.4 ± 0.1 (23.9) | 25.3 ± 0.3 (30.7) |
| Monounsaturated fat (g) | 3.1 ± 0.0 (3.3) | 4.5 ± 0.1 (5.3) |
| Polyunsaturated fat (% energy) | 2.7 ± 0.0 (1.7) | 6.4 ± 0.1 (6.1) |
| Polyunsaturated fat (g) | 0.4 ± 0.0 (0.2) | 1.1 ± 0.0 (0.6) |
| Protein (% energy) | 46.2 ± 0.3 (41.5) | 35.4 ± 0.5 (27.4) |
| Protein (g) | 13.6 ± 0.0 (13.5) | 9.8 ± 0.1 (8.8) |
| Sodium (mg) | 154.8 ± 3.4 (127.1) | 621.7 ± 7.6 (575.8) |
| Potassium (mg) | 161.0 ± 0.8 (152.8) | 170.2 ± 1.9 (153.6) |
| Cholesterol (mg) | 41.9 ± 0.2 (43.8) | 34.1 ± 0.3 (28.3) |
| Iron (mg) | 1.1 ± 0.0 (1.2) | 0.6 ± 0.0 (0.6) |
| Nitrates (mg) | 3.3 ± 0.0 (2.9) | 4.6 ± 0.1 (3.0) |
| Nitrites (mg) | 0.5 ± 0.0 (0.7) | 0.8 ± 0.0 (0.6) |
| Nitrosamines (μg) | 0.1 ± 0.0 (0.2) | 0.3 ± 0.0 (0.2) |
Based on data from the 2005–06 US National Health and Nutrition Survey (NHANES) and a report of published nitrate, nitrite, and nitrosamine contents of foods(26), each analyzed according to actual US consumption levels and accounting for the NHANES sampling and weighting strategies.
All mean differences were significant at the.05 level.
DISCUSSION
Whereas meat consumption is commonly considered a risk factor for cardiovascular and metabolic diseases, our findings indicate that the effects and magnitudes may vary depending on both the type of meat consumed and the outcome considered. This first systematic review and meta-analysis of these relationships, including 1,218,380 individuals from 10 countries on 4 continents with 23,889 cases of CHD, 2,280 cases of stroke, and 10,797 cases of diabetes, provides the most robust and reliable evidence to-date of how unprocessed red and processed meat consumption may influence risk of cardiometabolic diseases. Consumption of processed meats was associated with significantly higher incidence of both CHD and diabetes, with 42% and 19% higher risk, respectively, per 50 g serving/day. In contrast, consumption of unprocessed red meats was not associated with CHD and was associated with a nonsigificant trend toward higher risk of diabetes. Associations were intermediate for total meat intake.
Our extensive search of multiple databases and direct contact with authors resulted in the identification of 17 prospective cohorts and 3 case-control studies; no RCTs were identified that evaluated effects of red, processed, or total meat consumption on CVD or diabetes events. This is not surprising considering that trials of such effects can be very challenging and costly to conduct, with limitations of nonblinding and noncompliance over the long periods of time required to detect clinical endpoints. In this setting, the best available evidence is derived from long-term prospective cohorts of disease endpoints such as those identified here, although such studies can be limited by misclassification and residual confounding. Retrospective case-control studies may have additional potential limitations (e.g., recall and selection bias).
Thus, each of these individual studies have potential limitations, and our findings should be interpreted in that context. On the other hand, this represents the most complete worldwide evidence to-date of the potential effects of red and processed meat consumption on incidence of CHD, stroke, and diabetes. We also performed multiple sensitivity analyses to evaluate the extent to which our findings might vary depending on underlying study design (cohort vs. case-control), presence or absence of prespecified analyses, geographic region (e.g., US vs. other), overrepresentation of one large study, or identified other sources of heterogeneity. Generally, findings were consistent in each of these sensitivity analyses and similar to the overall pooled results. Thus, although limitations of the individual studies should not be ignored, our results provide the best current evidence for how red, processed, and total meat consumption relate to CHD, stroke, and diabetes and highlight specific gaps in knowledge that are essential for policy decisions relating to these important diet-disease relationships.
For example, our findings of different relationships of red vs. processed meat consumption with incident CHD and diabetes events support the need to better characterize which particular components of meats may increase cardiometabolic risk. At least in the US, where most of the studies were performed, processed meats contain, on average, similar saturated fat and lower cholesterol and iron compared with red meats, suggesting that differences in these constituents may not account for different associations with disease risk. Other constituents may be relevant in determining health effects. In particular, the observed substantially higher sodium and nitrate preservative levels in processed meats could plausibly contribute to increased CVD and diabetes risk and account, at least in part, for the present findings. Dietary sodium significantly increases blood pressure(30–32), and habitual consumption may also worsen arterial compliance and promote vascular stiffness(33). Nitrates and their byproducts (e.g., peroxynitrite) experimentally promote atherosclerosis and vascular dysfunction(34), reduce insulin secretion(35; 36), and impair glucose tolerance(36),while streptozotocin, a nitrosamine-related compound, is a known diabetogenic compound(37). In observational studies in children, nitrites and nitrous compounds are associated with type 1 diabetes(38; 39), and nitrite concentrations in adults have been used as a biomarker of endothelial dysfunction(40) and impaired insulin response(41). Differences in types of foods commonly replaced when individuals consume red vs. processed meats could also partly account for their different associations with risk.
Our study had several strengths. We reviewed multiple databases broadly and systematically for all investigations of meat consumption and incidence of CHD, stroke, or diabetes, making it likely that we identified all major published reports. Multiple authors were directly contacted and clarified findings or provided additional data, minimizing both misclassification and effects of publication bias. Study inclusion/exclusion and data extraction were performed independently and in duplicate by two investigators, increasing validity of results. Studies were identified from the US, Europe, Asia, and Australia, increasing generalizability. Large numbers of disease endpoints were identified, providing substantial statistical power to detect clinically meaningful associations. We used generalized least squares models for trend estimation, that explicitly assesses dose-response rather than simply categorical comparisons. We carefully identified and separately evaluated red, processed, and total meat consumption; in particular, relatively few prior reports have separately considered unprocessed red meats. Indeed, several key reports on “red meat” consumption included processed meats in this category(3; 7; 8; 19; 24), limiting inference on effects of unprocessed red meats alone. For example, a systematic review by the World Cancer Research Fund and American Institute for Cancer Research concluded that both “red” and processed meat consumption increased colorectal cancer(24); however, “red” meats in several of the included studies were the sum of unprocessed and processed meats. Interestingly, their identified relationship of “red” (commonly total, i.e., unprocessed red plus processed) meat intake with colorectal cancer (22% higher risk per 100 g/day) was approximately half that as for processed meat alone (46% higher risk per 100 g/day), consistent with our results that much of the association between total meat intake and CHD and diabetes may result from effects of processed meats.
Potential limitations should also be considered. As with all meta-analyses, analyses were restricted to available published and unpublished data. Most of these studies did not separately assess extensive details about specific subcategories of deli meats consumed. Processed meats may have included small amounts of processed poultry, which could theoretically have smaller effects and cause underestimation of effects of processed red meats. We did not have data on cooking methods that could alter health effects of red or processed meats(42–44). Both red and processed meats represent somewhat heterogeneous categories, and thus our findings should be interpreted as the average overall association rather than the particular effect of one specific subtype of such meats. This interpretation would be similar, for example, to analyses or meta-analyses of effects of other classes of dietary factors, such as fruits, vegetables, fish, whole grains, alcohol, etc. A recent meta-analysis of relationships between meat consumption and diabetes has been reported(45); this study also found higher risk with processed meat intake, but included crude (unadjusted) risk estimates and also did not separately evaluate unprocessed red meats.
All studies were observational, and residual confounding by imprecisely or unmeasured factors cannot be excluded. In particular, several studies did not adjust for other dietary habits or socioeconomic status. Thus, associations of processed meat consumption with diabetes or CHD could relate to generally less healthy diet or lifestyle, rather than causal effects of processed meats. Conversely, most studies adjusted for at least several major demographics and other risk factors; the reported potential confounding factors related to red vs. processed meat consumption were similar, yet only the latter was related to risk; and specific ingredients in processed meats (e.g., salt, other preservatives) provide biologic plausibility for the observed relationships. Several studies adjusted for factors that could be either confounders or intermediates in the causal pathway, that could potentially attenuate the observed risk estimates between meat consumption and disease risk. We standardized all servings to 100 g for red and total meat and 50 g for processed meat, and risks could vary when serving sizes are lower or higher. Representative nutrient and preservative data were available only for the US, and such values should be considered illustrative rather than definitive for other countries. Too few studies were present to formally exclude publication bias with sufficient statistical power. On the other hand, our extensive direct contact with multiple authors and inclusion of unpublished findings minimizes the potential impact of publication bias. Notably, if publication bias were present, it might cause overestimation of harmful associations between processed meats and diabetes or CHD (i.e., identified harmful associations might more likely be published), but would unlikely contribute to null associations between red meats and CHD or diabetes or between meats and stroke (i.e., publication bias is unlikely to favor reporting of null associations).
Our findings demonstrate that consumption of processed meat in particular is associated with incidence of CHD and diabetes, highlighting the importance of separate consideration of health effects, underlying mechanisms, and policy implications of different types of processed vs. unprocessed meats. Our findings also identify critical gaps in our understanding of how meat consumption influences cardiometabolic risk, including potential effects of red meat consumption on diabetes or CHD; of any meat consumption on stroke risk; and of specific ingredients that could be underlying these relationships. Based on our evaluation of average nutrient and preservative contents of red and processed meats, constituents in meats other than fats may be especially relevant to health effects. Based on this systematic review and meta-analysis of all available data, future research should carefully distinguish between different types of meats, and policy measures for improving cardiometabolic health should focus particularly on reducing processed meat consumption, including consideration of recommendations for specific quantitative limits. These findings are particularly timely to current efforts to update the US Dietary Guidelines for Americans that are also often a reference for other countries around the world.
Acknowledgments
We thank Eric Ding, Tao Hou, and Jacob Sattelmair for providing statistical advice. We thank the following authors for clarifying definitions in published papers and/or providing additional unpublished data: Alberto Ascherio; Valerie Burke; James Cerhan and Linda Kelemen on behalf of the IWHS investigators; Aaron Folsom; Teresa Fung; Eric Grant; Yoshihide Kinjo; Paul Knekt; Janine Kroger; Carlo La Vecchia; Laurie Lambert; Miguel Martinez-Gonzalez; Katie Meyer; Dr Jun Nagano; Demosthenes Panagiotakos; Catherine Sauvaget; Matthias Schulze; Duc Son Le; Alessandra Tavani; Rob van Dam; Raquel Villegas; Jyrki Virtanen; Eberhard Windler.
Funding Sources
Supported by the Bill & Melinda Gates Foundation/World Health Organization Global Burden of Diseases, Risk Factors, and Injuries Study; the National Heart, Lung, and Blood Foundation, NIH (R01 HL 085710); the Searle Scholars Program.
Footnotes
Conflict of Interest Disclosures
None.
Author Contributions: Renata Micha: Conception and design, data collection, statistical analysis, interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and approval of final manuscript for submission.
Sarah Wallace: Data collection, interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and approval of final manuscript for submission. Dariush Mozaffarian: Conception and design, obtained funding, statistical advice, interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and approval of final manuscript for submission.References
Full text links
Read article at publisher's site: https://doi.org/10.1161/circulationaha.109.924977
Read article for free, from open access legal sources, via Unpaywall:
https://www.ahajournals.org/doi/pdf/10.1161/CIRCULATIONAHA.109.924977
Free to read at intl-circ.ahajournals.org
http://intl-circ.ahajournals.org/cgi/content/abstract/121/21/2271
Free after 12 months at intl-circ.ahajournals.org
http://intl-circ.ahajournals.org/cgi/content/full/121/21/2271
Free after 12 months at intl-circ.ahajournals.org
http://intl-circ.ahajournals.org/cgi/reprint/121/21/2271.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/101833643
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1161/circulationaha.109.924977
Article citations
Validity of a four-item questionnaire in French assessing attachment to meat.
Front Med (Lausanne), 11:1383825, 04 Oct 2024
Cited by: 0 articles | PMID: 39430593 | PMCID: PMC11486648
Association between dietary patterns and glycemic control in type II diabetes mellitus patients.
Aten Primaria, 57(2):103075, 16 Sep 2024
Cited by: 0 articles | PMID: 39288729 | PMCID: PMC11421999
Ultra-processed foods and cardiovascular disease: analysis of three large US prospective cohorts and a systematic review and meta-analysis of prospective cohort studies.
Lancet Reg Health Am, 37:100859, 02 Sep 2024
Cited by: 0 articles | PMID: 39286398 | PMCID: PMC11403639
Integration of epidemiological and blood biomarker analysis links haem iron intake to increased type 2 diabetes risk.
Nat Metab, 6(9):1807-1818, 13 Aug 2024
Cited by: 1 article | PMID: 39138340
Dose-response relationship between physical activity and frailty: A systematic review and meta-analysis.
Heliyon, 10(13):e33769, 27 Jun 2024
Cited by: 0 articles | PMID: 39050432 | PMCID: PMC11267014
Go to all (573) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Associations of the consumption of unprocessed red meat and processed meat with the incidence of cardiovascular disease and mortality, and the dose-response relationship: A systematic review and meta-analysis of cohort studies.
Crit Rev Food Sci Nutr, 63(27):8443-8456, 01 May 2022
Cited by: 12 articles | PMID: 35491892
Review
Letter by Bryan regarding article, "red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis".
Circulation, 123(3):e16; author reply e17, 01 Jan 2011
Cited by: 6 articles | PMID: 21263003
Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes--an updated review of the evidence.
Curr Atheroscler Rep, 14(6):515-524, 01 Dec 2012
Cited by: 235 articles | PMID: 23001745 | PMCID: PMC3483430
Review Free full text in Europe PMC
Associations of processed meat and unprocessed red meat intake with incident diabetes: the Strong Heart Family Study.
Am J Clin Nutr, 95(3):752-758, 25 Jan 2012
Cited by: 53 articles | PMID: 22277554 | PMCID: PMC3278249
Funding
Funders who supported this work.
NHLBI NIH HHS (3)
Grant ID: R01 HL 085710
Grant ID: R01 HL085710-01
Grant ID: R01 HL085710