Abstract
Purpose
To design a glucocorticoid-inducible virus vector overexpressing recombinant matrix metalloproteinase 1 (MMP1) and counteract extracellular matrix deposition in the trabecular meshwork only when steroid is present.Methods
Endogenous MMP1 expression was measured in primary human trabecular meshwork cells (HTM) treated with dexamethasone (DEX), triamcinolone acetate, and prednisolone acetate by TaqMan PCR. Wild-type and mutant MMP1 cDNAs were cloned downstream of a glucocorticoid response element (GRE) and P(TAL) promoter. Adenoviruses AdhGRE.MMP1 and AdhGRE.mutMMP1 were generated by homologous recombination. HTM cells and perfused human anterior segments were infected with the viruses, with and without DEX. MMP1 mRNA and protein were analyzed by TaqMan PCR, Western blot analysis, and ELISA. Activity of secreted MMP1 was evaluated by FRET and rat tail collagen type I assays. Immunohistochemistry was performed by double-labeling with anti-human MMP1 and collagen type I antibodies.Results
Endogenous MMP1 expression was greatly downregulated by the steroids. DEX-treated cells and perfused organ cultures infected with AdhGRE.MMP1 secreted high levels of MMP1. Induction of MMP1 cycled on and off with the addition or removal of DEX. Secreted wild-type MMP1 degraded collagen type I after activation, whereas secreted mutMMP1 did not. Immunohistochemistry showed faint staining of collagen type I in areas of trabecular meshwork with high MMP1 transgene expression.Conclusions
The authors have developed a novel glucocorticoid-inducible adenovirus vector that overproduces MMP1 only in the presence of DEX. The availability of this vector sets up the foundation for the development of gene therapy drugs for the potential treatment of ocular hypertension in steroid-responsive patients.Free full text

Development of a Gene Therapy Virus with a Glucocorticoid-Inducible MMP1 for the Treatment of Steroid Glaucoma
Associated Data
Abstract
Purpose.
To design a glucocorticoid-inducible virus vector overexpressing recombinant matrix metalloproteinase 1 (MMP1) and counteract extracellular matrix deposition in the trabecular meshwork only when steroid is present.
Methods.
Endogenous MMP1 expression was measured in primary human trabecular meshwork cells (HTM) treated with dexamethasone (DEX), triamcinolone acetate, and prednisolone acetate by TaqMan PCR. Wild-type and mutant MMP1 cDNAs were cloned downstream of a glucocorticoid response element (GRE) and PTAL promoter. Adenoviruses AdhGRE.MMP1 and AdhGRE.mutMMP1 were generated by homologous recombination. HTM cells and perfused human anterior segments were infected with the viruses, with and without DEX. MMP1 mRNA and protein were analyzed by TaqMan PCR, Western blot analysis, and ELISA. Activity of secreted MMP1 was evaluated by FRET and rat tail collagen type I assays. Immunohistochemistry was performed by double-labeling with anti-human MMP1 and collagen type I antibodies.
Results.
Endogenous MMP1 expression was greatly downregulated by the steroids. DEX-treated cells and perfused organ cultures infected with AdhGRE.MMP1 secreted high levels of MMP1. Induction of MMP1 cycled on and off with the addition or removal of DEX. Secreted wild-type MMP1 degraded collagen type I after activation, whereas secreted mutMMP1 did not. Immunohistochemistry showed faint staining of collagen type I in areas of trabecular meshwork with high MMP1 transgene expression.
Conclusions.
The authors have developed a novel glucocorticoid-inducible adenovirus vector that overproduces MMP1 only in the presence of DEX. The availability of this vector sets up the foundation for the development of gene therapy drugs for the potential treatment of ocular hypertension in steroid-responsive patients.
Glucocorticoids are potent immunosuppressants commonly used for the treatment of many inflammatory disorders, including ocular inflammation. Administration of glucocorticoids can cause elevated intraocular pressure (IOP) and can lead to open-angle glaucoma in steroid-responsive persons. Topical ocular treatment with corticosteroids produces a dose-dependent IOP increase in 30% to 40% of the general population1,2 and in 90% of patients with primary open-angle glaucoma (POAG).3 The ocular hypertension effect of the glucocorticoids is significantly greater in older age groups and is completely reversed after cessation of the treatment.1,4 Steroid-responsive persons (steroid-responders) are more likely to develop POAG than their nonresponder counterparts.5
Glaucoma is a multifactorial ocular disease characterized by the death of retinal ganglion cells and degeneration of the optic nerve. Glaucoma affects 70 million people worldwide and is the second leading cause of irreversible blindness.6 It is well established that the major risk factor for the development of glaucoma is elevated IOP7 and that this elevated IOP is caused by increased resistance to the aqueous humor outflow exerted by the trabecular meshwork tissue.8 Potential mechanisms of steroid-induced glaucoma have been extensively studied.9,10 Glucocorticoid exposure affects the expression of numerous genes in the trabecular meshwork cells,11,12 and these changes translate to characteristic morphologic and biochemical changes of the outflow tissue.13 Glucocorticoids induce the formation of cross-linked actin networks in trabecular meshwork cells and tissues.14 In vitro, dexamethasone (DEX) decreases trabecular meshwork phagocytosis,15 increases extracellular matrix (ECM) deposition,16 induces the glaucoma-linked gene myocilin,17 and decreases the expression and activity of matrix metalloproteinases (MMPs). Together these changes lead to impairment of the aqueous humor drainage and contribute to the increased outflow resistance observed in steroid-responder subjects.1
Glucocorticoid response occurs by the binding of the steroid hormone to the intracellular glucocorticoid receptor alpha (GRα). The ligand-receptor complex dimerizes, translocates to the nucleus, and binds to a DNA cis-acting glucocorticoid response element (GRE) to modulate the expression of target genes.18 A recent study on glucocorticoid responsiveness of primary and transformed trabecular meshwork cells has shown that treatment with DEX activates a GRE element inserted into a transfected plasmid vector and is able to drive expression of the GRE-driven reporter gene.19 These results indicate that the transfected GRE regulatory element is active in the trabecular meshwork and may induce the expression of other eukaryotic genes.
The modulation of outflow resistance by medications with longer durations of action than those of conventional drugs remains the goal of glaucoma treatment. In the search for an alternative treatment of glaucoma, our laboratory first developed the delivery and overexpression of transgenes to the trabecular meshwork by the use of viral vectors.20,21 Our studies have since been extensively reproduced by other laboratories.22–24
Recently, we have demonstrated that after a single intracameral injection, some viral vectors can overexpress the delivered transgene in the trabecular meshwork of primates for more than 2 years.25 However, continuous unregulated overexpression of transgene products may result in an unwanted physiological or toxic effect. A successful overexpressing, insult-induced glaucoma gene drug would be one that could increase the levels of its therapeutic product when the agent triggering the disease is present and would stop its mode of action when it is no longer needed. Therefore, in this study, we hypothesized that the GRE regulatory element could be used to drive the expression of a gene that, in turn, could counteract the detrimental effects of glucocorticoids in the trabecular meshwork. Because of the inducible nature of the GRE element, the selected gene would then be expressed only when the trabecular meshwork tissue was exposed to the glucocorticoid.
Because of the relevance of the ECM in the regulation of outflow facility26 and because of the well-established fact that the administration of glucocorticoids leads to increased ECM deposition,16 we chose to test our hypothesis by focusing on MMPs. These enzymes comprise a family of zinc-containing proteases that are secreted as inactive proenzymes and are frequently regulated at the level of transcription. MMPs play a key role in the turnover and maintenance of the trabecular meshwork's ECM and have been shown to be involved in outflow facility.26,27 For this study, we selected MMP1. The member MMP1 is an interstitial collagenase that breaks down ECM collagens types I, II, and III. Collagen type I is an integral component of the trabecular meshwork extracellular scaffold, and it forms part of the central core of the trabecular meshwork beams. In the organ-cultured trabecular meshwork, collagen type I protein is upregulated by DEX,28 whereas C-propeptides of collagen type I are downregulated in a rat steroid-induced ocular hypertension.29 MMP1 is a well-known factor associated with tumor invasion and angiogenesis and has been the subject of outflow facility studies.27,30 In the trabecular meshwork in vitro, MMP1 expression is downregulated by DEX11 and by overexpression of a nonsecreted myocilin mutant.31 MMP1 is also downregulated by transforming-growth factor β (TGFβ), a growth factor that induces fibrosis and upregulates collagen type I,32 and delivery of MMP1 by infection with Ad5MMP1 attenuated liver fibrosis in the living rat.33 MMP1 is upregulated by wild-type myocilin,31 which is apparently inconsistent with the fact that DEX also upregulates myocilin. Experiments addressing this lack of correlation are ongoing in our laboratory and are under the scope of a different study. MMP1 is also upregulated by latanoprost.34 Interestingly, transgenic mice containing a targeted mutation in the gene for α1 subunit of collagen type I experienced elevated IOP. This mutation codes for five amino acid substitutions adjacent to the MMP1 cleavage site and blocks MMP1 activity, which results in collagen type I accumulation and ocular hypertension.35
With the intent of developing a gene therapy vector for the treatment of steroid-induced glaucoma, we designed a recombinant adenoviral vector containing the full coding human MMP1 cDNA under control of a GRE element and a basal promoter. Delivery of this vector (AdhGRE.MMP1) to primary human trabecular meshwork cells (HTM) and perfused postmortem human eye organ cultures secreted active MMP1 with the ability to degrade collagen type I. In a second, coupled study, delivery of the vector was able to reduce the steroid-induced ocular hypertension in a large animal model.36 The availability of this vector provides proof of concept and the basis for development of gene therapy drugs for the treatment of steroid-induced elevated IOP.
Materials and Methods
Primary Culture of Human Outflow Facility Cells
To generate primary HTM cells, the trabecular meshwork tissue was dissected from residual cornea rims after surgical corneal transplantation at the University of North Carolina Eye Clinic. Trabecular meshworks were isolated from surrounding tissue, cut into small pieces, and treated with 1 mg/mL collagenase type IV (Worthington, Lakewood, NJ), as described.37 Cells were maintained at 37°C, 7% CO2, in MEM Richter's modification medium (HyClone; Thermo Fisher Scientific, Waltham, MA) supplemented with 20% FBS and 50 μg/mL gentamicin (Gibco Invitrogen, Carlsbad, CA). At confluence, cells were passed and maintained in the same medium but with 10% FBS (complete medium). All cells were used at passages 4 to 6. These outflow pathway cultures include cells from the three distinct regions of the trabecular meshwork plus cells lining the Schlemm's canal (SC). Because most of the cells in these cultures come from the trabecular meshwork, they are commonly referred to as trabecular meshwork cells. The cells used in this study were from a 15-year-old Caucasian boy (HTM-109), a 2-year-old Caucasian girl (HTM-95), a 19-year-old Caucasian man (HTM-106), and a 54-year-old Caucasian woman (HTM-140).
Drug Treatments
Drug treatments on the HTM cells were conducted in complete media. HTM cells were grown to preconfluence and were exposed to drugs as follows: treatment with DEX (Sigma, St. Louis, MO) was conducted at a final concentration of 0.1 μM. DEX was reconstituted in absolute ethanol at 0.1 mM and diluted 1000-fold into complete media every 48 to 72 hours for the duration of the experiment. Treatment with triamcinolone acetonide (Kenacort-A; Bristol-Myers Squibb, New York, NY) was performed at a final concentration of 0.1 mg/mL. Kenacort-A 40 mg/mL suspension was mixed well and diluted into complete medium 400-fold at the time of use. The concentration of 0.1 mg/mL triamcinolone acetonide was chosen because intravitreal injections of 1 mg/mL are widely used in the clinical setting and result in a lower concentration of the steroid in the aqueous humor. In addition, the concentration of 0.1 mg/mL has been successfully studied in trabecular meshwork cells.38 Treatment with prednisolone was conducted at a final concentration of 200 μM (80 μg/mL). Prednisolone 21-acetate (Sigma) was reconstituted in ethanol at 200 mM (80 mg/mL) as a suspension and was mixed well before dilution of 1000-fold into the culture medium. The drugs were exposed to the cells for the period described in Results. Untreated control dishes received the same volume of absolute ethanol (drug vehicle) under identical conditions.
RNA Extraction, Reverse Transcription, and cDNA Quantification
HTM cells were scraped from tissue culture dishes with guanidine thiocyanate buffer (RLT; Qiagen, Valencia, CA). Total RNA was extracted by loading the solution onto a column (QIA Shredder; Qiagen) and was continued by the use of a kit with on-column RNase-free DNase digestion (RNeasy Mini kit; Qiagen) in accordance with the manufacturer's recommendations. Purified RNA was eluted in 30 μL RNase-free water and concentration measured with a spectrophotometer (NanoDrop ND-100; Thermo Fisher Scientific, Pittsburgh, PA). For the tissue, human trabecular meshworks were excised from 1-week RNA stabilization reagent (RNAlater; Ambion Applied Biosystems, Austin, TX) immersed anterior segments. Half the isolated trabecular meshwork tissue was homogenized on 350 μL RLT, and RNA extraction continued as described for the cells. Recoveries were between 1.4 to 2 μg RNA per human trabecular meshwork.
Reverse transcription (RT) reactions were conducted with 1 μg (HTM cells) or 400 ng (tissue) RNA in a 20-μL total volume of proprietary RT buffer with RNase inhibitor (High Capacity cDNA kit; Applied Biosystems [ABI], Foster City, CA) in accordance with the manufacturer's recommendations (25°C 10 minutes, 37°C 2 hours, and 85°C 5 seconds). Fluorescence-labeled TaqMan probe/primer sets for human MMP1 and 18S RNA were purchased from the ABI TaqMan gene expression collection (http://www.allgenes.com). The human MMP1 probe corresponded to sequences from exons 6 and 7 (Hs00233958_m1), and the 18S RNA probe corresponded to sequences surrounding position nucleotide 609 (Hs99999901_s1). Reactions were performed in 20-μL aliquots (TaqMan Universal PCR Master mix No AmpErase UNG; ABI), run on a PCR system (7500 Real-Time; ABI), and analyzed with ABI software (7500 System SDS). Relative quantity (RQ) values between treated and untreated samples were calculated by the formula 2-ΔΔCT, where CT is the cycle at threshold, ΔCT is CT of the assayed gene minus CT of the endogenous control (18S), and ΔΔCT is the ΔCT of the normalized assayed gene in the treated sample minus the ΔCT of the same gene in the untreated sample (calibrator). Because of the high abundance of the 18S rRNA used as the endogenous control and to get a linear amplification, RT reactions from treated and untreated samples were diluted 104 times before hybridization to the 18S TaqMan probe.
Protein Extraction, Western Blot Analysis, and Protein Quantification
Serum-containing culture medium from treated and untreated HTM cells was collected, cleared of cellular debris by centrifugation at 1500 rpm for 10 minutes, and concentrated 40× (10-kDa cutoff; Amicon Ultra-4 Centrifugal Filter Device Ultracel; Millipore, Billerica, MA) at 3500 rpm, 4°C. After the removal of medium, HTM cells were washed with cold phosphate-buffered saline (PBS) and harvested in 150 μL cold RIPA buffer (0.15 M NaCl, 0.02 M Tris-HCl, pH 8, 1% NP40, 1% sodium deoxycholate, 0.1% SDS) supplemented with 1× protease inhibitor cocktail (Roche Applied Biosciences, Indianapolis, IN). Cells were disrupted with a sonicator (Microson Ultrasonic XL 2000; Misonix, Farmingdale, NY) equipped with a 2.4-mm microprobe (Misonix) at setting 3 for five pulses. The sonicate was then centrifuged at 14,000g for 20 minutes at 4°C, and supernatants (soluble fraction) were collected and stored at −80°C until use. Serum-free effluents from perfused organ cultures were concentrated 40× in the same manner as media from the cultured cells.
Equivalent volumes from treated and untreated protein extracts, concentrated media, or effluents were mixed 1:2 (vol/vol) with loading Laemmli buffer (Bio-Rad, Hercules, CA) containing 5% β-mercaptoethanol and boiled for 5 minutes. Protein samples were separated on a 4% to 15% SDS-PAGE precast gel (Bio-Rad) and electrotransferred to a polyvinylidene difluoride membrane (Bio-Rad). After blocking with 5% nonfat dry milk in 0.01 M Tris, pH 8.0, 0.1% Tween for 1 to 2 hours at room temperature, membranes were incubated overnight at 4°C with rabbit anti-human MMP1 (1:1000, AB8105; Millipore) or goat anti-human collagen type I (1:200, AB758; Millipore) primary antibodies. Membranes were then washed and incubated with anti-rabbit or anti-goat IgG secondary antibodies conjugated to horseradish peroxidase (HRP) (1:5000; Pierce Thermo Fisher Scientific, Rockford, IL) for 1 hour at room temperature. Immunoreactive bands were visualized by chemiluminescence (ECL Plus; GE Healthcare, Piscataway, NJ) and exposed to X-ray film (BioMax MR Film; Kodak, Rochester, NY). To reprobe membranes with other primary antibodies, membranes were stripped in 0.01 M Tris, 0.1% Tween, pH 2.0, for 15 minutes, washed, and neutralized in the same buffer at pH 8.0. For controls, membranes were incubated with mouse monoclonal anti-human β-actin for 1 hour at room temperature (1:5000, A5441; Sigma) or rabbit anti-human myocilin (1:50, sc-21243; Santa Cruz Biotechnology, Santa Cruz, CA), washed, and incubated with HRP-conjugated anti-mouse or anti-rabbit IgG, respectively (1:5000; Pierce Thermo Fisher Scientific) for 1 hour at room temperature.
Levels of secreted MMP1 in concentrated HTM-cultured medium and effluents were determined by ELISA using a human MMP1 ELISA kit (RayBiotech, Norcross, GA) in accordance with the manufacturer's recommendations. At the end of the incubation, immunoplates were read at 450 nm in a microplate reader (FLUOstar Optima; BMG Labtech, Cary, NC).
Adenovirus Construction and Titration
The details of adenovirus constructions are provided as Supplementary Material, available at http://www.iovs.org/cgi/content/full/51/6/3029/DC1. Physical particles were titered as virus genomes (vg)/milliliter by real-time PCR using the MMP1 fluorescent TaqMan primers/probe described (Hs00233958_mL; ABI). For this, viral DNA was extracted from 5 μL purified virus (DNeasy tissue kit; Qiagen), and amplification reactions were set in triplicate. A standard curve was generated by amplifying known copy numbers of MMP1 plasmid pMG19 (see Supplementary Material) and plotting them against their threshold cycle (CT) values. The number of virus genomes was then determined comparing the CT values of the viral DNA to the standard curve. Viral lots used in this study had concentrations of 3.1 × 1011 (wild-type) and 4.0 × 1011 (mutant) virus genomes per milliliter, respectively.
Viral infectivity (infectious units [IFU]/mL) was measured with a rapid titer kit (AdenoX; Clontech, Mountain View, CA) containing an antibody specific to the adenovirus hexon capsid protein produced only in infected cells. QBI-HEK293A cells were seeded in 12-well plates to 90% confluence and were infected with serial dilutions (10−4–10−6) of the adenovirus stock in duplicate wells. At 48 hours, cells were fixed with ice-cold 100% methanol for 10 minutes at −20°C, washed with PBS/1% BSA, and incubated with a mouse anti-hexon antibody (1:2000) for 1 hour. Positive brown color spots were detected by incubation with HRP-conjugated rat anti-mouse (1:1000, 1 hour at 37°C), washing, and developing with 3,3′-diaminobenzidine substrate. Brown spots were counted with a 20× objective in an inverted microscope (IX71; Olympus, Tokyo, Japan) equipped with a digital camera (DP70; Olympus). Each brown-stained cell corresponds to one infectious viral particle (IFU); wells from dilutions with approximately 50 spots per field were chosen for counting. Quantification of the IFUs per milliliter was made by averaging the number of spots in three to four fields per well and applying the following formula: brown spots/field × fields/well divided by virus volume used/well (mL) × virus dilution factor. Under our conditions, there were 400 fields/well on a 12-well plate. A correction factor for the area of the captured image (1.84×) was obtained by the use of a calibrated slide and introduced in the formula to obtain the final titer. Viral lots used in this study had 1.8 × 1011 (wild-type) and 2.1 × 1011 (mutant) IFU/mL, respectively.
Delivery of Recombinant Adenoviruses to HTM Cells
HTM primary cells at passage 4 seeded on six-well dishes were grown to 90% confluence, washed twice with PBS, and exposed to the recombinant adenoviruses AdhGRE.MMP1 and AdhGRE.mutMMP1 in 1 mL serum-free medium. After exposure to the virus for 90 minutes, complete media containing 0.1 μM DEX was added, and incubation continued for 3 to 5 days. Fresh DEX medium was replaced at 48- to 72-hour intervals, as indicated. Viral concentrations were at a multiplicity of infection (MOI) ranging from 2.3 to 2.6 × 103 IFU/cell.
Measurement of MMP1 Activity by Collagen Degradation Assays
To determine the collagenase activity of the human recombinant MMP1 secreted in the media, we performed two assays: the fluorescence resonance energy transfer (FRET) assay, which incorporates the FRET pair labeling technology in an MMP peptide substrate, and the digestion of native rat tail collagen type I measured by gel electrophoresis. Conditioned media from postinfected dishes treated with steroids were cleared of cellular debris and concentrated 40×, as indicated. To activate pro-MMP1 from its latent state, samples were incubated at 37°C for 3 hours in 1 mM p-aminophenylmercuric acetate (APMA)39 in a total volume of 50 μL. Commercially available purified pro-MMP1 (AnaSpec, Fremont, CA) was used as a positive control.
For the FRET assay, 10 μL concentrated activated serum-containing media were incubated with the 5-FAM/QXL 520–labeled peptide for 40 minutes at 37°C in accordance with the manufacturer's recommendations (SensoLyte 520 MMP -1 Assay Kit; AnaSpec). Enzyme activity was determined by measuring the fluorescence released on proteolytic cleavage of the fluorescent peptide in a microplate reader (FLUOstar Optima) using 480/520-nm excitation/emission filters. To test the enzymatic activity against native collagen, 5 μL activated serum-free media were incubated with 10 μg native rat tail collagen type I (BD Biosciences, San Jose, CA) for 2 hours at 37°C in a total volume of 28 μL.40 Half the reaction volume was analyzed in a 4% to 15% Tris-HCl PAGE gel (Bio-Rad) at 100 V for 1.5 hours. Gels were subsequently washed with water and stained (Biosafe Coomassie G-250; Bio-Rad) at 4°C overnight. Bands were visualized after a final wash and were photographed with a digital camera (SD850 IS; Canon, Lake Success, NY).
Perfused Human Anterior Segment Organ Cultures
Three pairs of normal, nonglaucomatous human eyes from donors ages 72 to 74 were obtained from the National Disease Research Interchange (Philadelphia, PA) with the signed consent of the patients' families. All procedures were conducted in accordance with the tenets of the Declaration of Helsinki. Whole eye globes within 22 to 43 hours of death were dissected at the equator, cleaned, and mounted on custom-made perfusion chambers, as described previously.20,41 These anterior segments were perfused at constant flow (3–6 μL/min) through one of chamber's two cannulas with serum-free, high-glucose DMEM (Gibco Invitrogen) containing antibiotics, using a Harvard microinfusion pump (Harvard Bioscience, South Natick, MA). HPLC pumps (MX7900–000; Rheodyne, Rhonert Park, CA) equipped with a 20-μL loop were intercalated between perfusion syringes and chambers so that virus could be administered without injection through the cornea. All pumps were controlled by a custom-made computer program (Infusion Pump Control Program, University of North Carolina Chemistry Department, Electronic Design Facility). Anterior segments were maintained at 37°C, 5% CO2, and perfused for 24 hours to establish a stable baseline (steady pressure recordings for at least 10 hours). Outflow facility (flow/pressure in μL/min/mm Hg) was calculated from the average of three values obtained from pressure readings recorded at 30-minute intervals. Baseline values were taken just before the steroid treatment and sample delivery. The outflow facility at baseline for the eyes used in this study was C = 0.29 ± 0.03 (n = 6).
After obtaining a stable baseline, the perfusion syringes and eyes' anterior chambers were exchanged with fresh media containing 0.1 μM DEX. At the same time, HPLC loops were loaded with AdhGRE.MMP1 (for OS) or virus vehicle (for OD), which were delivered into the eyes by remote control loop injection from the computer. Fresh DEX media were changed approximately every 36 hours, and effluents were collected from the chamber reservoirs at different time points and saved at −20°C for analysis of secreted proteins. At the end of the experiment, anterior segments were cut in several wedges that were immersed in either 4% paraformaldehyde or RNA stabilization reagent (RNAlater; Ambion ABI) for immunohistochemistry and transgene expression.
Immunocytochemistry, Immunohistochemistry, and Light Microscopy
Cells were cultured on glass coverslips precoated with poly-d-lysine, fixed, and fluorescence double labeled for the MMP1 and collagen type I proteins. Cells were washed, fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton X-100/PBS for 10 minutes, washed, and blocked with 2% donkey serum/PBS for 30 minutes. Coverslips were simultaneously incubated with rabbit anti-human MMP1 antibody (1:500, AB8105; Millipore) and goat-anti collagen type I (1:100, AB758; Millipore) for 1 hour at room temperature, followed by an additional 1 hour with a mixture of donkey anti-rabbit Alexa Fluor 555 and donkey anti-goat Alexa Fluor 488, respectively (1:400; Molecular Probes Invitrogen). All antibody solutions were made in 2% donkey serum, and three washes were performed between incubation steps. All secondary antibodies were tested for cross-reactivity by incubating coverslips in the absence of the primary antibodies. Cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for 3 minutes before the coverslips were mounted with a drop of water-soluble, nonfluorescing compound (Fluoromount G; Southern Biotechnology Associates, Birmingham, AL).
Eyes from pairs 2 and 3 were fixed by immersion in 4% paraformaldehyde in PBS at room temperature overnight. Specimens were then rinsed in distilled water for 10 minutes and transferred to 70% ethanol for delivery to the University of North Carolina Histology Core for paraffin embedding. Meridional 10-μm sections from opposite quadrants of each eye were mounted on microscope slides (Superfrost/Premium; Thermo Fisher Scientific). For MMP1 and collagen type I detection, sections were first incubated at 60°C for 1 hour, deparaffinized with xylene (2 × 7 minutes), rinsed with descending concentrations of ethanol (100% for 5 minutes, 95% for 4 minutes, 75% for 3 minutes), and rehydrated with distilled water for 2 minutes. Heat-induced antigen retrieval was achieved by treating the sections with unmasking solution (Vector Laboratories, Burlingame, CA) for 30 seconds at 125°C in a decloaking chamber (Biocare Medical, Concord, CA). Slides were then cooled off, washed with PBS, permeabilized with 0.1% Triton X-100/PBS for 10 minutes, washed again, and blocked with 2% donkey serum/PBS for 30 minutes. Tissue sections were incubated with the same MMP1 (1:500; 2 hours at room temperature) and collagen type I (1:200, overnight at 4°C) primary antibodies used for the cells, followed by incubation with donkey anti-rabbit Alexa Fluor 555 and donkey anti-goat Alexa Fluor 488 secondary antibodies (1:200; Molecular Probes Invitrogen) at room temperature for 1 hour. Sections were mounted with coverslips and water-soluble, nonfluorescing compound (Fluoromount G; Southern Biotechnology Associates) and sealed with clear enamel. Fluorescence imaging was conducted with a fluorescence microscope (IX71; Olympus), and images were captured with a camera (DP70; Olympus) and accompanying software. Images from corresponding viral- and vehicle-treated sections were taken at the same exposure. Digital images were arranged with image analysis software (Photoshop CS; Adobe, Mountain View, CA). Negative controls were run in parallel but were incubated in blocking buffer in place of the primary antibody.
Results
Effect of Steroids on Endogenous MMP1 Expression
Previous studies have shown that DEX treatment of cultured HTM cells decreases the expression of several MMPs, including MMP1, 2, 3, and 9.11,42,43 To specifically confirm and readdress the effect of glucocorticoid treatment on MMP1 expression, we treated primary HTM-109 cells with DEX, triamcinolone acetonide, and prednisolone and measured levels of 18S normalized MMP1 cDNA by real-time TaqMan PCR. Treatment with 0.1 μM DEX in a representative experiment reduced MMP1 expression to relative quantification values of 0.03 ± 0.0002 at 3 days (n = 3; P = 7 × 10−7) and of 0.002 ± 0.0002 at 6 days (n = 3; P = 1 × 10−6; Fig. 1A). Removal of DEX from the cultured medium at 3 days prevented the MMP1 reduction observed at day 6 (0.03 ± 0.002; n = 6; P = 2 × 10−9; Fig. 1A). Analysis of secreted proteins by Western blot conducted in a different experiment showed that MMP1 protein was also reduced in the DEX-treated sample at 5 days while cross-reaction of the stripped blot with myocilin (internal control) was increased (Fig. 1B). These DEX findings were confirmed in four additional mRNA and two additional protein experiments with similar results. Treatment of the cells with 0.1 mg/mL triamcinolone acetonide and 80 μg/mL prednisolone in a representative experiment reduced MMP1 cDNA levels to 0.42 ± 0.1 (n = 3; P = 0.013) and 0.16 ± 0.02 (n = 3; P = 0.002) at 12 and 24 hours, respectively (Fig. 1C). The experiment was repeated once in an HTM-140 cell line with similar findings. These results indicate that treatment with glucocorticoids commonly used in a clinical setting specifically reduced the expression of MMP1 in primary HTM cells.
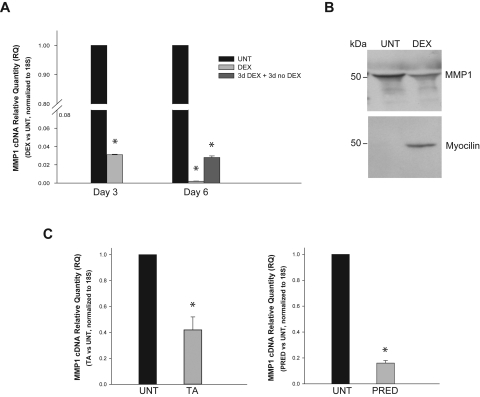
Glucocorticoid effect on endogenous MMP1 expression in human trabecular meshwork cells. Primary HTM cells were treated with the indicated glucocorticoids in serum-containing medium. Control cells were treated with drug vehicle (untreated [UNT]). After treatment, cells were processed for RNA extraction, RT reaction, and real-time TaqMan PCR using MMP1 and 18S TaqMan probes. Changes in gene expression were measured in relative quantity units (RQ) to the vehicle-treated cells (n = number of measurements in a representative experiment). (A) Confluent cells were exposed to 0.1 μM DEX for up to 6 days (n = 3 each). In one well, DEX was removed at 3 days and harvested 3 days later (n = 6). (B) Media from DEX-treated cells were assayed in 4% to 15% SDS-PAGE gels and probed with anti-human MMP1 antibody; after stripping, the membrane was reprobed with anti-human myocilin antibody as internal control. (C) Subconfluent cells were treated with triamcinolone acetonide (TA; 0.1 mg/mL) for 12 hours (left) or prednisolone 21-acetate (PRED; 80 μg/mL) for 24 hours (right; n = 3 each). *P ≤ 0.013. HTM cells treated with three different glucocorticoids; greatly downregulated expression of endogenous MMP1.
Design of a Glucocorticoid-Inducible Vector to Counteract the DEX Effect on Trabecular Meshwork ECM
Given a previous microarray report11 and our results regarding the specific downregulation of MMP1, we hypothesized that this enzyme could be a key mediator of increased ECM deposition caused by glucocorticoids in the trabecular meshwork.16 We then reasoned that increasing the level of MMP1 expression only at the time of glucocorticoid treatment could counteract its negative effects on the ECM and possibly lead to lowering IOP. To achieve this regulated expression, we designed a glucocorticoid-inducible vector in which human recombinant MMP1 cDNA expression would be under the control of the cis-acting GRE.
RNA was reverse transcribed, and the full-coding MMP1 sequence (1410 nucleotides) was amplified with restriction site-ended primers containing a Kozak sequence, as indicated in the Supplementary Material. The MMP1 cDNA was inserted downstream of three tandem copies of the GRE consensus sequence fused to a TATA-like promoter region from the HSV-thymidine kinase gene available in the commercial vector pGRE.Luc (Clontech). The GRE consensus element consists of a nonperfect palindromic sequence (GGTACAnnnTGTTCT) that is part of a GC regulatory response unit and can involve more than one GRE, half-site GREs, and even negative GREs.44 To avoid spurious transcription from upstream sequences, the vector contains a transcription blocker (TrBlk) upstream of the GRE element. This 154-bp TrBlk contains a synthetic polyA site and a transcription pause site from the α2 globin gene.45 The entire MMP1 expression cassette (TrBlk.GRE.PTAL.MMP1.pA) was then inserted into a shuttle vector to generate the recombinant adenovirus vector, as indicated in the Supplementary Material (AdhGRE.MMP1; Figs. 2A, A,22B).
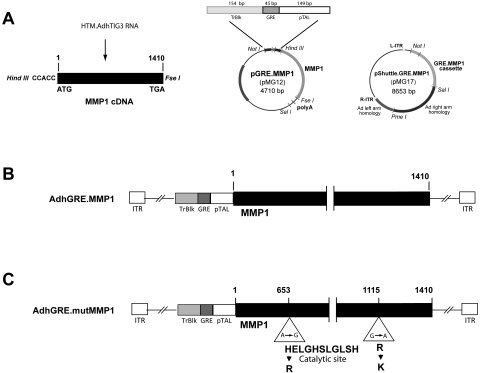
Schematic representation of the construction of glucocorticoid-inducible virus vectors expressing MMP1. (A) A glucocorticoid-inducible shuttle vector containing the full-coding MMP1 (pMG17) was generated by first inserting the MMP1-amplified RT from AdhTIG3-infected cells downstream of the TrBlk.GRE.pTAL element of plasmid vector pGRE-Luc (pMG12); this was followed by subcloning the full GRE.MMP1 cassette into pShuttle vector using NotI/SalI enzymes. (B) AdhGRE.MMP1 recombinant virus DNA was generated from a plasmid obtained by overlapping recombination of electroporated linear pMG17 DNA and the adenovirus backbone vector inside the BJ5183-Ad1 cells. (C) AdhGRE.mutMMP1 recombinant virus DNA was generated in a similar matter, except that the full-coding MMP1 cDNA contained two single-point mutations; one of the mutations is at the catalytic site. Ad-recombinant plasmid DNAs from wild-type and mutant MMP1 were subsequently digested with PacI and transfected into QBI-HEK293A cells for the generation of the viral particles.
A negative functional control vector containing a mutation in the MMP1 active catalytic site was also generated (AdhGRE.mutMMP1; Fig. 2C). This mutant MMP1 cDNA contains two nucleotide changes at positions 653 and 1115 cDNA that render histidine-to-arginine and arginine-to-lysine amino acid changes, respectively. The first of the two changes affects His199, which is one of the three essential zinc-binding ligands present in the active site. This change leads to improper folding of the protein and destroys the catalytic activity of MMP1.46 The details of viral constructions are provided in the Supplementary Material.
DEX-Regulated Induction of Viral-Transferred MMP1
To evaluate the expression levels of recombinant MMP1 in response to DEX treatment, we infected 80% confluent HTM-109 cells with either AdhGRE.MMP1 or AdhGRE.mutMMP1 (MOI 5000 and 6600 vg/cell, respectively) for 5 days in the presence or absence of 0.1 μM DEX. Results from a representative experiment showed that the normalized level of MMP1 mRNA in the wild-type–infected, DEX-treated samples was 79.2 ± 9.8-fold of the infected untreated controls (n = 3; P = 5 × 10−6). MMP1 expression of the mutant-infected, DEX-treated cells was 83.2 ± 10.1-fold of the infected untreated controls (n = 3; P = 1 × 10−6). This high value of expression by the mutant MMP1 virus is expected because the MMP1 mutations are not supposed to affect gene transcription (Fig. 3A). The experiment was repeated once with similar findings.
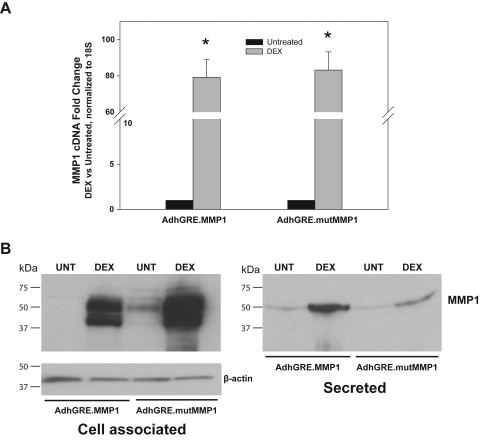
DEX-induced overproduction of recombinant MMP1 in HTM cells. Subconfluent primary HTM cells were infected with adenovirus vectors AdhGRE.MMP1 (wild-type) and AdhGRE.mutMMP1 (mutant). Cells were treated with 0.1 μM DEX at infection and every 2 to 3 days thereafter. Control cells were treated with vehicle (untreated [UNT]). Five days after infection and treatment, cells were harvested for RNA or protein and were processed for TaqMan assays or Western blot analysis (n = number of measurements in a representative experiment). (A) Fold changes of wild-type and mutant MMP1 cDNA expression in infected/DEX-treated cells over infected/untreated controls normalized to 18S (n = 3 each). (B) Western blot analysis of cell-associated and secreted MMP1 in infected/DEX-treated and infected/untreated samples probed with anti-human MMP1 (n = 3 each). *P ≤ 5 × 10−6. Expression of MMP1 in infected cells (mRNA and protein) is highly upregulated in the presence of DEX but is barely expressed in the absence of glucocorticoid treatment. High levels of mutant mRNA and protein were expected because the mutation only blocks MMP1 activity.
To determine the levels of recombinant MMP1 protein in HTM cells infected with AdhGRE.MMP1, immunoblotting analysis was carried out for both conditioned media and cell lysates. Our results showed that infected cells treated with DEX produced high levels of recombinant MMP1 protein in the cell-associated and secreted fractions compared with infected, untreated cells (Fig. 3B). Additional quantification of secreted MMP1 levels after AdhGRE.MMP1 infection was determined by ELISA. At 5 days after infection, recombinant MMP1 levels in DEX-treated HTM cells were 6491 ± 169 ng/mL 9 (n = 2) compared with 282 ± 32 ng/mL (n = 2) in infected untreated cells, respectively, which correspond to a 23-fold induction on DEX treatment. Repeated experiments, also including infection with the mutated virus, confirmed these findings. They further revealed that the level of secreted protein in the AdhGRE.mutMMP1-infected cells was reduced 8.0 ± 2.4 times (n = 3), suggesting that the mutated protein might be either secretion impaired or subjected to easier degradation than the wild type.
Taken together, the results indicate that cells treated with DEX can induce high expression of MMP1 in cells infected with adenovirus vector at both the mRNA and the protein levels.
Sequential ON and OFF Regulation of Delivered MMP1 by Dexamethasone
Once a gene is delivered, one of the goals of using an inducible vector is to be able to turn its expression off and back on again when the corticosteroid stimuli is reapplied. To test the ability of AdhGRE.MMP1 to express the gene under those conditions, we infected HTM-109 cells with 5.1 × 103 vg/cell and treated them with 0.1 μM DEX. In two wells, DEX was removed at day 3 for 3 days, and added back in one of them for an additional 3 days (3d DEX, 3d noDEX, 3d DEX). Results of MMP1 cDNA levels from a representative experiment are shown in Figure 4. DEX induction for 3 days increased the expression of recombinant MMP1, as expected, to 11.7 ± 1.4-fold (n = 3; P = 2 × 10−5). Removal of DEX from the media reversed the induction to 3.4 ± 0.29-fold (n = 3; P = 0.0002), which was 11.7% of the dish that received DEX continuously for the 6 days (29.1 ± 2.0-fold; n = 3; P = 5 × 10−6). Reinduction with DEX for another 3 days restored the levels of MMP1 close to the original level (14.8 ± 1.2-fold; n = 3; P = 0.0003). Two more experiments confirmed the findings. These results indicate that during periods of DEX absence, the vector does not induce overexpression of the recombinant gene. This is important because overexpression under normal physiological conditions could be damaging for the cells and tissues.
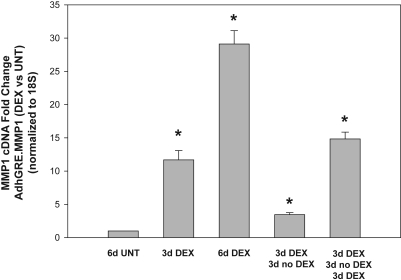
Reversal of DEX induction of MMP1 in wild-type virus-infected cells on removal of the glucocorticoid. Subconfluent primary HTM cells in six-well plates were infected with AdhGRE-MMP1 and treated with 0.1 μM DEX at t = 0. One well was left untreated (UNT). DEX was removed from two infected wells at t = 3 days and added again to one of the two at 6 days. Cells were harvested at the indicated times and were processed for RNA, RT, and TaqMan real-time with MMP1 and 18S probes (n = number of measurements in a representative experiment). MMP1 was upregulated at 3 and 6 days after DEX treatment. This transgene MMP1 upregulation disappeared on the removal of DEX and returned on the reapplication of the corticosteroid (n = 3 each). *P ≤ 0.0002. Cells continuously carrying the MMP1 gene transfer vector can turn on and off the transgene in the presence or absence of the glucocorticoid.
Collagenase Activity of the HTM Adenovirus-Infected Culture Medium
To determine the enzymatic activity of the recombinant MMP1 protein and its ability to degrade collagen, we measured collagen breakdown of the steroid-treated infected cells' media on two independent assays. On the first assay, we measured the ability of the DEX-induced infected media to degrade native rat collagen type I by gel electrophoresis. HTM-109 cells at 80% to 90% confluence in six-well dishes were infected with AdhGRE.MMP1 and AdhGRE.mutMMP1 (MOI 5000 and 6600 vg/cell, respectively) and were treated with DEX for 5 to 7 days (n = 3). Serum was removed for the last 3 days of the experiment, and media were collected for Western blot analysis with MMP1 antibody, as indicated in Materials and Methods. Incubation of the concentrated media with APMA for 3 hours resulted in the switch of the pro-MMP1 band (51 kDa) to the lower, active form of the human MMP1 (41 kDa; Fig. 5A). Incubation of 25 ng purified pro-MMP1 (AnaSpec) was used in parallel as a positive control (Fig. 5A).
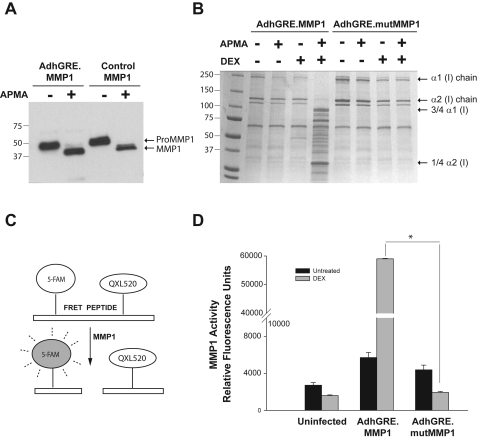
Enzymatic activity of secreted recombinant MMP1 in HTM cells. Subconfluent primary HTM cells were infected with wild-type or mutant adenovirus vectors (AdhGRE.MMP1 and AdhGRE.mutMMP1) and were treated with 0.1 μM DEX at t = 0. Media were concentrated 40×. (A) Ten microliters of media, collected 5 days after wild-type infection, were incubated with 1 mM APMA for 3 hours to cleave pro-MMP1 enzyme-inhibitor complex (51 kDa) and release the active MMP1 (41 kDa). Commercial purified pro-MMP1 protein was used as positive control. Western blot analysis was probed with an anti-MMP1 antibody that detected both the latent and the active form. (B) Five microliters of serum-free media, collected 7 days after infection, untreated and treated with DEX, were activated and incubated with rat tail native collagen type I (10 μg) for 2 hours. Samples were run on a 4% to 15% PAGE gel and were stained with Coomassie blue. (C) Schematic representation of the FRET assay: a MMP substrate peptide labeled with a 5-FAM (fluorophore) and QXL52 (quencher) releases the fluorophore after cleavage of the peptide by MMP1. Fluorescence is read using 490/520 nm EX/EM filters. (D) Ten microliters of serum-containing media, collected 5 days after infection, untreated or treated with DEX, were activated and incubated with the FRET peptide for 40 minutes at 37°C (n = 3 independent experiments). MMP1 activity was expressed in relative fluorescence units of the sample with higher activity. Although the MMP1 produced by the mutant was inactive, the MMP1 produced by the wild-type adenovirus was activated by APMA, degraded native collagen type I, and cleaved the FRET peptide with high efficiency. *P = 1 × 10−9.
Activated media from the AdhGRE.MMP1-infected dishes treated with DEX digested native collagen type I into smaller fragments (Fig. 5B, lane 5). The digested products are the ¾ and the ¼ fragments of the α1 and α2 chains, respectively, and correspond to those previously defined as cleaved by MMP1.40 In contrast, medium from cells infected with AdhGRE.mutMMP1 and overexpressing MMP1 did not exhibit MMP1 enzymatic activity and was unable to cleave collagen (Fig. 5B, lane 9), consistent with the expression of a recombinant mutant MMP1 protein lacking an active catalytic site. In the absence of DEX, the sensitivity of the gel assay was insufficient to detect collagen digestion by the secreted wild type (Fig. 5B, lane 3), which was nonetheless observed by the high sensitivity fluorescence assay (Figs. 5C, C,55D).
On this second assay, we measured the potential of the conditioned media to degrade a fluorescence-labeled MMP1 substrate FRET peptide (AnaSpec). HTM-109 cells at 80% to 90% confluence in six-well dishes were infected with AdhGRE.MMP1 and AdhGRE.mutMMP1 (MOI 5000 and 6600 vg/cell respectively) and were treated with DEX or left untreated for 5 days (n = 3). Media were cleared of cellular debris, concentrated 40×, and treated with APMA, as indicated in Materials and Methods. Equivalent aliquots of treated and control dishes were incubated with the labeled collagen FRET peptide, and the released fluorescence of the cleaved peptide was read in the fluorophotometer (Fig. 5C). The average of three independent experiments is shown in Figure 5D. Relative fluorescence units of the media of uninfected dishes were 2719 ± 292 and 1607 ± 63 for the untreated and DEX-treated cells, respectively. Media from wild-type (AdhGRE.MMP1)-infected dishes showed 5712 ± 550 in the untreated and 59,013 ± 148 in DEX-treated cells. In contrast, media from cells infected with AdhGRE.mutMMP1 gave 4384 ± 512 and 1945 ± 101 in the untreated and DEX-treated samples, respectively. The statistical comparison between the DEX-treated wild-type and mutant was highly significant (P = 1 × 109; Fig. 5D). Validation of equivalent cell numbers in treated and untreated dishes was performed in one experiment by measuring intracellular lactate dehydrogenase (LDH) levels (0.96 vs. 1.27 OD492/mL in uninfected dishes, 1.29 vs. 1.24 OD492/mL, and 1.30 vs. 1.23 OD492/mL in wild-type and mutant virus infected, respectively) using an LDH assay kit (Promega, Madison, WI). Altogether these results indicate that only the recombinant virus with the GRE element driving the wild-type MMP1 secretes the active collagenase when exposed to the glucocorticoid. In addition, the data confirm that, in addition to MMP1 mRNA and protein levels (Figs. 1A, A,1B),1B), DEX also downregulated the activity of MMP-1 (Fig. 5D). Altogether these data indicate that the recombinant MMP1 produced by the AdhGRE.MMP1 virus is able to cleave collagen type I in vitro.
Local Effect of MMP1 Overexpression on Primary HTM Cells
The effect of overexpressing MMP1 in situ was assessed by immunocytochemistry. Primary HTM-106 cells grown in coverslips were treated with 0.1 μM DEX and infected with 2.5 × 103 vg/cell AdhGRE.MMP1. Localization of collagen type I and MMP1 were detected at 48 hours after infection by double labeling with the corresponding primary and secondary antibodies described in Materials and Methods. Figure 6 demonstrates that individual cells overexpressing MMP1 (infected by the virus) exhibited lower levels of collagen type 1 than did cells that were not infected. Conversely, cells with lower MMP1 expression exhibited higher staining intensity for collagen type I. These results indicate that the recombinant MMP1 has a local effect on the collagen of HTM cells. In addition, some of the recombinant MMP1 appeared associated with the cell nuclei. Although this localization was originally unexpected, it has been reported that the transfection of recombinant MMP1 did associate with nuclei and mitochondria in human Müller cells.47
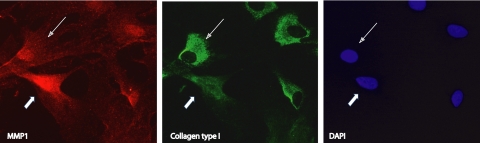
Local effect of overexpressing MMP1 in HTM cells. Forty percent confluent primary HTM cells in coverslips were infected with 2.5 × 103 vg/cell AdhGRE.MMP1 in the presence of 0.1 μM DEX. After 48 hours, cells were fixed and incubated simultaneously with human anti-MMP1 and anti-collagen type I antibodies, followed by fluorescence-tagged Alexa Fluor 555 (for MMP1, red) and Alexa Fluor 488 (for collagen type I, green) antibodies. Cells were counterstained with DAPI (blue). Shown is a single frame captured under Texas Red, FITC, and DAPI filters. A representative infected cell (thick arrows) showed increased MMP1 and decreased collagen type I. A representative uninfected cell (thin arrows) showed higher levels of collagen type I in the presence of lower levels of MMP1.
Effect of MMP1 Overexpression on Perfused Human Anterior Segments
To best mimic an in vivo situation, we next assessed the delivery and DEX induction of the AdhGRE.MMP1 vector to the intact human TM in the perfused organ culture system. We examined the expression levels of MMP1 and measured the ability of the secreted MMP1 to degrade collagen by the FRET assay and immunohistochemistry. Three nonglaucomatous eye pairs from postmortem donors were perfused at constant flow and treated with 0.1 μM DEX, as indicated in Materials and Methods. The HPLC valve connected to one eye was loaded with 6.2 × 109 vg AdhGRE.MMP1 at the time of DEX treatment (t = 0; eye pair 1) or twice (t = 0 and t = 24 h; eye pairs 2 and 3). The HPLC connected to the contralateral eye was loaded with virus vehicle at the same times.
Examination of the delivered MMP1 cDNA in the dissected tissue showed that the mRNA level in the viral injected eye was increased 3977 ± 220-fold over that of the vehicle-injected one (eye 1, n = 3; P = 1 × 10−8; Fig. 7A). Equivalent aliquots from 40× concentrated effluents from the same eye pair analyzed by Western blot analysis showed similar MMP1 band intensities at preinjection time, whereas the intensity increased considerably in the eye injected with AdhGRE.MMP1 at 3 and 5 postinjection days (Fig. 7B). Both pro-MMP1 and MMP1 were observed after perfusion with DEX. Quantification of MMP1 levels in these effluents was determined by ELISA at dilutions of 1:1000, each in duplicate. At preinjection, MMP1 levels were similar in the effluent of both eyes (310 and 317 ng/mL, respectively). At 3 days postinjection, MMP1 levels in the effluent of the AdhGRE.MMP1-injected eye increased 2.4-fold over those in the vehicle-injected eye (409 vs. 168 ng/mL). At 5 days, MMP1 levels in the AdhGRE.MMP1-injected eye reached 4.9-fold over those in the vehicle-injected eye (842 vs. 143 ng/mL).
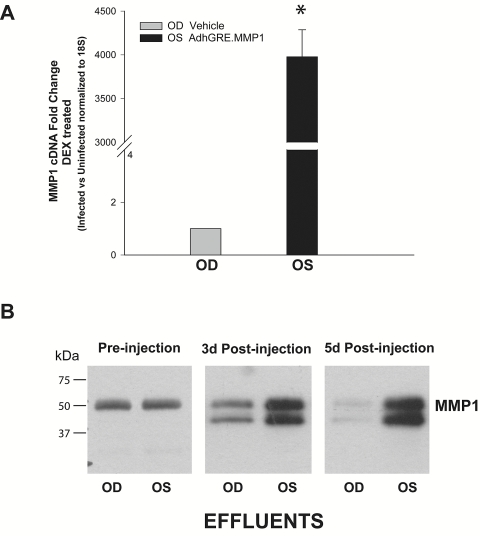
Characterization of adenovirus-delivered MMP1 in perfused human anterior segments. Eye pairs from nonglaucomatous donors were perfused to stable baseline with DMEM and followed by media exchange containing 0.1 μM DEX in both eyes. At this time, eyes were injected through an HPLC loop, and perfusion continued with DMEM/DEX media. One eye received virus vehicle (OD), whereas the contralateral eye received 6.2 × 109 vg AdhGRE.MMP1 (OS). The trabecular meshwork tissue was dissected at the end of the experiment and harvested for RNA and TaqMan assays. Effluents were collected at 3 and 5 days after infection and were processed for the analysis of secreted MMP1. (A) Fold changes of MMP1 transgene (OS) over vehicle-injected (OD) expression using MMP1 and 18S TaqMan probes (eye pair 1, 5 days after injection; n = 3; *P = 1 × 10−8). (B) Equivalent aliquots of concentrated effluents from the same eye pair analyzed by Western blot analysis with a human anti-MMP1 antibody. Delivery of the MMP1 transgene to the OS eye by the adenovirus vector is highly efficient. Perfusion with DEX results in the secretion of pro-MMP1 and MMP1 forms (latent and active).
Activity of the MMP1 enzyme by two different assays is shown in Figure 8. The FRET assay was performed on eye pairs 2 and 3. The ratio of relative fluorescence units in viral- and vehicle-injected eyes (OS/OD) effluents from pair 2 was 1.3-fold at preinjection time and reached 4.7- and 8.7-fold at 2 and 3 days postinjection, respectively (Fig. 8A). In effluents from eye pair 3, the ratio was 1.3-fold at preinjection time and reached 2.8- and 20.0-fold at 1 and 2 days postinjection, respectively. Concentrated effluents from eye pair 1 were run on PAGE gels, electroblotted, and sequentially probed with MMP1 and collagen type I antibodies, as described in Materials and Methods. Results in Figure 8B showed that the intensity of MMP1 protein bands was higher in the effluent of the viral-injected eye than in that of the vehicle-injected control. Conversely, collagen type I bands appeared to be more intense in the vehicle-injected than in the virus-injected eye, concomitant with the presence of a higher activity of the collagenase. Together, these results indicate that the vector successfully enters the cells of the trabecular meshwork tissue, is overexpressed in the presence of DEX, and secretes active MMP1 into the effluent media.
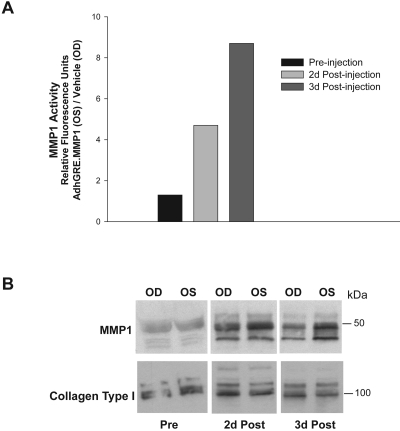
Functional activity of transgene MMP1 delivered to perfused human anterior segments. Eye pairs from nonglaucomatous donors were perfused to stable baseline with DMEM and followed by media exchange containing 0.1 μM DEX in both eyes (t = 0). Eyes were injected through an HPLC loop, and perfusion continued in DMEM/DEX media. One eye (OD) received virus vehicle while the contralateral eye (OS) received 6.2 × 109 vg/dose AdhGRE.MMP1. Effluents were collected at preinjection and postinjection times, concentrated 40×, activated with APMA, and assayed for MMP1 activity. (A) Ten-microliter effluents from OD (vehicle) and OS (wild-type MMP1 adenovirus) were incubated with the FRET peptide for 40 minutes at 37°C. MMP1 activity was measured by quantifying the released fluorescence from the substrate peptide, and it was expressed in ratio of relative fluorescence units of the viral- treated over vehicle (OS/OD; eye pair 2, injected twice at t = 0 and t = 24 hours). (B) Equivalent aliquots of effluents from eye pair 1 (injected once at t = 0) analyzed by Western blot analysis with human anti-MMP1 and anti-collagen type I antibodies. The MMP1 protein produced by the transgene had high enzymatic activity.
At 60 hours after injection, the average of the percentage changes of outflow facility from baseline of the three eye pairs treated with AdhGRE.MMP1 increased 17.9% ± 5.9% μL/min/mm Hg over that of the vehicle-treated eyes. These preliminary results, showing an increase in outflow facility with overexpression of MMP1, were the first indication of this vector's potential for a physiological effect in lowering IOP (see Ref. 36).
The increase in MMP1 and the decrease in collagen could also be observed by immunohistochemistry of the trabecular meshwork tissue by MMP1/collagen type I double labeling of sections from different quadrants in eye pairs 2 and 3 (Fig. 9). In regions of the trabecular meshwork in which MMP1 is intensively stained, collagen type I staining is faint, especially on the inner wall of the SC and in the juxtacanalicular region (Fig. 9). These images also show that the architecture and cell number of the trabecular meshwork tissue were not detrimentally affected by infection with the virus and subsequent overexpression of MMP1. All regions of the outflow tissue appeared healthy and conserved the canonical layered trabecular meshwork structure and well-formed SC.
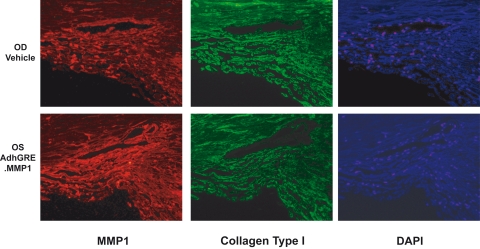
Immunohistochemistry evaluation of MMP1 activity. Eye pairs from nonglaucomatous donors were perfused to stable baseline with DMEM and followed by media exchange containing 0.1 μM DEX in both eyes (t = 0). Eyes were injected twice through an HPLC loop, and perfusion continued in DMEM/DEX media. One eye (OD; top) received virus vehicle (t = 0 and t = 24 hours) while the contralateral eye (OS; bottom) received 6.2 × 109 vg/dose AdhGRE.MMP1 at the same time points. At t = 6 days, anterior segments were fixed and embedded in paraffin. Immunohistochemistry was conducted by double labeling with human anti-MMP1 and anti-collagen type I, followed by fluorescence-tagged Alexa Fluor 555 (for MMP1, red) and Alexa Fluor 488 (for collagen type I, green). Tissues were counterstained with DAPI (blue). Shown is a single representative frame from eye pair 2 captured under Texas Red, FITC, and DAPI filters. Collagen type I was degraded where MMP1 was higher, especially on the SC wall and juxtacanalicular region. Note the apparent widening of the SC area because of the degradation of collagen type I (bottom middle).
Discussion
One of the significant side effects of corticosteroid therapy is the induction of ocular hypertension that, if untreated, would result in the development of steroid-induced glaucoma.10,48 To address this unwanted clinical effect at the molecular level, in the present study we report the development of a gene therapy vector for the potential treatment of steroid-induced glaucoma.
Our results first showed that human primary trabecular meshwork cells treated with corticosteroids greatly downregulated MMP1, a metalloproteinase shown to be involved in the ECM turnover of the trabecular meshwork. MMP1 is required for the maintenance of outflow facility and is upregulated by conditions that lower resistance of the trabecular meshwork and IOP.9 The three steroids tested here, DEX, triamcinolone acetonide (Kenacort-A; Bristol-Myers Squibb), and prednisolone acetate, are widely used in the clinical setting. In particular, the use of intravitreal triamcinolone acetonide (Kenacort-A; Bristol-Myers Squibb) has become very popular for the treatment of macular edema and choroidal neovascularization, and, as a result of this heavy use, more patients are experiencing elevated IOP.49 At the transcriptional level, our experiments showed a downregulation of MMP1 of 500-fold on DEX-treated cells for 6 days and of 2.4- and 6.2-fold on cells treated with the other two steroids for shorter time periods. We also found that the lower transcription of MMP1 resulted in decreased levels of secreted MMP1 protein, a finding that fits nicely with the decreased turnover and increased ECM deposition known to occur in the outflow pathway on corticosteroid treatment.10 All these findings support the concept that downregulated MMP1 protein has detrimental consequences for trabecular meshwork function. Thus, to overcome the MMP1 deficiency that occurs during a steroid administration episode, we designed and engineered a delivery vector with an inducible cassette upstream of the cDNA encoding MMP1. The cassette included a GRE element to respond to the steroid, a basal promoter, and an upstream blocker to avoid the generation of other, nonspecific transcripts.
We found that when primary cells of the human trabecular meshwork were transduced with this vector (AdhGRE.MMP1), they increased their expression of MMP1 mRNA 757-fold in the presence of DEX but not in its absence. This is an indication that, in a clinical setting, the vector would be active only during steroid treatment. Most important, the cycle of induction/noninduction was carried over more than once in the same cells. That is, cells transduced with the vector overexpressed MMP1 under the first exposure to DEX, returned to basal level when the steroid was removed, and overexpressed the enzyme again when exposed to DEX for a second time. This cycled induction/silencing of the vector activity would be of great value if a patient required separate steroid administrations. Because some gene therapy vectors have been shown to remain intracellular (as episomes) and to have the ability to express their transgene for as long as 5 years,50 having this inducible vector would mean that a single dose would be sufficient and that further doses would not have to be reapplied when the next steroid treatment is required. During the vector expression term, its transgene DNA would be present in the eye but latent during periods when steroids are not administered.
Extensive characterization of the MMP1 protein produced by this vector in human primary HTM cells and intact tissue showed that the recombinant enzyme seems to have the same characteristics as endogenous MMP1. In the primary HTM cells, the protein is secreted as a proenzyme that is cleaved and activated by APMA, a thiol-blocking reagent known to activate latent procollagenases, which are enzyme-inhibitor complexes.39 After incubation with APMA and after using an anti-human MMP1 antibody that detects pro-MMP1 and MMP1, we observed the shift from the 51-kDa pro-MMP1 to the lower 41-kDa active form of the human enzyme. Interestingly, the intracellular MMP1 associated with cultured cell extracts contained both forms of the enzyme, pro-MMP1 and MMP1. Regarding the determination of the recombinant enzyme functional activity, our results showed that the liberated active secreted MMP1 retained its full ability to degrade collagen type I. This activity was measured with a classic assay using exogenous native rat collagen and with a state-of-the-art FRET technology assay using a fluorescence-labeled MMP peptide substrate. We further showed the specificity of our results by comparing the expression behavior of the wild-type MMP1 adenovirus with that of a parallel control carrying an identical inducible cassette but with a mutant MMP1 cDNA containing a point mutation in the cDNA region encoding the catalytic site of the MMP1, which theoretically would produce an inactive enzyme. As hypothesized, we found that the levels of mutant MMP1 mRNA and protein were similar to those produced by the wild-type vector. However, the recombinant mutant protein was unable to degrade collagen in both assays. Together these findings demonstrate that the enzyme produced by the wild-type recombinant vector has the specific ability to degrade components of the trabecular meshwork ECM. Although the main role attributed to MMP1 is that of degradation of the ECM, this enzyme could perform yet unidentified intracellular functions in the human trabecular meshwork. The observation that some of the recombinant enzyme appears associated with the nuclei is intriguing and raises the possibility that MMP1 may play a role in other cell functions as it has been shown in other cell types.47
The MMP1 encoded by the AdhGRE.MMP1 vector was also induced by steroids in a model of perfused human anterior segments from postmortem donors. In these organ cultures, the trabecular meshwork maintains its natural architecture, and the perfused media flows in a manner that mimics the flow of aqueous humor through the tissue. Organ cultures also have the advantage of their serum-free culture conditions (important for the study of secreted proteins) and the characteristic of maintaining expression of many genes that get downregulated once the cells are placed in standard tissue cultures. Most important, experiments with paired eyes allow the comparison of vehicle- and vector-injected trabecular meshworks from identical genetic backgrounds. Our results with organ cultures confirmed all findings first observed on the HTM cultured cells. The steroid-regulated increase of recombinant MMP1 was observed at the level of transcription in the dissected tissue and at the level of enzyme secretion in the effluents, showing a further increase with perfusion time. Interestingly, at pre-DEX perfusion, the trabecular meshwork secreted only the pro-MMP1 form of the protein, whereas after perfusion with DEX both vector and vehicle-injected eyes secreted the latent and the active forms. At this time, we do not know why DEX perfusion resulted in the secretion of the active form, which was not observed in the cultured cells. The collagen activity of the effluents measured by FRET was also found to be greatly increased in the eye injected with the gene therapy vector. Double-labeling immunohistochemistry showed that the intensely stained regions of the delivered MMP1 overlapped with the weak staining of collagen type I. To a lesser extent, Western blot analysis revealed overall lower levels of collagen type I in the eyes with higher MMP1 levels. Last, although the number of eyes used in this study was not sufficient to assess a significant change in outflow facility, we observed an increasing trend in the eyes overexpressing MMP1. We interpreted this result as a prelude of the possibility of lowering IOP in vivo, which we subsequently demonstrated to occur in a large animal model of steroid-induced hypertension (see Ref. 36).
Our study, though in a less advanced stage, could be compared with those studies that use inducible gene therapy vectors to treat disease rather than to replace genes and correct genetic defects. Several inducible vectors are being engineered for cancer treatment, some in advanced clinical trials. For example, the TNFerade is an adenovirus vector in which radio- and chemo-inducible elements from the early growth response gene (Egr-1) promoter have been inserted upstream of a cDNA encoding human tumor necrosis factor α (TNFα).51 Local injection of the vector into the tumor, followed by treatment with radiation or chemotherapy, triggers TNFα secretion and enhances antitumor activity (ongoing phase III clinical trial). In the same vein, vectors combining the heat shock protein promoter with a catalytic subunit of diphtheria toxin are inducing cell growth arrest on transfected cells after application of heat.52 Gene therapy for the treatment of acquired diseases continues to advance. Today, more than 60% of ongoing gene therapy trials are designed to treat cancer53 with encouraging results.54 Limitations and challenges relate primarily to the immune responses to vectors and to the high cost of the technology. However, some of the challenges of gene therapy are no different from those involving translational research of other complex medical technologies.
The engineered vector used here was specifically designed to be delivered into the anterior chamber to treat steroid-induced glaucoma. Although this route of administration favors gene delivery to the trabecular meshwork,21 the vector does not yet contain trabecular meshwork target sequences. Once we show that this vector is properly regulated by steroids and is able to secrete an ECM deposition enzyme, additional improvements will follow. Experiments to switch the basal generic promoter by a trabecular meshwork-specific promoter are under way. With the same adenovirus-type vector, we have already determined that the −700/+44-bp promoter region of the αB-crystallin gene targets the expression of a reporter gene to the trabecular meshwork in organ cultures, living rats, and monkeys (Spiga MG et al. IOVS 2008;48:E-Abstract 1612). This αB-crystallin promoter has been selected as a good candidate.
Finally, our laboratory has previously shown that adenovirus vectors deliver transgenes to the trabecular meshwork of living animals with high efficiency and that they do not interfere with outflow facility of the aqueous humor.20,21 For these reasons, combined with our longstanding adenoviral expertise, we first selected an adenovirus for the testing of our new strategy. However, adenovirus vectors might not be the ideal final gene therapy vector to translate this strategy to the ophthalmology clinic for humans. Although highly efficient in transduction, they have a relatively short-term transgene expression and can induce an inflammatory response under certain concentration conditions.21 Fortunately, we have recently shown that a second-generation, self-complementary adenoassociated virus (scAAV) was able to override the lack of trabecular meshwork transduction of their AAV parent vectors. This scAAV vector transduced the trabecular meshwork of living monkeys for more than 2 years.25 Most significantly, the safety of AAV vectors in the eye has been recently corroborated by their success in clinical trials that restored sight in patients with Leber amaurosis.55
In summary, our study provides the first gene therapy strategy for the treatment of steroid-induced glaucoma. Our vector design and engineering showed that a key ECM remodeling enzyme, MMP1, can be induced and silenced by the presence or absence of the steroid. The enzyme produced by the vector under steroid conditions is similar, if not identical, to the endogenous enzyme and retains the activity to degrade the collagen type I MMP1 substrate. Overproduction of the enzyme counteracts its downregulation by corticosteroids. Our findings are further strengthened by the ability of this gene transfer vector to reduce elevated IOP in a model of steroid ocular hypertension in sheep (see Ref. 36). Continuing development of this vector would provide the opportunity to translate our findings to the clinical setting and has the potential to lead to the development of a treatment for steroid-induced hypertension.
Acknowledgments
The authors thank LaKisha K. Buie, K. David Kennedy, and Zahidul Karim for critical reading of the manuscript and for helpful comments.
Footnotes
Supported by National Institutes of Health Grants EY11906 (TB) and EY13126 (TB) and by an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology at the University of North Carolina.
Disclosure: M.-G. Spiga, P; T. Borrás, P
References
Articles from Investigative Ophthalmology & Visual Science are provided here courtesy of Association for Research in Vision and Ophthalmology
Full text links
Read article at publisher's site: https://doi.org/10.1167/iovs.09-4918
Read article for free, from open access legal sources, via Unpaywall:
https://iovs.arvojournals.org/data/journals/iovs/933456/z7g00610003029.pdf
Free to read at intl.iovs.org
http://intl.iovs.org/cgi/content/abstract/51/6/3029
Free after 12 months at intl.iovs.org
http://intl.iovs.org/cgi/content/full/51/6/3029
Free after 12 months at intl.iovs.org
http://intl.iovs.org/cgi/reprint/51/6/3029.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1167/iovs.09-4918
Article citations
Development and testing of a metabolic chamber for effluent collection during whole eye perfusions.
Exp Eye Res, 236:109652, 16 Sep 2023
Cited by: 1 article | PMID: 37717688 | PMCID: PMC10842592
The Role of Metalloproteinases and Their Tissue Inhibitors on Ocular Diseases: Focusing on Potential Mechanisms.
Int J Mol Sci, 23(8):4256, 12 Apr 2022
Cited by: 11 articles | PMID: 35457074 | PMCID: PMC9026850
Review Free full text in Europe PMC
Long-Term Decrease of Intraocular Pressure in Rats by Viral Delivery of miR-146a.
Transl Vis Sci Technol, 10(8):14, 01 Jul 2021
Cited by: 7 articles | PMID: 34254987 | PMCID: PMC8287046
Novel Drug Delivery Systems Fighting Glaucoma: Formulation Obstacles and Solutions.
Pharmaceutics, 13(1):E28, 26 Dec 2020
Cited by: 16 articles | PMID: 33375224 | PMCID: PMC7824381
Review Free full text in Europe PMC
Cathepsin B Localizes in the Caveolae and Participates in the Proteolytic Cascade in Trabecular Meshwork Cells. Potential New Drug Target for the Treatment of Glaucoma.
J Clin Med, 10(1):E78, 28 Dec 2020
Cited by: 11 articles | PMID: 33379277 | PMCID: PMC7795952
Go to all (36) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Treatment of sheep steroid-induced ocular hypertension with a glucocorticoid-inducible MMP1 gene therapy virus.
Invest Ophthalmol Vis Sci, 51(6):3042-3048, 20 Jan 2010
Cited by: 55 articles | PMID: 20089869 | PMCID: PMC2891463
Glucocorticoid induction of the glaucoma gene MYOC in human and monkey trabecular meshwork cells and tissues.
Invest Ophthalmol Vis Sci, 42(8):1769-1780, 01 Jul 2001
Cited by: 96 articles | PMID: 11431441
Regulation of glucocorticoid responsiveness in glaucomatous trabecular meshwork cells by glucocorticoid receptor-beta.
Invest Ophthalmol Vis Sci, 46(12):4607-4616, 01 Dec 2005
Cited by: 42 articles | PMID: 16303956
Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma.
Prog Retin Eye Res, 18(5):629-667, 01 Sep 1999
Cited by: 140 articles | PMID: 10438153
Review
Funding
Funders who supported this work.
NEI NIH HHS (4)
Grant ID: R01 EY011906
Grant ID: EY13126
Grant ID: EY11906
Grant ID: R01 EY013126






